Abstract
Coronaviruses (CoVs) possess large RNA genomes and exist as quasispecies, which increases the possibility of adaptive mutations and interspecies transmission. Recently, CoVs were recognized as important pathogens in captive wild ruminants. This is the first report of the isolation and detailed genetic, biologic, and antigenic characterization of a bovine-like CoV from a giraffe (Giraffa camelopardalis) in a wild-animal park in the United States. CoV particles were detected by immune electron microscopy in fecal samples from three giraffes with mild-to-severe diarrhea. From one of the three giraffe samples, a CoV (GiCoV-OH3) was isolated and successfully adapted to serial passage in human rectal tumor 18 cell cultures. Hemagglutination assays, receptor-destroying enzyme activity, hemagglutination inhibition, and fluorescence focus neutralization tests revealed close biological and antigenic relationships between the GiCoV-OH3 isolate and selected respiratory and enteric bovine CoV (BCoV) strains. When orally inoculated into a BCoV-seronegative gnotobiotic calf, GiCoV-OH3 caused severe diarrhea and virus shedding within 2 to 3 days. Sequence comparisons and phylogenetic analyses were performed to assess its genetic relatedness to other CoVs. Molecular characterization confirmed that the new isolate belongs to group 2a of the mammalian CoVs and revealed closer genetic relatedness between GiCoV-OH3 and the enteric BCoVs BCoV-ENT and BCoV-DB2, whereas BCoV-Mebus was more distantly related. Detailed sequence analysis of the GiCoV-OH3 spike gene demonstrated the presence of a deletion in the variable region of the S1 subunit (from amino acid 543 to amino acid 547), which is a region associated with pathogenicity and tissue tropism for other CoVs. The point mutations identified in the structural proteins (by comparing GiCoV-OH3, BCoV-ENT, BCoV-DB2, and BCoV-Mebus) were most conserved among GiCoV-OH3, BCoV-ENT, and BCoV-DB2, whereas most of the point mutations in the nonstructural proteins were unique to GiCoV-OH3. Our results confirm the existence of a bovine-like CoV transmissible to cattle from wild ruminants, namely, giraffes, but with certain genetic properties different from those of BCoVs.
Coronaviruses (CoVs) are enveloped, 80- to 160-nm diameter particles with helical nucleocapsids and positive-sense single-stranded RNA genomes of 26 to 32 kb. They belong to the Coronaviridae family of the Nidovirales order (22, 40). CoVs cause a broad spectrum of diseases in domestic and wild animals, including poultry and rodents, ranging from mild to severe enteric, respiratory, or systemic disease, as well as common colds in humans (8, 14, 22, 31-34). Based on antigenic and genetic similarities, CoVs have been classified into three groups that are known to infect and produce disease in multiple species of animals, including human beings (groups 1 and 2) and birds (group 3).
Bovine CoV (BCoV) is a member of group 2, along with human CoVs (HCoV-OC43 [3, 45] and HCoV-HKU1 [48, 49]), mouse hepatitis virus (MHV) (14), rat CoV (1), porcine hemagglutinating encephalomyelitis virus (30), equine CoV (13), and canine respiratory CoV (11, 12, 31). BCoV is an important agent of neonatal calf diarrhea and is also associated with an acute diarrhea of adult cattle referred to as winter dysentery (34, 35). Besides infecting the small and large intestines of calves, BCoV also possesses a tissue tropism for the upper and lower respiratory tracts (15, 23) and has recently been associated with the bovine respiratory disease complex in feedlot cattle (6, 15, 17, 23, 41). Based on experimental and field studies, it was suggested that both the fecal-oral and nasal modes of transmission for BCoV might be important in the field (6, 8, 15, 23, 31, 32, 34, 41).
CoVs, like other RNA viruses, represent a quasispecies, increasing the possibility of adaptive mutations and interspecies transmission (9). Prior and recent findings support the likelihood that the group 2b CoV severe acute respiratory syndrome (SARS) is a zoonotic infection of animal origin (21, 24, 25, 29, 31, 32, 46, 50). Recently, CoVs have been recognized as important pathogens in captive or wild ruminants in the United States, including sambar deer (Cervus unicolor), white-tailed deer (WTD; Odocoileus virginianus), waterbuck (Kobus ellipsiprymnus), and elk (Cervus elephus) (26, 42). Additionally, some wild ruminants such as caribou (Rangifer tarandus) and musk oxen (Ovibus moschatus) were found to be BCoV seropositive (10), and CoVs were detected in fecal samples from sitatunga (Tragelaphus spekei) and waterbuck (K. ellipsiprymnus) by electron microscopy (EM) and a BCoV-specific enzyme-linked immunosorbent assay (ELISA) (4). In these studies, the captive and wild ruminant CoV isolates showed close antigenic relationships to BCoV strains in vitro (4, 10, 42) or in vivo (42). However, some of the CoV strains (from sitatunga and waterbuck in England) detected by EM failed to replicate or induce clinical signs after experimental oral inoculation of gnotobiotic (Gn) calves, and these strains could not be adapted to growth in BCoV-susceptible cell cultures (4).
In this paper, we describe the isolation of a bovine-like CoV from a giraffe fecal sample and comparative analyses of its antigenic and genomic properties with respect to those of BCoV strains.
MATERIALS AND METHODS
Diarrhea outbreak and giraffe samples.
Fecal samples from three (two male, one female) adult giraffes (Giraffa camelopardalis) (OH1, OH2, and OH3) with clinically mild-to-severe and sometimes bloody diarrhea were submitted within 1 to 2 days after diarrhea onset from a wild-animal park in Ohio to our laboratory in 2003. One of the affected giraffes, which was pregnant, died because of the prolonged (17 days) diarrhea, anorexia, and weight loss; the other two diarrheic giraffes did not develop anorexia and recovered within a 2-week period. The three clinically affected giraffes ranged in age from 14 to 23 years; two developed diarrhea initially, and the third did so several days later. Two other, younger (1- to 9-year-old), giraffes were housed in the park in the same giraffe barn (in separate stalls but with nose-to-nose contact), but they showed no clinical signs.
Of interest, other ruminant species (banteng, sable antelope) at the park that were housed in a separate barn about 0.5 mi from the giraffe barn developed a similar outbreak of diarrhea within 1 to 2 weeks preceding the giraffe outbreak. Feces collected from the sable antelope and tested by immune EM (IEM) with hyperimmune antiserum to BCoV confirmed the presence of a CoV reactive with antiserum to BCoV in this animal (L. J. Saif, unpublished data, 2003).
There were cattle farms about 2 to 5 miles from the wild-animal park, but there were no shared services or direct traffic between the cattle farms and the park and no information about diarrhea outbreaks in the cattle farms. However, within the park, the same individuals were responsible for feeding the various animals and the same dump truck that was used to haul the manure was also used to haul fresh bedding. Also, starlings were present in the giraffe and other ruminant barns.
Reference viruses and antisera.
The BCoV strains and corresponding hyperimmune antisera prepared in guinea pigs included calf diarrhea strains (Mebus and DB2), winter dysentery strains (DBA and TS), and respiratory strains (67NS, 220NS, and 440NS), as described previously (17, 43). The DB2 strain was isolated from a calf with diarrhea, and its virulence was maintained by serial passage of infected feces in Gn calves. The transmissible gastroenteritis virus (TGEV) Purdue-P115 and porcine respiratory CoV (PRCV) ISU-1 strains were described previously (37). All viruses were titrated in fluorescent focus neutralization (FFN) assays. Reference TGEV Purdue-P115 and PRCV ISU-1 antisera were also produced in seronegative guinea pigs or Gn pigs as described previously (37, 47).
Gn calves.
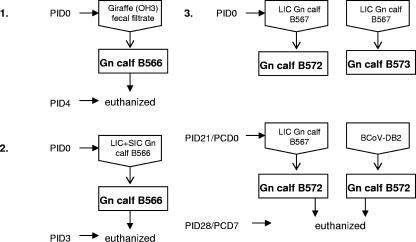
Preparation of fecal sample inocula, inoculation methods, and sample collection were described previously (5). A diagram of the experimental design illustrating the initial giraffe CoV inoculum and subsequent serial passage in Gn calves is shown in Fig. 1. Briefly, 3 ml of a giraffe fecal sample (GiCoV-OH3-FS/WD1421) was diluted 1:10 with minimum essential medium (MEM; Invitrogen Corp., Carlsbad, CA), supplemented with 1% antibiotic-antimycotic solution (Invitrogen Corp., Carlsbad, CA) and 1% NaHCO3 (pH 7.2), and filtered sequentially through 0.8-μm, 0.45-μm, and 0.22-μm syringe filters (Nalge Nunc International, Rochester, NY). The fecal filtrate was used to orally inoculate an 8-day-old Gn calf (B566). All calves were fed with human infant formula (Similac; Ross Laboratories, Columbus, OH), and fecal samples were collected daily as described previously (5). Calf B566 was euthanized at postinoculation day 4 (PID4), and small and large intestinal contents (SIC and LIC, respectively) were collected. A second 13-day-old Gn calf (B567) was orally inoculated with the GiCoV-OH3-containing SIC-plus-LIC sample from the first calf. The calf-passaged sample was diluted 1:5 in MEM supplemented with antibiotic-antimycotic solution. After necropsy (at PID3), LIC collected from this calf was used as GiCoV source material for RNA extraction and sequencing (GiCoV-OH3-Gn calf). Two additional calves, B572 and B573 (8 to 9 days of age), were inoculated as described for B567, with the GiCoV-OH3 LIC sample from Gn calf B567, and fecal samples from these calves were collected daily. From diarrhea onset (PID3) through PID5 (end of severe diarrhea), these two calves were orally given Bounce Back Electrolyte-Energy Supplemented for dehydrated calves (The Manna Pro Corporation, Chesterfield, MO) to alleviate the virus-induced dehydration. At PID21, calf B572 was challenged with virulent BCoV-DB2 (maintained by serial passage in Gn calves) and calf B573 was rechallenged with the GiCoV-OH3-containing LIC sample from Gn calf B567. Blood was collected at PID0, PID14, and PID21 and at postchallenge day 7.
FIG. 1.
Diagram of the experimental design, illustrating the initial giraffe CoV inoculum and its subsequent serial passage in Gn calves. Parts: 1, inoculation of the original GiCoV-OH3-containing feces into a Gn calf (B566); 2, second passage of GiCoV-OH3 in a Gn calf (B567); 3, demonstration of cross-protection between GiCoV-OH3 and BCoV-DB2 in which two Gn calves (B572 and 573) were initially inoculated with GiCoV-OH3 and then either rechallenged with GiCoV (B572) or challenged with BCoV-DB2 (B573).
IEM.
The IEM techniques used were described previously (35). Briefly, supernatants from 20% fecal samples or purified (as described subsequently) cell culture-passaged GiCoV-OH3 were mixed with Gn calf antiserum to BCoV-Mebus and reacted at 4°C for 18 h. Samples were ultracentrifuged (35,000 × g for 30 min), and the pellets were mixed with filtered distilled water and an equal volume of 3% phosphotungstic acid (pH 7.0) and placed on Formvar carbon-coated grids, which were evaluated with an electron microscope (Philips 201; Norelco, Eindhoven, The Netherlands).
ELISA.
An indirect antigen capture ELISA and an antibody capture ELISA with a pool of three monoclonal antibodies (MAbs) directed against the S, N, and HE structural proteins of BCoV strain DB2 were used to detect GiCoV in fecal suspensions and GiCoV-specific antibody in serum samples as previously described (16, 17, 38, 39).
Western blotting.
Polyacrylamide gel electrophoresis and Western blot assays of GiCoV- or mock-infected human rectal tumor 18 (HRT-18) cell supernatants containing viral proteins were performed according to standard protocols as described by Sambrook et al. (36). Clarified virus- or mock-infected HRT-18 cell supernatants were lysed in 1× loading buffer (Fermentas, Hanover, MD) in the presence of 200 mM dithiothreitol. After proteins were separated on gels and transferred to nitrocellulose membranes, they were stained with a pool of BCoV-Mebus spike and nucleoprotein MAbs and a horseradish peroxidase-conjugated anti-mouse immunoglobulin G serum was used as the secondary antibody.
RT-PCR.
The total RNA was extracted from giraffe fecal samples with TRIZOL LS reagent (Gibco, Life Tech, Grand Island, NY) according to the manufacturer's instructions. A one-step reverse transcription (RT)-PCR assay was performed as previously described (5). The oligonucleotide primers used in the RT-PCR were designed from the published sequence of the polymerase and nucleoprotein genes of the CoV strains. The following primer pairs were designed or modified and used for genome detection of BCoV and the related ruminant CoVs. Pan-CoV-specific forward (IN-2deg; GGGDTGGGAYTAYCCHAARTGYGA) and reverse (IN-4deg; TARCAVACAACISYRTCRTCA) universal primers targeting a 452-bp fragment of the polymerase gene were modified in our laboratory from those of Ksiazek et al. (20). Group 2-specific forward (Gr2F; GAAGGCTCDGGAARGTCTG) and reverse (Gr2R; CCTCTYTTHCCAAAACACTG) primers capable of detection of all group 2 CoVs targeting a 300-bp fragment of the nucleocapsid gene were developed and used. BCoV-specific forward (NOF; GCAATCCAGTAGTAGAGCGT) and reverse (NOR; CTTAGTGGCATCCTTGCCAA) primers targeting a 729-bp fragment of the nucleocapsid gene and highly specific for BCoV and bovine-like CoVs were developed and used.
Virus isolation.
Monolayers of HRT-18 cell cultures from cells cloned in our laboratory were used for virus isolation as described previously (2, 17). Cells were inoculated, in duplicate wells, with ELISA- and RT-PCR-positive fecal supernatants, adsorbed at 37°C for 1 h, and then advanced minimum essential medium (AMEM) containing 5 μg/ml pancreatin (Sigma Chemical Co., St. Louis, MO) was added. Cultures were incubated at 37°C in a 5% CO2 atmosphere and examined daily for evidence of cytopathic effects (CPE) or assayed for the presence of CoV by RT-PCR (as described above).
Plaque induction.
The detailed procedures for BCoV plaque induction have been described previously (2, 17). After addition of serial virus dilutions (10−1 to 10−10) in AMEM to HRT-18 cell monolayers in six-well plates (Costar, Cambridge, MA), monolayers were incubated at 37°C for 1 h to allow virus adsorption. The plates were then overlaid with AMEM containing 1.6% Noble agar (BBL, Cockeysville, MD) plus 0.1% neutral red (Sigma Chemical Co., St. Louis, MO), 0.1% pancreatin, and 1% DEAE dextran (Sigma Chemical Co., St. Louis, MO) (AMEM plus). Plates were inverted and incubated at 37°C in a 5% CO2 atmosphere for 3 to 5 days, and induction of plaques was assessed.
Virus purification.
The cloned GiCoV-OH3 isolate was purified from the HRT-18 cell culture supernatants as described previously (17). Briefly, the clarified, virus-infected cell culture supernatants were purified by ultracentrifugation (112,000 × g for 2 h) on 20% to 50% sucrose density gradients in an ultracentrifuge (L8-M; Beckman, Schaumburg, IL).
Hemagglutination (HA) and receptor-destroying enzyme (RDE) activity tests.
HA tests were conducted by the microtiter method with V-bottom plates (Dynex Tech Inc., Chantilly, VA) as described previously (16, 17). Briefly, serial twofold dilutions of the purified CoV strains were prepared in 25 μl of Veronal-buffered saline solution (pH 7.2) and mixed with 25-μl suspensions of mouse (0.8%) or chicken (0.4%) erythrocytes. Mixtures were incubated (4°C or 37°C for 1 h). After incubation, titers were expressed as the reciprocal of the highest dilution of virus that caused complete HA. Plates were incubated at 37°C for an additional 4 h to assess RDE activity, which was expressed as the reciprocal of the highest dilution of virus resulting in complete disappearance of the HA patterns (17).
HA inhibition (HI) assay.
The procedure for the HI test has been described elsewhere (17). Briefly, all serum samples were treated with kaolin and packed mouse erythrocytes to remove nonspecific hemagglutinins. Then, 25 μl of antiserum (in serial twofold dilutions) was mixed with 25 μl of 8 HA units of purified CoV strains and incubated at 22°C for 1 h. After incubation, 50 μl of a 0.8% suspension of mouse erythrocytes was added and the mixture was incubated at 22°C for 2 h. Results were considered positive when HA (pellet formation) was inhibited.
FFN assay.
The FFN test was performed with 50% fluorescent focus units (50% FFU/0.1 ml) of GiCoV or the BCoV strains, which was calculated by performing cell culture immunofluorescence (CCIF) tests with 96-well microtiter plates (Costar, Cambridge, MA) as described previously (17). Briefly, to define the virus titer in the CCIF assay, 7-day-old monolayers of HRT-18 cell cultures in 96-well plates were inoculated with 10-fold serial dilutions of virus in AMEM containing 0.025 μg/ml pancreatin. After incubation for 18 h at 37°C in a 5% CO2 atmosphere, cells were fixed with 80% acetone and stained with fluorescein isothiocyanate-conjugated hyperimmune Gn calf antiserum to BCoV (Mebus strain). The cells were considered positive for CoV antigen when they showed specific cytoplasmic fluorescence distinct from any background reactions in controls. The percentage of positive cells in each specimen was determined after the examination of five different fields of view with a fluorescence microscope (Olympus, Tokyo, Japan), and the number of FFU per milliliter was calculated. In the FFN test, fourfold serially diluted guinea pig anti-BCoV and anti-TGEV (group 1 CoV control) sera and bovine anti-GiCoV-OH3 serum samples were mixed with 50% FFU/0.1 ml of the virus isolate or BCoV strain, incubated for 60 min at 37°C, and added to 7-day-old monolayers of HRT-18 cells in 96-well plates. The next steps were the same as for the CCIF assay described above. The FFN test for TGEV Purdue-P115 and PRCV ISU-1 was performed similarly to that for BCoV, except that 3-day-old swine testicular cells were used and virus was detected with mouse MAbs against TGEV, followed by fluorescein isothiocyanate-conjugated anti-mouse immunoglobulin G (KPL, Gaithersburg, MD) (47).
Sequencing. (i) RNA extraction.
RNA was extracted as described earlier from the original giraffe fecal sample (GiCoV-OH3-FS), the supernatant of HRT-18 cell cultures from the fourth serial passage (two passages on HRT-18 cells, followed by plaque purification and one more passage of plaque-purified virus) of the GiCoV-OH3 strain (GiCoV-OH3-CC) and an LIC sample from the second passage of GiCoV-OH3 in a Gn calf (GiCoV-OH3-Gn calf).
(ii) cDNA synthesis.
Specific oligonucleotide primers were designed with BCoV (NC_003045) as a reference genome and purchased from Invitrogen (Carlsbad, CA). Primers were designed every 500 bp along the genome. An M13 sequence tag was added to the 5′ end of each primer to be used for sequencing (F primers, TGTAAAACGACGGCCAGT; R primers, CAGGAAACAGCTATGACC). Primer sequences are available from the authors. RT-PCRs were performed with 50 to 200 ng of CoV RNA with QIAGEN OneStep RT-PCR kits (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Duplicate reaction mixtures were analyzed by agarose gel electrophoresis for quality control. Amplicons were prepared for sequencing by incubation at 37°C for 60 min with 0.5 U of shrimp alkaline phosphatase (USB, Cleveland, OH) and 1 U of exonuclease I (USB) to inactivate the remaining deoxynucleoside triphosphates and to digest the single-stranded primers. The enzymes were inactivated by incubation at 72°C for 15 min.
(iii) Sequencing and sequence editing.
Sequencing reactions were performed with a standard high-throughput sequencing system by using Big Dye Terminator chemistry (Applied Biosystems) with 2 μl of template cDNA. Each amplicon was sequenced from each end with M13 primers (F primer, TGTAAAACGACGGCCAGT; R primer, CAGGAAACAGCTATGACC). Sequencing reactions were analyzed on an ABI 3730 sequencer.
Sequencing reads were downloaded, trimmed to remove amplicon primer-linker and low-quality sequences, and assembled with TIGR Assembler (from The Institute for Genomic Research [TIGR], www.tigr.org/software/assembler/). To close gaps between assembled contigs, strain-specific primers were designed, RT-PCRs were performed, and amplicons were sequenced as described above. Additional primer design, cDNA synthesis, and sequencing were performed to ensure greater-than-fourfold sequence coverage along the CoV genomes.
Assemblies were manually edited with CloE (Closure Editor), a TIGR program for editing assemblies. All apparent polymorphisms were checked against reference data, and ambiguities were analyzed by RT-PCR and cloning. Each assembly was analyzed with Viral Genome ORF Reader (VIGOR), a program designed at TIGR to predict viral protein sequences. VIGOR checked segment length, alignments with reference sequences, and fidelity of reading frames; correlated amino acid mutations with nucleotide polymorphisms; and detected potential sequence errors.
Sequence analyses.
The reference CoV genome sequences from GenBank compared for phylogenetic analyses are as follows. The group 1 CoVs were HCoV-229E (NC_002645), HCoV-NL63 (NC_005831), PEDV (NC_003436), FIPV (NC_007025), and TGEV (NC_002306). The group 2a CoVs were HCoV-0C43 (NC_005147), HCoV-HKU1 (NC_006577), MHV-A59 (NC_001846), BCoV-Mebus (U00735), BCoV-ENT (NC_003045), and BCoV-DB2 (DQ811784). The sole group 2b CoV was SARS-Tor2 (NC_004718). The sole group 3 CoV was infectious bronchitis virus strain Beaudette (NC_001451). Sequence alignment and phylogenetic analysis were performed by the ClustalW method of the Lasergene Biocomputing Software (DNASTAR Inc., Madison, WI). The GiCoV-OH3 sequence was compared with the human and animal CoV strains in GenBank. The deduced amino acid sequences were then assembled and analyzed with the MegAlign module of the Lasergene Biocomputing Software.
Nucleotide sequence accession numbers.
The sequences determined in this study were submitted to GenBank and assigned accession numbers EF424623, EF424622, and EF424624.
RESULTS
Biological and antigenic characterization of the GiCoV-OH3 isolate.
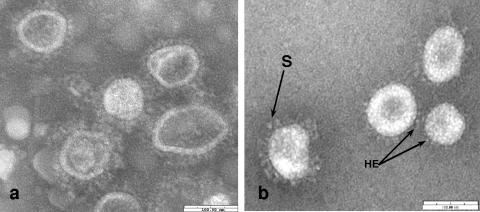
Three fecal specimens from giraffes were tested by IEM, antigen capture ELISA, and RT-PCR with pan-CoV-, group 2-, and BCoV-specific primers. The RT-PCR results were positive for all three test samples. By ELISA, one (OH3) of the three samples showed a high absorbance value. Virus isolation for all three samples was attempted by serial passage of the fecal filtrates on HRT-18 cell monolayers. The OH-1 and OH-2 samples did not show CPE after 10 blind serial passages, whereas OH-3 showed CPE after the second passage. The CPE, characterized by enlarged, rounded, detached cells, was observed approximately 48 h after inoculation. Confirmation of virus replication in cell culture was performed for each passage by RT-PCR with pan-CoV-specific primers. After plaque isolation of the giraffe CoV (GiCoV-OH3 strain), a single plaque-purified virus clone was inoculated onto HRT-18 cell monolayers for virus amplification and subsequent purification. Plaques observed were circular, 1 to 1.5 mm in diameter, and very similar to BCoV in morphology. In addition, 10-fold serial dilutions of the plaque-purified cell culture-passaged virus tested by CCIF assay demonstrated a viral titer of 4.7 × 109 FFU/ml. After purification on a sucrose density gradient, typical negatively stained CoV particles with an approximate diameter of 100 to 120 nm were clearly visible by IEM for the GiCoV-OH3 plaque-isolated strain (Fig. 2b). Purified virus was then used for assessment of HA and RDE activities, and the results were compared with those of BCoV strains (Table 1). In the HA test with mouse erythrocytes, the results for the GiCoV-OH3 strain were similar to those for BCoV strains at both 4°C and 37°C, whereas the ability to agglutinate chicken erythrocytes by GiCoV-OH3 was observed only at 4°C. This result is consistent with similar results for one enteric BCoV strain (WD BCoV-TS) and two respiratory BCoV strains (67NS and 220NS). In addition to HA of mouse erythrocytes at 37°C, the GiCoV isolate showed RDE activity (with mouse erythrocytes), which was evident as a loss of the HA patterns formed at 4°C.
FIG. 2.
IEM of (a) the giraffe CoV (GiCoV-OH3) in feces from the second passage in a Gn calf (B567) and (b) plaque-isolated, sucrose-purified GiCoV-OH3 from an HRT-18 cell culture. Both samples were incubated with Gn-calf hyperimmune antiserum to BCoV, leading to the specific viral-antibody aggregates with an antibody fringe evident on particles in panel a. S denotes longer spikes, and HE indicates a shorter hemagglutinin layer. The bar represents 100 nm.
TABLE 1.
HA and RDE titers of giraffe CoV (GiCoV-OH3) and BCoV strains
| Virus strain (origin)a | HA titer at:
|
RDE titer
|
||||
|---|---|---|---|---|---|---|
| 4°C
|
37°C
|
Mouse | Chicken | |||
| Mouse | Chicken | Mouse | Chicken | |||
| BCoV-Mebus (CD) | 1,600 | 400 | 1,600 | 400 | <25 | <25 |
| BCoV-DB2 (CD) | 800 | 400 | 800 | 400 | <25 | <25 |
| BCoV-DBA (WD) | 1,600 | 400 | 1,600 | 400 | <25 | <25 |
| BCoV-TS (WD) | 800 | 400 | 800 | <25 | <25 | ≥400 |
| GiCoV-OH3 | 3,200 | 400 | 3,200 | <25 | <25 | ≥400 |
| BCoV-67NS (resp) | 400 | 50 | 400 | <25 | <25 | <25 |
| BCoV-220NS (resp) | 800 | 100 | 800 | <25 | <25 | <25 |
| BCoV-440NS (resp) | 800 | 100 | 800 | 100 | <25 | <25 |
CD, calf diarrhea; WD, winter dysentery; resp, respiratory nasal sample.
By using HI in two-way tests to compare the GiCoV-OH3 isolate with enteric and respiratory BCoV strains, no differences greater than fourfold were detected (Table 2). Similar results were obtained with the FFN test. Antisera against the porcine CoV TGEV Purdue-P115 and PRCV strains (group 1 CoVs) were also included in the FFN test, but the results were negative (<4) (Table 2).
TABLE 2.
Two-way HI and FFN test results for GiCoV-OH3, BCoV, TGEV, and PRCV strains
| Virus strain (source) | HI or FFN titerb of antiserum to:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GiCoV-OH-3 | Mebus | DB2 | DBA | TS | 67NS | 220NS | 440NS | TGEV Purdue-P115 | PRCV ISU-1 | |
| GiCoV | 320 | 160 | 320 | 320 | 320 | 320 | 160 | 320 | NT | NT |
| GiCoV-OH3 (Gn calf) | ||||||||||
| BCoV | 1,024 | 1,024 | 256 | 1,024 | 1,024 | 256 | 256 | 1,024 | <4 | <4 |
| CD-Mebus (GP)a | 320 | 320 | ||||||||
| CD-Mebus (GP) | 256 | 1,024 | ||||||||
| CD-DB2 (GP) | 640 | 640 | ||||||||
| CD-DB2 (GP) | 1,024 | 1,024 | ||||||||
| WD-DBA (GP) | 640 | 640 | ||||||||
| WD-DBA (GP) | 256 | 256 | ||||||||
| WD-TS (GP) | 1,280 | 1,280 | ||||||||
| WD-TS (GP) | 4,096 | 4,096 | ||||||||
| Resp-67NS (GP) | 1,280 | 1,280 | ||||||||
| Resp-67NS (GP) | 1,024 | 1,024 | ||||||||
| Resp-220NS (GP) | 160 | 160 | ||||||||
| Resp-220NS (GP) | 256 | 256 | ||||||||
| Resp-440NS (GP) | 160 | 160 | ||||||||
| PRCV | 1,024 | 1,024 | ||||||||
| TGEV-P115 (GP) | NT | NT | ||||||||
| TGEV-P115 (GP) | <4 | 1,024 | ||||||||
| PRCV-ISU-1 (Gn pig) | NT | NT | ||||||||
| PRCV-ISU-1 (Gn pig) | <4 | 1,024 | ||||||||
GP, guinea pig.
Boldface values represent FFN test titers, nonbold values represent HI titers. NT, not tested.
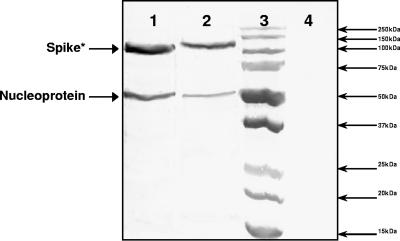
BCoV-DB2-, GiCoV-OH3-, or mock-infected HRT-18 cell supernatants were assayed in Western blot assays with a pool of BCoV-Mebus spike and nucleoprotein MAbs. Cross-reactivity was observed for both major CoV proteins, the spike protein and nucleoprotein (Fig. 3).
FIG. 3.
Demonstration of cross-reactivity between BCoV-DB2 and GiCoV-OH3 by Western blotting with a pool of BCoV-Mebus spike and nucleoprotein MAbs. Lanes: 1, supernatant of HRT-18 cells infected with BCoV-DB2; 2, supernatant of HRT-18 cells infected with GiCoV-OH3; 3, protein ladder (Precision Plus protein standards, dual color; Bio-Rad, Hercules, CA); 4, supernatant of mock-infected HRT-18 cells. *, the smaller size of the band corresponding to the spike protein is due to proteolytic cleavage between the S1 and S2 subunits. The glycosylated S1 subunit represented on the blot has a molecular mass of about 120 kDa.
After inoculation of a Gn calf (B566) with the original sample (GiCoV-OH3-FS), virus shedding and severe diarrhea were detected by PID2, and virus shedding in fecal samples was detected by ELISA and RT-PCR through the day of necropsy (PID4). The second Gn calf (B567) inoculated with the GiCoV-containing LIC-plus-SIC sample from the first calf developed similar clinical signs with severe diarrhea and virus shedding detected from PID2 through the day of necropsy at PID3. The calves were euthanized within 1 to 2 days after the onset of diarrhea due to humane considerations because of their deteriorating health. The presence of GiCoV-OH3 in Gn calf fecal samples was also confirmed by IEM (Fig. 2a). Gn calves B572 and B573 inoculated with the GiCoV-containing LIC sample from calf B567 demonstrated the same pattern of disease, with the only difference being that diarrhea and virus shedding started at PID3 and virus shedding was detectable by RT-PCR and ELISA for 4 days. The supplemental electrolytes helped to alleviate the effects of dehydration due to the pronounced diarrhea. Gn calves B572 and B573 seroconverted by PID14 after primary inoculation with GiCoV-OH3, and the highest BCoV antibody titer was demonstrated at postchallenge day 7 by ELISA. No diarrhea or virus shedding was detected after a challenge with either virulent BCoV-DB2 (B572) or a rechallenge with the GiCoV-OH3 inoculum (B573).
Genetic characterization of the GiCoV-OH3 isolate.
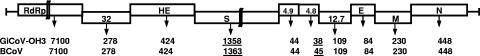
Genomic characterization was performed by full-length genome sequencing of the original GiCoV (GiCoV-OH3-FS), cell-adapted GiCoV (GiCoV-OH3-CC), and Gn-calf-passaged GiCoV (GiCoV-OH3-Gn). The GiCoV RNA genome was organized into 10 open reading frames and contains a total of 31,002 nucleotides. A comparison of GiCoV open reading frames with those of BCoVs revealed the same pattern of genome organization, with minor differences in the lengths of the spike and 4.8-kDa proteins (Fig. 4). The numbers of predicted structural and nonstructural proteins (NSPs) of the isolate are shown in Fig. 4.
FIG. 4.
Schematic diagram representing the genome organization of the GiCoV-OH3 isolate and BCoV. RdRp, RdRp replication protein complex; 32, 32-kDa NSP; HE, hemagglutinin-esterase protein; S, spike glycoprotein; 4.9, 4.9-kDa NSP; 4.8, 4.8-kDa NSP; 12.7, 12.7-kDa NSP; E, small membrane/envelope protein; M, membrane protein; N, nucleoprotein. Below the diagram, the length of each protein in amino acids is represented for the GiCoV-OH3 isolate and the BCoV-Mebus and BCoV-ENT strains. Protein lengths that differ between GiCoV-OH3 and BCoVs are underlined.
Differences (substitutions, deletions, and insertions) between the GiCoV-OH3 and BCoV-Mebus, BCoV-ENT, and/or BCoV-DB2 amino acid sequences were detected in each structural protein and NSP, with most of them concentrated in the spike protein (Tables 3, and 4). There were 5, 1, 3, and 7 polymorphic positions in the HE, E, M, and N structural proteins, respectively, and 4, 6, 2, 10, and 67 polymorphic positions in the 4.9-kDa, 4.8-kDa, 12.7-kDa, 32-kDa, and RdRp (RNA-dependent RNA polymerase) NSPs, respectively. Interestingly, most of the detected polymorphisms in the HE, E, M, and N structural proteins were common to BCoV-ENT (n = 13) and BCoV-DB2 (n = 11) and only three in the M and N proteins were common to GiCoV-OH3 and BCoV-Mebus. In contrast, several (n = 15) of those scattered in NSPs were unique to GiCoV-OH3 (Table 3). Nucleotide and amino acid sequence alignment also revealed closer relatedness between the GiCoV-OH3 and BCoV-ENT (99.6% amino acid identity) strains and the GiCoV-OH3 and BCoV-DB2 (99.3% amino acid identity) strains. The latter BCoVs were isolated more recently (BCoV-ENT in 2001) or were maintained by serial passage in calves since 1983 (BCoV-DB2), whereas GiCoV-OH3 was more distantly related (98.7% amino acid identity) to the BCoV-Mebus strain isolated several decades ago (1972) and passaged numerous times in various bovine cell types or lines (27). One hundred thirty-three amino acid differences were detected between GiCoV-OH3 and the BCoV-Mebus strain, whereas only 38 and 69 amino acid differences were found between the GiCoV-OH3 and BCoV-ENT and the GiCoV-OH3 and BCoV-DB2 sequences, respectively (Tables 3 and 4).
TABLE 3.
Amino acid sequence differences observed in ORF 1ab and HE, E, M, N, and nonstructural proteins of the GiCoV-OH3 isolate and those of BCoV-Mebus, BCoV-ENT, and BCoV-DB2
| Strain | Structural proteins
|
Nonstructural proteins
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HE
|
E, 53 | M
|
N
|
4.9 kDa
|
4.8 kDaa
|
|||||||||||||||||||||
| 5b | 49 | 66 | 367 | 392 | 38 | 57 | 221 | 15 | 53 | 146 | 249 | 386 | 423 | 441 | 2 | 11 | 19 | 23 | 8 | 9 | 10 | 18 | 21 | 28 | ||
| BCoV-ENT | P | T | G | P | I | V | V | V | L | S | Q | Y | A | T | I | F | K | T | S | N | E | G | A | I | L | V |
| BCoV-Mebus | L | N | D | S | L | G | I | I | M | F | L | F | T | M | S | Y | T | A | F | K | D | G | T | S | S | C |
| BCoV-DB2 | P | N | D | P | I | V | V | I | M | S | Q | F | T | M | S | F | T | A | F | K | D | G | T | I | S | V |
| GICoV-OH3 | P | T | G | P | I | V | V | I | L | S | Q | F | T | T | I | F | K | T | S | N | E | V | A | I | L | V |
| Nonstructural proteins
| ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12.7 kDa
|
32 kDa
|
ORF 1ab
|
||||||||||||||||||||||||
| 5 | 65 | 21 | 86 | 89 | 151 | 168 | 176 | 177 | 186 | 259 | 274 | 24 | 423 | 606 | 628 | 632 | 772 | 784 | 854 | 910 | 955 | 1010 | 1030 | 1035 | 1240 | 1141 |
| K | H | Q | E | H | N | L | E | R | A | S | S | A | G | D | S | H | A | S | R | I | S | D | E | A | V | E |
| R | H | E | D | L | S | L | E | K | V | S | P | A | G | G | G | H | T | S | C | I | P | D | Q | V | I | K |
| K | H | Q | E | H | S | L | V | K | A | S | S | T | V | G | S | H | A | S | R | V | P | D | E | V | I | K |
| K | R | Q | E | H | S | I | E | K | A | A | S | A | G | D | S | Y | A | T | R | V | S | N | E | A | V | E |
| Nonstructural proteins
| |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF 1ab
| |||||||||||||||||||||||||||
| 1582 | 1583 | 1884 | 1887 | 1930 | 2033 | 2084 | 2094 | 2134 | 2243 | 2257 | 2383 | 2395 | 2424 | 2494 | 2522 | 2544 | 2762 | 2781 | 2848 | 2921 | 2968 | 3144 | 3148 | 3279 | 3395 | 3398 | 3514 |
| P | S | I | N | I | S | Y | M | V | S | Q | I | P | V | E | G | A | L | H | L | E | S | A | R | D | G | L | R |
| P | S | I | K | V | P | C | R | V | N | Q | S | S | A | D | S | A | V | H | F | D | P | S | Q | G | V | I | H |
| P | N | L | K | I | S | Y | M | V | N | H | S | P | V | E | G | V | V | H | L | E | S | A | R | D | G | L | R |
| S | S | I | K | I | S | Y | M | M | S | Q | S | P | V | E | G | A | L | Q | F | E | S | A | R | D | G | L | R |
| Nonstructural proteins
| |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF 1ab
| |||||||||||||||||||||||
| 360 | 3682 | 3683 | 3951 | 4096 | 4120 | 4156 | 4296 | 4674 | 5024 | 5104 | 5259 | 5819 | 5888 | 5894 | 5954 | 5999 | 6263 | 6374 | 6390 | 6570 | 6638 | 6669 | 6792 |
| A | H | D | R | N | V | N | N | H | C | S | D | K | L | A | I | S | S | G | E | K | A | L | P |
| T | Q | Y | C | H | A | Y | S | R | Y | S | E | R | V | A | T | S | F | V | N | R | A | V | L |
| A | H | D | R | N | V | N | S | H | C | S | D | K | L | A | I | S | S | G | E | K | A | V | P |
| A | H | D | R | N | V | N | N | H | C | T | D | K | L | V | I | N | S | G | E | K | T | L | P |
The GiCoV-OH3 4.8-kDa NSP lacks 7 amino acids (FTCFSRY at the C-terminal end) that are present in the BCoV-ENT, BCoV-Mebus, and BCoV-DB2 4.8-kDa NSPs.
BCoV amino acid position.
TABLE 4.
Amino acid sequence differences between the spike glycoprotein of the GiCoV-OH3 isolate and those of BCoV-Mebus, BCoV-ENT, and BCoV-DB2
| Strain | Spike S1a
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11c | 33 | 40 | 88 | 100 | 115 | 146 | 148 | 154 | 169 | 173 | 175 | 179 | 248 | 253 | 256 | 370 | 458 | 465 | 470 | 483 | 484 | 499 | 501 | |
| BCoV-ENT | T | V | T | T | T | D | I | G | F | N | N | N | Q | M | N | L | Y | S | A | D | S | T | N | P |
| BCoV-Mebus | M | A | I | R | I | K | N | D | L | H | H | N | K | L | S | M | D | F | V | H | P | S | N | P |
| BCoV-DB2 | T | V | T | T | T | N | N | D | L | N | H | T | Q | M | D | M | D | S | A | A | P | T | N | P |
| GiCoV-OH3 | T | V | T | T | T | D | I | G | F | N | N | N | Q | M | N | L | D | S | A | D | P | T | S | S |
| Spike S1a
|
Spike S2b
|
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 509 | 510 | 531 | 540 | 542 | 543 | 544 | 545 | 546 | 547 | 571 | 578 | 769 | 778 | 792 | 942 | 965 | 984 | 988 | 1026 | 1100 | 1241 | 1242 | 1260 | 1341 | 1362 |
| T | S | D | T | K | A | T | G | P | Y | H | T | S | N | E | A | E | W | A | G | A | P | Y | D | K | E |
| N | S | N | T | K | S | T | G | P | Y | Y | T | A | T | E | A | V | L | V | D | V | H | D | D | I | D |
| T | S | D | S | K | A | T | G | P | Y | Y | T | S | N | Q | A | E | W | A | G | A | P | D | H | K | D |
| H | T | D | T | N | H | S | S | N | E | V | E | W | A | G | A | P | D | D | K | E | |||||
S1 subunit of the spike protein (amino acids 1 to 768). Amino acids 543 to 547 of GiCoV-OH3 S1 have been deleted.
S2 subunit of the spike protein (amino acids 769 to 1358 or 1363). The predicted hypervariable region comprises amino acids 456 to 592. Polymorphic amino acid positions unique to GiCoV-OH3 are underlined.
CoV amino acid position.
In the spike protein, 7 polymorphic amino acid positions were common to GiCoV-OH3 and BCoV-Mebus, 34 were common to GiCoV-OH3 and BCoV-ENT, 24 were common to GiCoV-OH3 and BCoV-DB2, and 7 were unique for the GiCoV-OH3 isolate. A major difference based on amino acid sequence analysis was observed in the hypervariable region of the S1 subunit of the GiCoV-OH3 spike gene: five amino acids (S/ATGPY), at positions 543 to 547, were deleted compared to BCoV strains (Table 4). Additionally, only one mutation, at position 509 of the GiCoV-OH3 spike protein, differed among all four CoVs whereas other mutations were common to the GiCoV amino acid sequence and either the BCoV-Mebus or the BCoV-ENT amino acid sequence (Table 4).
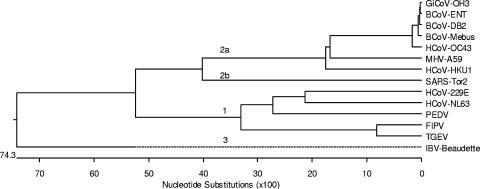
Phylogenetic analysis with the full-length nucleotide sequences of the CoV strain (GiCoV-OH3) newly isolated from giraffe feces and reference CoV strains from different groups demonstrated that GiCoV belongs to CoV group 2, forming a tight cluster with BCoV strains (Fig. 5).
FIG. 5.
Phylogenetic analysis of the GiCoV-OH3 isolate based on full-length genome sequencing. For phylogenetic tree construction, the following CoVs were used: group 1, HCoV-229E, HCoV-NL63, PEDV, FIPV, and TGEV; group 2a, BCoV-ENT, BCoV-DB2, BCoV-Mebus, HCoV-OC43, HCoV-HKU1, and MHV-A59; group 2b, SARS-CoV; group 3, infectious bronchitis virus (IBV).
Complete genome sequencing and comparison of the GiCoV-OH3-FS, GiCoV-OH3-CC, and GiCoV-OH3-Gn calf sequences demonstrated the presence of the only two silent nucleotide mutations in the RdRp gene, at positions 7033 and 10149 (C→T) of GiCoV-OH3-CC, compared to the original GiCoV-OH3-FS and the GiCoV-OH3-Gn calf fecal sample obtained after the second passage in a Gn calf.
DISCUSSION
In November 2002, a novel CoV, likely of animal origin, emerged in the human population in Guangdong Province, People's Republic of China, and spread globally, causing a severe respiratory disease (and often diarrhea) referred to as SARS (20, 21, 29). Recently, bats were shown to be a suspect wildlife reservoir for SARS-like CoVs (24, 25), with civet cats likely playing a role as an intermediate host (46, 50). The likelihood that SARS is a zoonosis transmitted from wild animals is not unprecedented for CoVs in view of previously documented interspecies transmission of animal CoVs and wildlife reservoirs for CoVs (19, 26, 31, 32, 42). The antigenically closely related group 1 CoVs cross-infect pigs, with variable disease expression and cross-protection. Bovine-like CoVs can naturally infect mammalian species (humans, dogs) (11, 12, 52), but they can also experimentally infect and cause disease even in diverse avian hosts (19). These findings raise the question of whether wild birds, ruminants, or other species could serve as reservoirs for transmission of bovine-like CoVs to bovine species (31, 32, 42) or even to humans (52).
Sambar deer (C. unicolor), WTD (O. virginianus), waterbuck (K. ellipsiprymnus), and elk or wapiti (C. elephus) were shown to harbor CoVs antigenically (cross-neutralizing) indistinguishable from BCoV, and these CoVs experimentally infect calves (26, 31, 32, 42). Unfortunately, complete genome sequencing of these CoVs has not been done to assess their genetic similarity to BCoV. To our knowledge, there is no report of the isolation and characterization of CoVs from giraffes. Here we report the first isolation and detailed characterization, including the complete genome sequence analysis, of a bovine-like CoV from feces of a giraffe with clinical signs of diarrhea. Although the source of the GiCoV-OH3 strain that caused the diarrheal outbreak among giraffes (in a wild-animal park in Ohio) is unknown, the virus may have originated from other wild ruminants in the park or outside the park (WTD) or even nearby (2 miles distant) cattle farms.
After the second passage in HRT-18 cell cultures, a typical CoV CPE was observed for GiCoV-OH3. Interestingly, the typical CPE appeared after only 36 to 48 h of inoculation, whereas it usually takes longer for BCoV strains (3 to 5 days). Also, the titer of the isolated virus was very high, 4.7 × 109 FFU/ml by CCIF assay, whereas other BCoV strains usually grow to titers of 106 to 109 FFU/ml (17, 43, 44). CoVs, like other RNA viruses, represent a quasispecies (9), which increases the potential for adaptive mutations and interspecies transmission. The isolate GiCoV-OH3 was from a 24-year-old male giraffe with diarrhea, and when inoculated into four BCoV-seronegative Gn calves, both the original isolate and the Gn-calf-passaged virus caused severe diarrhea with virus shedding within 2 to 3 days, although the infectious titer of the original giraffe feces was low. In comparison, diarrhea and virus shedding usually begin at PID3 to PID7 in Gn calves inoculated with BCoV (5). No virus shedding or diarrhea occurred after a challenge with virulent BCoV-DB2 in the calf initially inoculated with GiCoV-OH3. This complete cross-protection confirms the close antigenic relatedness in vivo between BCoV-DB2 and the giraffe CoV-OH3 isolate. Furthermore, in vitro cross-reactivity between the GiCoV-OH3 and BCoV-DB2 spike and nucleocapsid proteins was demonstrated by Western blotting with pooled BCoV-Mebus spike and nucleoprotein MAbs.
Sequence analysis demonstrated a deletion in the presumed hypervariable region (7, 28, 51) of the GiCoV-OH3 spike gene compared to that of the BCoV-Mebus, BCoV-ENT, and BCoV-DB2 strains. Yoo and Deregt (51) reported that a single point mutation in this region of the BCoV-Quebec strain could allow the virus to escape from immunological selective pressure, as tested in radioimmunoprecipitation assays. Giraffe isolate GiCoV-OH3 showed an increased level of replication in cell culture (compared to BCoV strains) and high virulence upon initial passage in a Gn calf and one of the original giraffe hosts. We speculate that the five-amino-acid deletion in the hypervariable region of the spike protein might influence viral replication in the Gn calf in vivo or in HRT-18 cells in vitro. In contrast, a large deletion in the N-terminal region of the spike protein was shown to be critical in the case of the naturally occurring S gene deletion mutant of the highly virulent porcine enteric TGEV, resulting in the attenuated PRCV strain that emerged independently in Europe and the United States in the 1980s (31, 32).
Comparisons of the entire genome sequences revealed the closest relatedness between GiCoV-OH3 and BCoV-ENT and slightly more distant relatedness between GiCoV-OH3 and BCoV-DB2, whereas GiCoV-OH3 and BCoV-Mebus were the most distantly related. The high homologies of nucleotide and amino acid sequences and the phylogenetic analysis suggest that GiCoV and BCoV strains may have evolved concurrently from a common ancestor. More amino acids were common to GiCoV-OH3 and BCoV-ENT and to GiCoV-OH3 and BCoV-DB2 than to the Mebus strain, suggesting that bovine CoVs have evolved since the time of isolation of BCoV-Mebus in 1972 or that the high passage level of the Mebus strain in multiple cell lines (27) may have selected for such genomic differences. Alignment of the amino acid sequences of the spike proteins confirmed previous findings (7) concerning a higher level of variability in the S1 subunit (33 polymorphic positions in S1) compared to the S2 subunit (12 in S2) (Table 4) and revealed that most of the detected substitutions were conserved among the enteropathogenic BCoV strains. Interestingly, the six mutations unique to GiCoV-OH3, including the five-amino-acid deletion, were clustered within the region that is identified as hypervariable for other CoVs (7, 28, 51), suggesting that this region in the GiCoV-OH3 spike protein is also prone to selective immunologic pressure. Previously, it was reported that one of the polymorphic positions in the spike protein, at amino acid 531, discriminated between enteric (aspartic acid [D] or asparagines [N]) and respiratory (glycine [G]) BCoV strains (7, 51). We confirmed the presence of an aspartic acid (D) in this position in enterotropic GiCoV-OH3. In our earlier investigations, we compared the antigenic and biological properties of respiratory and enteric BCoV strains (17, 43) and found that respiratory and enteric BCoVs differed genetically on the basis of the molecular analysis of the S1 subunit of the spike protein (18). Further genetic and antigenic studies are required to determine the biological role and importance of the identified genetic differences in the GiCoV-OH3 genome compared to BCoVs.
Antigenic variation by virus neutralization and HI tests and biological variation in HA and RDE activities were observed among enteric and respiratory BCoV strains but were independent of their clinical origin (enteric or respiratory) (17, 43). In this study, the GiCoV isolate also possessed biological (in HA and RDE activity tests) and antigenic (in HI and FFN tests) properties similar to those of both enteric and respiratory BCoV strains. These findings and the absence of reactivity in HI and FFN tests with TGEV or PRCV antiserum demonstrated that the newly isolated bovine-like GiCoV-OH3 strain belongs to group 2a and is distant from group 1 CoVs. This result was also confirmed by a phylogenetic analysis comparing the full-length genomic nucleotide sequences from all three groups of CoVs.
Our results have confirmed again that wild ruminants are an important natural reservoir for bovine-like CoVs and some of these CoVs can be transmitted experimentally to domestic cattle. Although the risk of transmission from giraffes to cattle may seem remote in the United States, this is likely not the case in Africa, where the grazing areas for giraffes and cattle overlap, allowing introduction of new strains of CoVs from wild ruminants into cattle and their possible establishment in the cattle population or vice versa, with cattle strains being transmitted to and becoming established in wild ruminants. Moreover, the area of Ohio where the captive giraffe was located (a wildlife park) also is in an area with cattle farms nearby. We previously showed that CoVs can be transmitted by aerosols and the fecal-oral route and that bovine CoVs can infect turkey poults (19). Thus, the risk of airborne, mechanical (feed, veterinary trucks, etc.), and wild-bird transmission of a GiCoV to cattle in the vicinity or vice versa is not improbable. Also, a bovine-like CoV was isolated from a captured WTD in Ohio and antibodies to BCoV were confirmed in the serum of the WTD (42). Because WTD are also present in high numbers throughout Ohio and in the vicinity of the wild-animal park, they could also be a source of CoV infection for the captive wild ruminants in the wild-animal park.
In summary, we demonstrated that wild-ruminant CoVs are biologically, antigenically, and genetically similar to bovine CoVs from domestic cattle, suggesting the possibility of interspecies transmission and adaptation of CoVs to new hosts among the ruminant species.
Acknowledgments
We thank M. Azevedo, G. Myers, J. Hanson, J. McCormick, T. Root, and Y. Tang for technical assistance. We also thank Tea Meulia (Molecular and Cellular Imaging Center of the Ohio Agricultural Research and Development Center) for assistance with electron microscopy. We also thank A. Rae Gandolf (Zanesville, OH), who provided the giraffe samples and detailed information on the diarrheal outbreak.
This work was supported by grant R21 AI062763 from the NIAID, NIH. Salaries and research support were provided by state and federal funds provided to the Ohio Agricultural Research and Development Center, The Ohio State University.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Barker, M. G., D. H. Percy, D. J. Hovland, and J. I. MacInnes. 1994. Preliminary characterization of the structural proteins of the coronaviruses, sialodacryoadenitis virus and Parker's rat coronavirus. Can. J. Vet. Res. 58:99-103. [PMC free article] [PubMed] [Google Scholar]
- 2.Benfield, D. A., and L. J. Saif. 1990. Cell culture propagation of a coronavirus isolated from cows with winter dysentery. J. Clin. Microbiol. 28:1454-1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch, C. J., H. J. Clothier, A. Seccull, T. Tran, M. C. Catton, S. B. Lambert, and J. D. Druce. 2005. Human coronavirus OC43 causes influenza-like illness in residents and staff of aged-care facilities in Melbourne, Australia. Epidemiol. Infect. 133:273-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasey, D., D. J. Reynolds, J. C. Bridger, T. G. Debney, and A. C. Scott. 1984. Identification of coronaviruses in exotic species of Bovidae. Vet. Rec. 115:602-603. [DOI] [PubMed] [Google Scholar]
- 5.Cho, K. O., M. Hasoksuz, P. R. Nielsen, K. O. Chang, S. Lathrop, and L. J. Saif. 2001. Cross-protection studies between respiratory and calf diarrhea and winter dysentery coronavirus strains in calves and RT-PCR and nested PCR for their detection. Arch. Virol. 146:2401-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho, K. O., A. E. Hoet, S. C. Loerch, T. E. Wittum, and L. J. Saif. 2001. Evaluation of concurrent shedding of bovine coronavirus via the respiratory tract and enteric route in feedlot cattle. Am. J. Vet. Res. 62:1436-1441. [DOI] [PubMed] [Google Scholar]
- 7.Chouljenko, V. N., K. G. Kousoulas, X. Lin, and J. Storz. 1998. Nucleotide and predicted amino acid sequences of all genes encoded by the 3′ genomic portion (9.5 kb) of respiratory bovine coronaviruses and comparisons among respiratory and enteric coronaviruses. Virus Genes 17:33-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, M. A. 1993. Bovine coronavirus. Br. Vet. J. 149:51-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domingo, E., E. Baranowski, C. M. Ruiz-Jarabo, A. M. Martin-Hernandez, J. C. Saiz, and C. Escarmis. 1998. Quasispecies structure and persistence of RNA viruses. Emerg. Infect. Dis. 4:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elazhary, M. A., J. L. Frechette, A. Silim, and R. S. Roy. 1981. Serological evidence of some bovine viruses in the caribou (Rangifer tarandus caribou) in Quebec. J. Wildl. Dis. 17:609-612. [DOI] [PubMed] [Google Scholar]
- 11.Erles, K., E. J. Dubovi, H. W. Brooks, and J. Brownlie. 2004. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 42:4524-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erles, K., C. Toomey, H. W. Brooks, and J. Brownlie. 2003. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310:216-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guy, J. S., J. J. Breslin, B. Breuhaus, S. Vivrette, and L. G. Smith. 2000. Characterization of a coronavirus isolated from a diarrheic foal. J. Clin. Microbiol. 38:4523-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haring, J., and S. Perlman. 2001. Mouse hepatitis virus. Curr. Opin. Microbiol. 4:462-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasoksuz, M., A. E. Hoet, S. C. Loerch, T. E. Wittum, P. R. Nielsen, and L. J. Saif. 2002. Detection of respiratory and enteric shedding of bovine coronaviruses in cattle in an Ohio feedlot. J. Vet. Diagn. Investig. 14:308-313. [DOI] [PubMed] [Google Scholar]
- 16.Hasoksuz, M., S. Lathrop, M. A. Al-dubaib, P. Lewis, and L. J. Saif. 1999. Antigenic variation among bovine enteric coronaviruses (BECV) and bovine respiratory coronaviruses (BRCV) detected using monoclonal antibodies. Arch. Virol. 144:2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasoksuz, M., S. L. Lathrop, K. L. Gadfield, and L. J. Saif. 1999. Isolation of bovine respiratory coronaviruses from feedlot cattle and comparison of their biological and antigenic properties with bovine enteric coronaviruses. Am. J. Vet. Res. 60:1227-1233. [PubMed] [Google Scholar]
- 18.Hasoksuz, M., S. Sreevatsan, K. O. Cho, A. E. Hoet, and L. J. Saif. 2002. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 84:101-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail, M. M., K. O. Cho, L. A. Ward, L. J. Saif, and Y. M. Saif. 2001. Experimental bovine coronavirus in turkey poults and young chickens. Avian Dis. 45:157-163. [PubMed] [Google Scholar]
- 20.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and S. W. Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 21.Kuiken, T., R. A. Fouchier, M. Schutten, G. F. Rimmelzwaan, G. van Amerongen, D. van Riel, J. D. Laman, T. de Jong, G. van Doornum, W. Lim, A. E. Ling, P. K. Chan, J. S. Tam, M. C. Zambon, R. Gopal, C. Drosten, S. van der Werf, N. Escriou, J. C. Manuguerra, K. Stohr, J. S. Peiris, and A. D. Osterhaus. 2003. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362:263-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai, M. M., and D. Cavanagh. 1997. The molecular biology of coronaviruses. Adv. Virus Res. 48:1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lathrop, S. L., T. E. Wittum, K. V. Brock, S. C. Loerch, L. J. Perino, H. R. Bingham, F. T. McCollum, and L. J. Saif. 2000. Association between infection of the respiratory tract attributable to bovine coronavirus and health and growth performance of cattle in feedlots. Am. J. Vet. Res. 61:1062-1066. [DOI] [PubMed] [Google Scholar]
- 24.Lau, S. K., P. C. Woo, K. S. Li, Y. Huang, H. W. Tsoi, B. H. Wong, S. S. Wong, S. Y. Leung, K. H. Chan, and K. Y. Yuen. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. USA 102:14040-14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, W., Z. Shi, M. Yu, W. Ren, C. Smith, J. H. Epstein, H. Wang, G. Crameri, Z. Hu, H. Zhang, J. Zhang, J. McEachern, H. Field, P. Daszak, B. T. Eaton, S. Zhang, and L. F. Wang. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676-679. [DOI] [PubMed] [Google Scholar]
- 26.Majhdi, F., H. C. Minocha, and S. Kapil. 1997. Isolation and characterization of a coronavirus from elk calves with diarrhea. J. Clin. Microbiol. 35:2937-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mebus, C. A., E. L. Stair, M. B. Rhodes, and M. J. Twiehaus. 1973. Neonatal calf diarrhea: propagation, attenuation, and characteristics of a coronavirus-like agent. Am. J. Vet. Res. 34:145-150. [PubMed] [Google Scholar]
- 28.Parker, S. E., T. M. Gallagher, and M. J. Buchmeier. 1989. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology 173:664-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peiris, J. S., Y. Guan, and K. Y. Yuen. 2004. Severe acute respiratory syndrome. Nat. Med. 10:S88-S97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pensaert, M. B. 2006. Porcine hemagglutinating encephalomyelitis virus, p. 353-359. In B. E. Straw, J. J. Zimmerman, S. D'Allaire, and D. J. Taylor (ed.), Diseases of swine, 9 ed. Blackwell Publishing, Ames, IA.
- 31.Saif, L. J. 2004. Animal coronaviruses: what can they teach us about the severe acute respiratory syndrome? Rev. Sci. Tech. 23:643-660. [DOI] [PubMed] [Google Scholar]
- 32.Saif, L. J. 2005. Comparative biology of animal coronaviruses: lessons for SARS, p. 84-89. In M. Peiris, L. J. Anderson, A. D. M. E. Osterhaus, K. Stohr, and K. Y. Yuen (ed.), Severe acute respiratory syndrome. Blackwell Publishing, Oxford, United Kingdom.
- 33.Saif, L. J. 1993. Coronavirus immunogens. Vet. Microbiol. 37:285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saif, L. J. 1990. A review of evidence implicating bovine coronavirus in the etiology of winter dysentery in cows: an enigma resolved? Cornell Vet. 80:303-311. [PubMed] [Google Scholar]
- 35.Saif, L. J., K. V. Brock, D. R. Redman, and E. M. Kohler. 1991. Winter dysentery in dairy herds: electron microscopic and serological evidence for an association with coronavirus infection. Vet. Rec. 128:447-449. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 37.Simkins, R. A., P. A. Weilnau, J. Van Cott, T. A. Brim, and L. J. Saif. 1993. Competition ELISA, using monoclonal antibodies to the transmissible gastroenteritis virus (TGEV) S protein, for serologic differentiation of pigs infected with TGEV or porcine respiratory coronavirus. Am. J. Vet. Res. 54:254-259. [PubMed] [Google Scholar]
- 38.Smith, D. R., P. R. Nielsen, K. L. Gadfield, and L. J. Saif. 1998. Further validation of antibody-capture and antigen-capture enzyme-linked immunosorbent assays for determining exposure of cattle to bovine coronavirus. Am. J. Vet. Res. 59:956-960. [PubMed] [Google Scholar]
- 39.Smith, D. R., H. Tsunemitsu, R. A. Heckert, and L. J. Saif. 1996. Evaluation of two antigen-capture ELISAs using polyclonal or monoclonal antibodies for the detection of bovine coronavirus. J. Vet. Diagn. Investig. 8:99-105. [DOI] [PubMed] [Google Scholar]
- 40.Spaan, W., D. Cavanagh, and M. C. Horzinek. 1988. Coronaviruses: structure and genome expression. J. Gen. Virol. 69:2939-2952. [DOI] [PubMed] [Google Scholar]
- 41.Storz, J., L. Stine, A. Liem, and G. A. Anderson. 1996. Coronavirus isolation from nasal swab samples in cattle with signs of respiratory tract disease after shipping. J. Am. Vet. Med. Assoc. 208:1452-1455. [PubMed] [Google Scholar]
- 42.Tsunemitsu, H., Z. R. el-Kanawati, D. R. Smith, H. H. Reed, and L. J. Saif. 1995. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J. Clin. Microbiol. 33:3264-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunemitsu, H., and L. J. Saif. 1995. Antigenic and biological comparisons of bovine coronaviruses derived from neonatal calf diarrhea and winter dysentery of adult cattle. Arch. Virol. 140:1303-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsunemitsu, H., H. Yonemichi, T. Hirai, T. Kudo, S. Onoe, K. Mori, and M. Shimizu. 1991. Isolation of bovine coronavirus from feces and nasal swabs of calves with diarrhea. J. Vet. Med. 53:433-437. [DOI] [PubMed] [Google Scholar]
- 45.Vijgen, L., E. Keyaerts, E. Moes, I. Thoelen, E. Wollants, P. Lemey, A. M. Vandamme, and M. Van Ranst. 2005. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 79:1595-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, M., M. Yan, H. Xu, W. Liang, B. Kan, B. Zheng, H. Chen, H. Zheng, Y. Xu, E. Zhang, H. Wang, J. Ye, G. Li, M. Li, Z. Cui, Y. F. Liu, R. T. Guo, X. N. Liu, L. H. Zhan, D. H. Zhou, A. Zhao, R. Hai, D. Yu, Y. Guan, and J. Xu. 2005. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 11:1860-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Welch, S. K., and L. J. Saif. 1988. Monoclonal antibodies to a virulent strain of transmissible gastroenteritis virus: comparison of reactivity with virulent and attenuated virus. Arch. Virol. 101:221-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo, P. C., S. K. Lau, C. M. Chu, K. H. Chan, H. W. Tsoi, Y. Huang, B. H. Wong, R. W. Poon, J. J. Cai, W. K. Luk, L. L. Poon, S. S. Wong, Y. Guan, J. S. Peiris, and K. Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo, P. C., S. K. Lau, H. W. Tsoi, Y. Huang, R. W. Poon, C. M. Chu, R. A. Lee, W. K. Luk, G. K. Wong, B. H. Wong, V. C. Cheng, B. S. Tang, A. K. Wu, R. W. Yung, H. Chen, Y. Guan, K. H. Chan, and K. Y. Yuen. 2005. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 192:1898-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, D., C. Tu, C. Xin, H. Xuan, Q. Meng, Y. Liu, Y. Yu, Y. Guan, Y. Jiang, X. Yin, G. Crameri, M. Wang, C. Li, S. Liu, M. Liao, L. Feng, H. Xiang, J. Sun, J. Chen, Y. Sun, S. Gu, N. Liu, D. Fu, B. T. Eaton, L. F. Wang, and X. Kong. 2005. Civets are equally susceptible to experimental infection by two different severe acute respiratory syndrome coronavirus isolates. J. Virol. 79:2620-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoo, D., and D. Deregt. 2001. A single amino acid change within antigenic domain II of the spike protein of bovine coronavirus confers resistance to virus neutralization. Clin. Diagn. Lab. Immunol. 8:297-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, X. M., W. Herbst, K. G. Kousoulas, and J. Storz. 1994. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J. Med. Virol. 44:152-161. [DOI] [PMC free article] [PubMed] [Google Scholar]