Abstract
Herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is one of four glycoproteins necessary and sufficient for HSV cellular entry. Recently, the crystal structures of HSV-1 gB and vesicular stomatitis virus glycoprotein G were determined. Surprisingly, the two proteins share remarkable structural homology. Both proteins are homotrimeric and center about a long alpha-helix, features reminiscent of class I fusion proteins, such as influenza virus hemagglutinin or paramyxovirus F. However, these structures revealed that G has internal fusion loops, similar to the fusion loops of the class II fusion proteins, and that these loops are structurally conserved in gB. To examine whether these putative fusion loops are important for gB function, we mutated potential membrane-interacting (hydrophobic) residues to charged amino acids. Of most interest were mutant gB proteins that were expressed on the cell surface and were recognized by monoclonal antibodies against conformational epitopes but lacked the ability to function in cell-cell fusion assays. We find that three of the five hydrophobic amino acids targeted in these loops, tryptophan 174, tyrosine 179, and alanine 261, are integral in the function of gB. Our data suggest that they are part of an important functional domain. We hypothesize that two loops in domain 1 of HSV gB function as fusion loops. Our data are further evidence that gB is a viral fusogen and suggest clues as to how gB may function.
Herpes simplex virus type 1 (HSV-1) 1 is the prototypic member of the Herpesviridae family. Its members are large-DNA viruses that infect a broad spectrum of organisms, ranging from invertebrates to humans. HSV displays 11 glycoproteins (g) on its envelope, and four of these, gB, gH, gL, and gD, are essential for virus entry into cells. It is well documented that gD is the receptor-binding protein for the majority of alphaherpesviruses, but it has no structural homologue among the beta- or gammaherpesviruses. Although the details of gD/receptor interaction are relatively well understood (9, 27, 28, 34, 47), the events that follow and lead to fusion are unclear. All herpesviruses use a combination of gB, gH, and gL to carry out fusion (7, 23, 42); they are considered the “core fusion machinery.” gH and gL form a heterodimer (23), and gB is a homotrimer (21). For HSV, binding of gD to either of its receptors likely transmits a signal to gB and/or gH/gL that triggers fusion (14, 28). In support of this, several mutations associated with syncytial phenotypes or that affect the viral rate of entry map to gB (15, 20, 22, 49).
HSV-1 gB is a 904-amino-acid (aa) polypeptide that binds heparan sulfate proteoglycans using a polylysine motif between aa 68 and 76 (30). Soluble truncated gB associates with lipid rafts and blocks virus entry into cells deficient in HPSG (5), suggesting it is the ligand of a non-HPSG cell surface receptor (4). We recently determined the crystal structure of residues 111 through 725 of the gB ectodomain (21). Simultaneously, Roche et al. determined the crystal structure of the vesicular stomatitis virus (VSV) G protein ectodomain in the low-pH form (41). Although there is no primary sequence conservation, the two proteins share a high degree of structural homology.
The crystal structure of VSV G is particularly enlightening because much is known about the function of this protein. As the only VSV glycoprotein, G mediates both cell attachment and fusion. Like gB, it is a homotrimer with a central coiled coil, reminiscent of class I fusion proteins (26, 29, 45). Unlike class I fusion proteins, G contains internal fusion loops, one of which was previously identified by photolabeling and mutagenesis to be critical for virus-cell fusion (11, 50). The fusion loops of G resemble those of class II fusion proteins (41). Because they have structural features of both class I and class II fusion proteins, G and gB have been proposed to represent a new class of viral fusion proteins (41).
Two loops in gB, aa 173 to 179 and 258 to 265, are structurally homologous to the fusion loops of VSV G (21, 41). We hypothesized that they function as fusion loops, and we carried out structure-based mutagenesis to test this hypothesis. Here we report that three of the five tested residues, W174, Y179, and A261, are essential for gB's function in cell-cell fusion. We propose that gB has internal fusion loops, functionally homologous to those of VSV G and class II fusion proteins, that are an important functional domain and possibly interact with the target cellular membrane.
MATERIALS AND METHODS
Cells and viruses.
Mouse fibroblast L cells were grown in Dulbecco's modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Mouse melanoma B78H1 cells, engineered to express the gD receptor nectin 1 (C10 cells), were grown in DMEM supplemented with 5% FBS and 500 μg/ml of G418 (33). For the fusion assay, CHO-K1 and CHO-HVEM12 (CHO-K1 cells engineered to express herpesvirus entry mediator [HVEM]) cells were grown in F12 medium containing 10% FBS and 250 μg/ml G418. CHO-K1, CHO-HVEM12, and C10 cells were kindly provided by P. G. Spear. L cells were a generous gift from F. Tufaro (2).
Construction of gB mutant molecules.
The QuikChange site-directed mutagenesis kit (Stratagene Cloning Systems, La Jolla, CA) was used to generate mutant gB constructs as recommended by the manufacturer and as described previously (10). Briefly, primers designed to mutate individual gB residues were used to amplify the entire pPEP98 plasmid (gB in the pCAGGS background) by PCR (38). The reaction products were then treated with DpnI to digest methylated template DNA and used to transform competent bacteria (Invitrogen One Shot Top10F′). The mutations were confirmed by sequencing of the entire gB gene. pBH730 encodes gBW174Y, pBH732 encodes gB A261D, pBH733 encodes gB F262L, pBH736 encodes gB F262D, pBH739 encodes gB W174R, pBH750 encodes gB A261W, pBH776 encodes gB W174K, pBH777 encodes gB Y179K, and pBH778 encodes gB W174R/A261D.
Antibodies and reagents.
Rabbit serum R69 was raised against full-length gB-1 purified from infected cells (24). Monoclonal antibody (MAb) DL16 was generated using extracts of cells infected by HSV-1 and -2 as immunogens. It recognizes a trimer-specific conformational epitope on gB (3). Hybridoma cell lines expressing MAbs to gB, numbered SS10 to SS145, were generated. SS55 and SS145 both react with different conformational epitopes on gB (3). SS55 also has potent virus-neutralizing activity. SS63 recognizes a linear epitope at the C terminus of the ectodomain (aa 695 to 725). Hybridoma cells were injected into mice to raise ascites. Immunoglobulin G (IgG) was purified from sera and ascites fluid with HiTrap protein G 1-ml columns (Amersham Pharmacia Biotech) and dialyzed against phosphate-buffered saline (PBS) (3). Antimouse and antirabbit secondary antibodies coupled to horseradish peroxidase were purchased from Kirkegaard & Perry Laboratories. The anti-c-myc MAb 9E10 (12) was used as a negative control for immunoprecipitation.
Western blotting and immunoprecipitation.
L cells growing at 37°C in six-well plates were transfected with gB mutant-expression plasmids using 3 μg of DNA/well and 20 μl of GENEporter (Genlantis, San Diego, CA)/well for 3 h before the transfection medium was replaced with growth medium. Cells were incubated at 37°C and were lysed after 48 h in a buffer consisting of 10 mM Tris, pH 8, 150 mM NaCl, 10 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, and 1 mM phenylmethylsulfonyl fluoride (lysis buffer). Nuclei and debris were removed by centrifugation. Proteins in the cell lysates were separated by electrophoresis on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel under denaturing and reducing conditions. Separated proteins were transferred to nitrocellulose, probed using the SS63 IgG, and incubated with secondary antibody (goat antimouse) coupled to horseradish peroxidase. Blots were washed with 0.2% Tween 20 (Bio-Rad) in PBS and visualized by exposure to film after addition of a chemiluminescent substrate (ECL; Amersham).
The MAbs were also used to immunoprecipitate gB from total cell lysates of transfected L cells. Cell extracts were diluted in lysis buffer and incubated with either DL16, SS55, SS145, or anti-c-myc (negative control) IgG for 18 h at 4°C. Proteins were precipitated with protein A-agarose beads (Gibco BRL) for 2 h at 4°C, subjected to electrophoresis on a 10% SDS-polyacrylamide gel, and detected by Western blotting with R69 IgG.
CELISA.
To detect gB cell surface expression, we used a modification of a cell-based enzyme-linked immunoabsorbent assay (CELISA) method (16, 34). CHO-K1 cells growing in 96-well plates were transfected with the T7 polymerase and gD, gH, gL, and gB plasmids by using 40 ng of each plasmid/well and 0.5 μl of Lipofectamine 2000 (Invitrogen), both diluted in Opti-MEM1 (Gibco). Cells were exposed to the DNA-Lipofectamine 2000 mix for 5 h before it was replaced with growth medium. Cells were grown overnight at 37°C, fixed in 3% paraformaldehyde, and rinsed three times with PBS (Ca+/Mg+). Cells were incubated for 1 h at room temperature (RT) with polyclonal antibody (PAb) R69 IgG diluted in 3% bovine serum albumin in PBS, rinsed with PBS three times, and incubated for 30 min at RT with goat antirabbit antibodies coupled to horseradish peroxidase. Following another three washes with PBS, cells were rinsed with 20 mM citrate buffer (pH 4.5). 2,2′-Azino-di(3-ethylbenzthiazoline) sulfonic acid peroxidase substrate (Moss, Inc.) was added, and the absorbance at 405 nm was recorded using a microtiter plate reader.
Quantitative fusion assay.
To detect cell-cell fusion, we modified the luciferase reporter gene activation assay described previously (37, 38). Effector cells (4 × 104 CHO-K1 cells per well) growing in 96-well plates were transfected with plasmids encoding T7 RNA polymerase, gD, gH, gL, and one of the mutant gB plasmids described above. Forty nanograms of each plasmid and 0.5 μl of Lipofectamine 2000/well (Invitrogen), both diluted in Opti-MEM1 (Gibco), were added to cells in each well. Transfections were performed in triplicate. To prepare receptor-bearing target cells, CHO-HVEM12 cells growing in six-well plates were transfected with 10 μl of Lipofectamine 2000/well and 4 μg/well of a plasmid encoding the firefly luciferase gene under control of the T7 promoter. Cells were exposed to the DNA-Lipofectamine 2000 mix for 5 h at 37°C, after which the transfection mixes were replaced with fresh medium and incubated for another hour at 37°C. Target cells were trypsinized, and 4 × 104 cells/well were added to the effector cells and cocultivated at 37°C for 18 h. Cells were washed once with PBS, lysed with 30 μl/well of 1× reporter lysis buffer (luciferase assay system; Promega), and then frozen at −80°C. To measure the extent of fusion, 25 μl of each sample was mixed with 100 μl of the luciferase substrate (Promega) and immediately assayed for light output with a Luminoskan Ascent (Thermo Labsystems). Plasmids encoding the firefly luciferase gene (pT7EMCLuc), T7 RNA polymerase (pCAGT7), gB (pPEP98), gD (pPEP99), gH (pPEP100), and gL (pPEP101) were gifts of P. Spear (37, 38).
Syncytium formation assay.
C10 cells growing in 24-well plates were cotransfected with plasmids encoding gD (pPEP99), gH (pPEP100), gL (pPEP101), and one of the gB mutants. Five hundred nanograms of each plasmid and 5 μl of GENEPorter reagent in 600 μl DMEM/well were added to each well of cells and incubated for 3 h at 37°C, after which the medium was replaced with growth medium. After an 18-h incubation at 37°C, cells were fixed with methanol, stained with Giemsa (Gibco BRL) for 10 min, and examined for syncytium formation by microscopy (8, 10).
Immunofluorescence.
We used a modification of a previously described technique (8). C10 cells were seeded onto glass coverslips in 24-well plates at 7.5 × 104 cells/well and cultured overnight. Cells were transfected with mutant gB expression plasmids using GENEporter (Invitrogen). Eighteen hours posttransfection, cells were fixed with 3% paraformaldehyde for 20 min, quenched with 50 mM NH4Cl for 20 min, and then incubated for 30 min with 0.5% bovine serum albumin in PBS (blocking solution). Cells were incubated with PAb R69 IgG (diluted 1:1,000 in blocking solution) at RT for 30 min, washed with PBS, and then incubated with antirabbit secondary antibody coupled to Alexa 488 (Molecular Probes) (1:1,000 in blocking solution) for 30 min. Coverslips were washed three times with PBS and once with water and mounted on slides in ProLong Antifade mounting solution (Molecular Probes). 4′,6′-Diamidino-2-phenylindole was added to the mounting solution to stain cell nuclei. Cells were observed under a Nikon Eclipse E600 microscope at ×400 magnification using a 488-nm filter.
RESULTS
Selection of target residues.
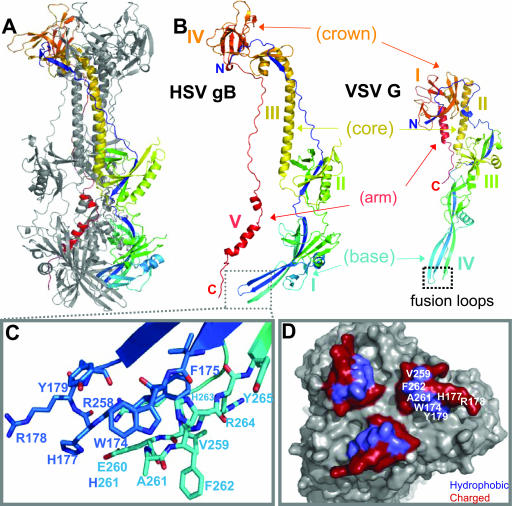
The similarity of the VSV G fusion loops to those of class II fusion proteins and the high degree of structural homology between G and gB guided selection of our targets for site-directed mutagenesis. Figure 1B illustrates the structural homology between the two proteins; the domains of each protomer are labeled according to the original publication (21, 41). Domain IV of VSV G, including the two loops, is conserved in gB as domain I (41). The primary sequence of gB domain I loop 1 is 173VWFGHRY179, and the primary sequence of loop 2 is 258RVEAFHRY265. Thirteen of these residues are labeled in Fig. 1C, and each is colored according to loop. Nine are hydrophobic or nonpolar, while the remainder are charged (Fig. 1D). Five of the hydrophobic residues (V259, F262, A261, W174, and Y179) are clustered and lie on the outer face of a ridge formed by the two loops of each protomer. V259 is conserved among all herpesvirus sequences analyzed, except that of VZV. W174 and Y179 are conserved among the gBs of all alphaherpesviruses analyzed, whereas A261 and F262 are conserved among all HSV-1 and -2 gB sequences analyzed (21). Introduction of a charged residue in place of a hydrophobic amino acid in class I and class II fusion peptides is detrimental to fusion (1, 13, 17). It has been postulated that charged residues cannot insert into the target membrane. To determine the functional importance of the five residues in the hydrophobic cluster, we mutated each to a charged residue. F262 was the most obvious choice, because its location and aromaticity resemble those of key residues in the fusion loops of the class II and VSV fusion proteins (35, 40, 41).
FIG. 1.
HSV gB and VSV G are structurally homologous, including the putative fusion loops. (A) Ribbon diagram of HSV-1 gB. (B) Comparison of HSV-1 gB and VSV G crystal structures (21, 41). One protomer of each trimer is shown, and they are labeled according to their respective domains. Four of the five domains of gB are structurally homologous to VSV G. (C) Closeup of the gB putative fusion loops of one protomer (21). The loops are colored to match panel A, with nitrogens in blue and oxygens in red. (D) A molecular surface representation of the putative fusion loops at the tip of domain I. This view was derived from the one in panel A by rotating the lower tip of the molecule by 90° towards the viewer. Hydrophobic residues are colored in purple and the surrounding charged residues in red. All structural figures were generated, in part, using PyMOL (PyMOL Molecular Graphics System software). Unless stated otherwise, all structures are colored by secondary structure succession.
Rationale for construction of additional mutants.
We mutated F262 to D, W174 to R and K, Y179 to K, V259 to R, and A261 to D. Based on evidence that previous mutagenesis of HSV gB yielded few properly processed proteins (32, 44), we made more conservative substitutions. We mutated A261 and F262 to W and L, respectively. W174 was mutated to Y, because although this residue is a tryptophan in alphaherpesviruses, it is a tyrosine in gB of cytomegalovirus, human herpes virus 6, Epstein-Barr virus, and human herpesvirus 8 (21). In addition, we generated the W174R/A261D double mutant so that one amino acid in each loop was altered simultaneously.
Expression of mutant proteins in mammalian cells.
We tested each mutant protein for expression by transfecting L cells with plasmids expressing each construct. Proteins in the total cell lysate were separated by SDS-polyacrylamide gel electrophoresis under denaturing and reducing conditions. We probed the blots with an anti-gB PAb (R69) and with a MAb that detects an epitope (aa 697 to 725) near the C terminus of the gB ectodomain (SS63) (3).
All mutant forms of gB migrated similarly to full-length wild-type (WT) gB and were detected by R69 and SS63 (data not shown). The level of W174K and A261W mutant expression was reduced with respect to those of the other mutants and to WT gB.
Mutant gB proteins are immunoprecipitated with MAbs against conformation-dependent epitopes.
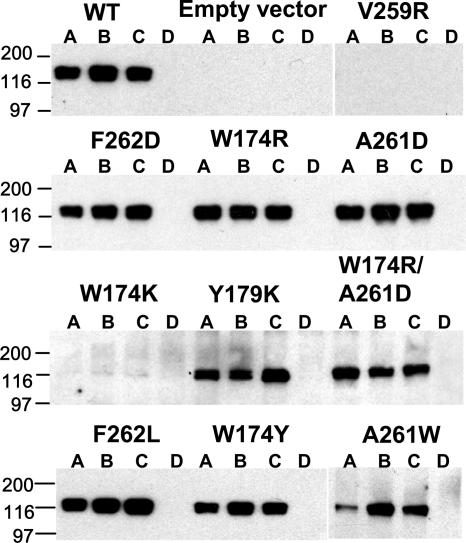
To ensure that the gB mutant proteins were properly folded, we immunoprecipitated each one with MAbs (DL16, SS55, and SS145) against conformational epitopes that map to different regions of gB (3). SS55 has strong virus-neutralizing activity and recognizes an epitope that includes domains I and V (Fig. 1A). Thus, we rationalized that it would be sensitive to any structural changes caused by the mutations. SS145 recognizes an epitope in domain IV (Fig. 1A). Because all fusion proteins function as trimers, it was important to ensure that none of the mutations in gB affected trimerization. This was tested with MAb D16, whose epitope has not been mapped but which recognizes only the gB trimer.
Mutant gB proteins were immunoprecipitated from transfected L-cell lysates (Fig. 2). All mutants except the W174K and V259R mutants were immunoprecipitated by DL16, SS55, and SS145. These data indicate that for eight of the ten mutants, all three epitopes were intact and none of the mutations prevented trimerization. Repetitions show that differences in amounts of the immunoprecipitated A261W mutant are the result of lower protein levels, not a property of the mutant gB. The W174K and V259R mutants were not immunoprecipitated by any of the MAbs, suggesting that they were not properly folded. These mutants were not studied further.
FIG. 2.
Immunoprecipitation of mutant gB proteins from transfected L cells. gB mutant proteins were immunoprecipitated from lysates of transfected L cells using MAbs DL16 (lanes A), SS55 (lanes B), or SS145 (lanes C) against conformational epitopes. A MAb against c-Myc was used as a negative control (lanes D) (12). gB mutations are indicated at the tops of the Western blots. WT gB is expressed from pPEP98, and the empty vector pCAGGS was used as a negative control. PAb R69 was used for detection of gB. Molecular weight markers are labeled on the left in thousands.
Cell-cell fusion and surface expression of mutant gB proteins in C10 cells.
As another way to assess folding as well as transport, we examined the cell surface expression of the mutant gB proteins by immunofluorescence of intact and permeabilized cells. C10 cells growing on duplicate coverslips were transfected with plasmids encoding each mutant. Cells on one coverslip were permeabilized (control for expression), and the others were not. Both sets of coverslips were stained with R69 (anti-gB PAb) and Alexa-488-coupled secondary antibody.
C10 cells were transfected with the WT gB, empty vector (control), or one of eight mutants. WT gB and the two gB mutants were displayed on the cell surface, as were the other six gB mutants (data not shown). Thus, the W174Y, W174R, A261W, A261D, Y179K, F262L, F262D, and W174R/A261D gB mutants are properly folded and expressed on the cell surface. These results are consistent with the notion that loops are more tolerant of mutations than other structural motifs, as 8 of 10 mutations in the putative fusion loops of gB yielded properly folded and processed proteins.
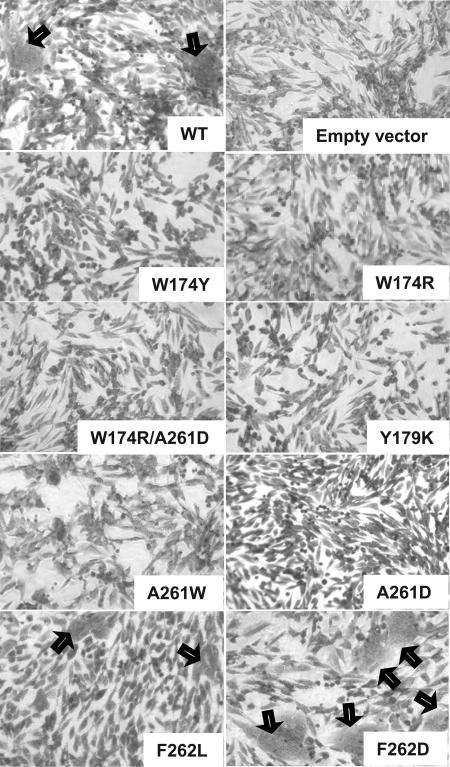
We carried out two types of cell-cell fusion assays to determine the abilities of the gB mutants to function. The first was a qualitative assessment of syncytium formation. C10 cells (stably expressing nectin 1) were transfected with expression plasmids for gD, gB, gH, and gL; their synthesis and cell surface expression result in cell-cell fusion events leading to the formation of large multinucleated cells, or syncytia (36). Syncytium formation requires functional forms of all four of the HSV glycoproteins and therefore can be used to test the effects of mutations in any one of the proteins. C10 cells were transfected with expression plasmids for WT gD, gH, gL, and WT gB or mutants. After 18 h at 37°C, cells were stained with Giemsa to visualize syncytia.
As expected, cells transfected with WT gD, gH, gL, and gB formed several large syncytia, and those transfected with empty vector formed none. Cells transfected with WT gD, gH, gL, and either the W174Y, W174R, Y179K, A261W, A261D, or W174R/A261D mutant were unable to form syncytia (Fig. 3). We expected F262 to be important for function, because its location in the putative fusion loops of gB best resembled the location of aromatic residues in the fusion loops of VSV G and the class II fusion proteins. However, the F262L and F262D mutants both supported syncytium formation. Nonetheless, these data suggest that W174, Y179, and A261 are important in gB function.
FIG. 3.
Syncytium formation assay. To determine the abilities of mutant gB proteins to participate in cell-cell fusion, C10 cells were transfected with plasmids encoding WT gD, WT gH, WT gL, and either WT gB or one of the gB mutants. The cells were incubated for 18 h, fixed with methanol, and stained with Giemsa. Syncytia are indicated by black arrows.
Cell-cell fusion and surface expression of gB mutant proteins in CHO cells.
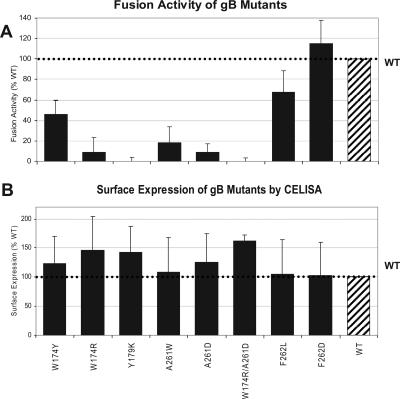
It was still possible that some of the mutant proteins might exhibit quantitative differences in function from wild-type gB. Therefore, we tested them using a quantitative luciferase-based fusion assay with CHO cells (38). Effector cells were prepared by transfecting CHO-K1 cells with plasmids encoding T7 RNA polymerase, gD, gH, gL, and either WT gB or one of the gB mutants in a 96-well plate. A second 96-well plate of effector cells was prepared in parallel to examine surface expression of each form of gB in the same experiment. Target cells were prepared by transfecting cells that stably express the gD receptor HVEM (CHO-HVEM12) with a plasmid encoding the luciferase open reading frame under control of the T7 promoter. The target and effector cells were cocultivated for 18 h and assayed for luciferase activity as a measure of cell-cell fusion. As a control, effector cells that substituted empty vector for WT gB were prepared. These cells were cocultured with target cells, and the luciferase activity was subtracted as the background level.
The fusion activity of the mutants was expressed as a percentage of fusion facilitated by WT gB (Fig. 4A; Table 1). The Y179K and W174R/A261D mutants had no detectable activity in this assay, in agreement with results of the syncytium formation assay (Fig. 3). The W174R, A261D, and A261W mutants functioned minimally, also in agreement with results shown in Fig. 3. The two mutants with substitutions for F262 functioned at least as well as WT gB, confirming this same surprising result seen for syncytium formation (Fig. 3). In fact, the only discrepancy noted was in the case of the W174Y mutant, which did not promote syncytium formation yet exhibited 46% of WT activity in the luciferase assay. Since this mutant, like the others, was expressed on the cell surface, we considered the possibility that fusion might be gD receptor dependent. However, we obtained the same results when we did the syncytium formation assay using B78H1 cells expressing HVEM and the luciferase fusion assay using CHO cells expressing nectin 1 (data not shown).
FIG. 4.
Cell-cell fusion activity and surface expression of gB mutants in CHO cells. (A) Luciferase fusion assay. CHO cells stably expressing HVEM (CHO-HVEM12) were transfected with a plasmid encoding the luciferase gene under the control of the T7 promoter and cocultivated with CHO-K1 cells transfected with plasmids encoding T7 polymerase along with plasmids encoding gD, gH, gL, and either WT gB or the indicated gB mutant. The cells were lysed and assayed for luciferase activity as a measure of cell-cell fusion. The luciferase value for the empty vector was subtracted (background), and all data are represented as percentages of WT activity. Each experiment was performed in triplicate. The data and standard deviations were derived from a minimum of three experiments. (B) CELISA was used as a measure of cell surface expression of gB mutant proteins in CHO cells. Transfected cells were seeded into 96-well plates, and expression of the indicated gB mutant on the cell surface was detected using PAb R69.
TABLE 1.
Summary of gB mutant propertiesa
| Mutation(s) | Fusion loop | Expression in L cells | MAb reactivity
|
Surface expression
|
Fusion
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| DL16 | SS55 | SS145 | Anti-Myc | IFA | CELISA (% WT) | Luc (% WT) | Syncytia | |||
| None | + | + | + | + | − | + | 100 | 100 | + | |
| W174Y | I | + | + | + | + | − | + | 123 ± 47 | 46 ± 14 | − |
| W174K | I | + | − | − | − | − | + | 55 ± 23 | 0 ± 0.25 | − |
| W174R | I | + | + | + | + | − | + | 146 ± 57 | 9 ± 14 | − |
| Y179K | I | + | + | + | + | − | + | 142 ± 45 | 0 ± 10 | − |
| V259R | II | + | − | − | − | − | − | 22 ± 23 | 4 ± 4 | − |
| A261W | II | + | + | + | + | − | + | 108 ± 60 | 18 ± 16 | − |
| A261D | II | + | + | + | + | − | + | 125 ± 50 | 9 ± 8 | − |
| W174R/A261D | I and II | + | + | + | + | − | + | 162 ± 10 | 0 ± 10 | − |
| F262L | II | + | + | + | + | − | + | 105 ± 59 | 68 ± 21 | + |
| F262D | II | + | + | + | + | − | + | 103 ± 57 | 115 ± 23 | + |
IFA, immunofluorescence assay; Luc, luciferase.
However, in spite of this one puzzling observation, the results of the two assays suggest that mutations of residues W174, Y179, and A261 seriously impair or ablate gB function, whereas mutations of F262 do not.
DISCUSSION
Our goal in this study was to determine whether hydrophobic residues in the putative fusion loops of domain I of HSV-1 gB have a role in fusion. These loops are structurally homologous to the known fusion loops of VSV G (Fig. 1 and 5). We carried out site-directed mutagenesis of 5 residues from 2 loops of HSV-1 gB to generate 10 mutants. Two did not fold correctly and were not further characterized. The remaining eight mutants folded correctly and were expressed on the cell surface. Of those, all but two failed to function in cell-cell fusion, suggesting the importance of the mutated residues in fusion. Two of them, W174 and Y179, are in one loop, and the third, A261, is in the other loop. Interestingly, in the structure, side chains of W174, Y179, and A261 are adjacent on the tip of domain I and lie on a hydrophobic ridge formed by the two putative fusion loops (Fig. 5A).
FIG. 5.
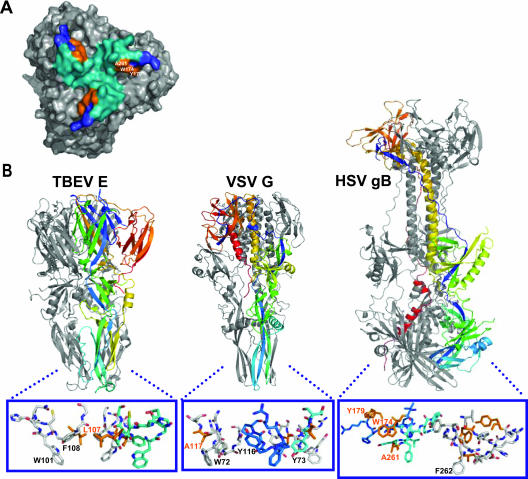
Comparison of HSV gB with VSV G and TBE E. (A) Bottom view of gB. Loop 1 is colored blue, and loop 2 is colored teal. A261, W174, and Y179 (all in orange) are adjacent and form a solvent-accessible region on the outer face of the ridge formed by the putative fusion loops. (B) Crystal structures of TBEV E, VSV G, and HSV gB (21, 40, 41). The fusion loops are boxed and magnified, with key residues labeled. Those that were shown by mutagenesis to be important in function are colored in orange. For clarity, one protomer of gB has been excluded in the magnification. The TBEV E fusion loop has W101 and F108 in an orientation favorable for insertion into a target membrane; L107 is orange (1). VSV G has W72, Y73, and/or Y116 in positions similar to those of W101 and F108 of TBEV E (6). VSV G A117 is orange (13). Mutation of VSV G A177 to K abolished fusion activity (green). One protomer of each trimer is colored by secondary structure succession. gB W174, Y179, and A261 are important in fusion, but only F262 is positioned similarly to the aromatic residues in the fusion loops of VSV G and TBE E.
Contribution of gB W174, Y179, A261, and F262 to fusion.
The crystal structures of class II and VSV fusion proteins in their postfusion states, as well as some functional data, have identified the fusion loops of these proteins (1, 6, 11, 13, 19, 31, 41). It is hypothesized that aromatic amino acids (W, Y, and F) are paramount in their function. It is presumed that their side chains, along with their carbon backbones, are inserted into the target membrane (35, 40, 41). In the case of gB, W174 and Y179 are consistent with this hypothesis. Interestingly, tyrosines and tryptophans are commonly found at the interface between fatty acid chains and head group layers of lipids (48). However, where mutagenesis has been applied (in Semliki Forest virus E1, tick-borne encephalitis virus [TBEV] E, and VSV G), only one nonaromatic amino acid in the fusion loop was changed (1, 13, 25). This is the first report that examines the surface accessible aromatic residues in putative fusion loops.
It is interesting that the A261D mutant was severely impaired in cell-cell fusion. This result suggests two possibilities. Although the alanine side chain is not large enough to be inserted into a target membrane, perhaps the backbone of this residue must be inserted. Similarly, mutation of VSV G A177 to K abolished fusion activity (13) (Fig. 5B). Although it is unclear what role alanine plays in the function of fusion loops, it is clear that it is important in at least VSV G and gB. Somewhat surprisingly, the substitution of a tryptophan at this position did not enhance fusion. We propose that both the position and the context of amino acid side chains are important.
Equally interesting is the result that the F262D mutant supported cell-cell fusion in C10 and CHO-K1 cells at levels similar to those for the WT (Fig. 3 and 4A; Table 1). These results were surprising to us, because we expected F262 to be a critical residue based on its position in the structure (Fig. 1 and 5) (21) and because of the fact that charged residues, such as aspartic acid, are unlikely to be inserted into target membranes (13). It is intriguing that the hydrophobic residues in the putative fusion loops of gB are surrounded exclusively by charged residues (Fig. 1D). Perhaps F262 has no role in gB fusion, or perhaps only the carbon backbone is important.
Final remarks.
It must be considered that gB has three hydrophobic regions downstream of amino acid 725. The third (aa 774 to 795) is the transmembrane anchor (39), and glycines in the second and third (aa 752 to 772 and 774 to 795) have been shown to be important for gB function (46). None of these hydrophobic domains were included in the protein used to solve the crystal structure. At least one of these regions could mask the hydrophobic loops of domain I in the prefusion state. In class II fusion proteins, the fusion loop is located at the tip of the prefusion structure, masked at the dimer interface (18, 43, 51). Such an arrangement would keep gB from aggregating with itself through hydrophobic interactions and mask a potential neutralizing epitope from immune detection. A conformational change, triggered through gD or gH/gL, would then relocate the fusion loops to the target membrane, with the aforementioned hydrophobic ridge inserting into the target membrane.
It is important to note that HSV fusion is more complex than most other viral fusion systems. Because the gH/gL complex is also indispensable for HSV entry, there is likely to be some cooperation between gB and the gH/gL heterodimer. The homology between G and gB suggests that although HSV uses a multicomponent fusion system, some components have developed in a manner similar to that of other viruses. At the same time, gB and gH/gL coevolved to function together in a complex multipartite fusion machine. Our data show that residues W174, Y179, and A261 of HSV gB are key participants in HSV fusion. Are the fusion loops of gB the only HSV fusion peptide? What triggers them to be inserted into the target membrane? These are only two of the many questions that remain for future studies.
Acknowledgments
This work was supported by Public Service grant NS-36731 from the National Institute of Neurological Disease and Stroke to R.J.E. and grants AI-18289, AI-056045, and AI-065886 from the National Institute of Allergy and Infectious Diseases to G.H.C., R.J.E., and E.E.H., respectively. B.P.H. has received support from NIH training grants T32-AI-08034 and T32-GM07229.
We are grateful to P. G. Spear and F. Tufaro for reagents. We also thank all other members of our laboratory for critical advice and helpful discussions.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.Allison, S. L., J. Schalich, K. Stiasny, C. W. Mandl, and F. X. Heinz. 2001. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J. Virol. 75:4268-4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banfield, B. W., Y. Leduc, L. Esford, K. Schubert, and F. Tufaro. 1995. Sequential isolation of proteoglycan synthesis mutants by using herpes simplex virus as a selective agent: evidence for a proteoglycan-independent virus entry pathway. J. Virol. 69:3290-3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, F. C., M. Samanta, E. E. Heldwein, M. Ponce de Leon, E. Bilman, H. Lou, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J. Virol. 81:3827-3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, F. C., J. C. Whitbeck, M. Ponce de Leon, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressanelli, S., K. Stiasny, S. L. Allison, E. A. Stura, S. Duquerroy, J. Lescar, F. X. Heinz, and F. A. Rey. 2004. Structure of a flavivirus envelope glycoprotein in its low-pH-induced membrane fusion conformation. EMBO J. 23:728-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 8.Cairns, T. M., M. S. Shaner, Y. Zuo, M. Ponce-de-Leon, I. Baribaud, R. J. Eisenberg, G. H. Cohen, and J. C. Whitbeck. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J. Virol. 80:2596-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carfi, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 10.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J. Virol. 76:10894-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrer, P., Y. Gaudin, R. W. Ruigrok, R. Graf, and J. Brunner. 1995. Photolabeling identifies a putative fusion domain in the envelope glycoprotein of rabies and vesicular stomatitis viruses. J. Biol. Chem. 270:17575-17581. [DOI] [PubMed] [Google Scholar]
- 12.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fredericksen, B. L., and M. A. Whitt. 1995. Vesicular stomatitis virus glycoprotein mutations that affect membrane fusion activity and abolish virus infectivity. J. Virol. 69:1435-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gage, P. J., M. Levine, and J. C. Glorioso. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 67:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geraghty, R. J., C. R. Jogger, and P. G. Spear. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268:147-158. [DOI] [PubMed] [Google Scholar]
- 17.Gething, M. J., R. W. Doms, D. York, and J. White. 1986. Studies on the mechanism of membrane fusion: site-specific mutagenesis of the hemagglutinin of influenza virus. J. Cell Biol. 102:11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbons, D. L., A. Ahn, M. Liao, L. Hammar, R. H. Cheng, and M. Kielian. 2004. Multistep regulation of membrane insertion of the fusion peptide of Semliki Forest virus. J. Virol. 78:3312-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibbons, D. L., M. C. Vaney, A. Roussel, A. Vigouroux, B. Reilly, J. Lepault, M. Kielian, and F. A. Rey. 2004. Conformational change and protein-protein interactions of the fusion protein of Semliki Forest virus. Nature 427:320-325. [DOI] [PubMed] [Google Scholar]
- 20.Goodman, J. L., and J. P. Engel. 1991. Altered pathogenesis in herpes simplex virus type 1 infection due to a syncytial mutation mapping to the carboxy terminus of glycoprotein B. J. Virol. 65:1770-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 22.Highlander, S. L., W. H. Cai, S. Person, M. Levine, and J. C. Glorioso. 1988. Monoclonal antibodies define a domain on herpes simplex virus glycoprotein B involved in virus penetration. J. Virol. 62:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isola, V. J., R. J. Eisenberg, G. R. Siebert, C. J. Heilman, W. C. Wilcox, and G. H. Cohen. 1989. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J. Virol. 63:2325-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kielian, M., M. R. Klimjack, S. Ghosh, and W. A. Duffus. 1996. Mechanisms of mutations inhibiting fusion and infection by Semliki Forest virus. J. Cell Biol. 134:863-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kielian, M., and F. A. Rey. 2006. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 4:67-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krummenacher, C., A. V. Nicola, J. C. Whitbeck, H. Lou, W. Hou, J. D. Lambris, R. J. Geraghty, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Herpes simplex virus glycoprotein D can bind to poliovirus receptor-related protein 1 or herpesvirus entry mediator, two structurally unrelated mediators of virus entry. J. Virol. 72:7064-7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb, R. A., R. G. Paterson, and T. S. Jardetzky. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laquerre, S., R. Argnani, D. B. Anderson, S. Zucchini, R. Manservigi, and J. C. Glorioso. 1998. Heparan sulfate proteoglycan binding by herpes simplex virus type 1 glycoproteins B and C, which differ in their contributions to virus attachment, penetration, and cell-to-cell spread. J. Virol. 72:6119-6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy-Mintz, P., and M. Kielian. 1991. Mutagenesis of the putative fusion domain of the Semliki Forest virus spike protein. J. Virol. 65:4292-4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, W., T. J. Minova-Foster, D. D. Norton, and M. I. Muggeridge. 2006. Identification of functional domains in herpes simplex virus 2 glycoprotein B. J. Virol. 80:3792-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 34.Milne, R. S., S. A. Connolly, C. Krummenacher, R. J. Eisenberg, and G. H. Cohen. 2001. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315-328. [DOI] [PubMed] [Google Scholar]
- 35.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313-319. [DOI] [PubMed] [Google Scholar]
- 36.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 37.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 38.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 39.Rasile, L., K. Ghosh, K. Raviprakash, and H. P. Ghosh. 1993. Effects of deletions in the carboxy-terminal hydrophobic region of herpes simplex virus glycoprotein gB on intracellular transport and membrane anchoring. J. Virol. 67:4856-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rey, F. A., F. X. Heinz, C. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 41.Roche, S., S. Bressanelli, F. A. Rey, and Y. Gaudin. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187-191. [DOI] [PubMed] [Google Scholar]
- 42.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roussel, A., J. Lescar, M.-C. Vaney, G. Wengler, G. Wengler, and F. A. Rey. 2006. Structure and interactions at the viral surface of the envelope protein E1 of Semliki Forest virus. Structure 14:75-86. [DOI] [PubMed] [Google Scholar]
- 44.Ruel, N., A. Zago, and P. G. Spear. 2006. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 346:229-237. [DOI] [PubMed] [Google Scholar]
- 45.Steven, A. C., and P. G. Spear. 2006. Biochemistry: viral glycoproteins and an evolutionary conundrum. Science 313:177-178. [DOI] [PubMed] [Google Scholar]
- 46.Wanas, E., S. Efler, K. Ghosh, and H. P. Ghosh. 1999. Mutations in the conserved carboxy-terminal hydrophobic region of glycoprotein gB affect infectivity of herpes simplex virus. J. Gen. Virol. 80:3189-3198. [DOI] [PubMed] [Google Scholar]
- 47.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wimley, W. C., and S. H. White. 1992. Partitioning of tryptophan side-chain analogs between water and cyclohexane. Biochemistry 31:12813-12818. [DOI] [PubMed] [Google Scholar]
- 49.Yuhasz, S. A., and J. G. Stevens. 1993. Glycoprotein B is a specific determinant of herpes simplex virus type 1 neuroinvasiveness. J. Virol. 67:5948-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang, L., and H. P. Ghosh. 1994. Characterization of the putative fusogenic domain in vesicular stomatitis virus glycoprotein G. J. Virol. 68:2186-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang, Y., W. Zhang, S. Ogata, D. Clements, J. H. Strauss, T. S. Baker, R. J. Kuhn, and M. G. Rossmann. 2004. Conformational changes of the flavivirus E glycoprotein. Structure 12:1607-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]