Abstract
Glycoprotein H (gH) is conserved among all herpesviruses and is essential for virus entry and cell fusion along with gL, gB, and, in most alphaherpesviruses, gD. Within the gH/gL heterodimer, it is thought that gH accounts for the fusion function and gL acts as a chaperone for the folding and transport of gH. Here, we found that the N terminus of gH2 contains important elements involved in both its folding and its transport. Our conclusions are based on the phenotypes of a series of gH deletion mutants in which the signal sequence (residues 1 to 18) was retained and N-terminal residues were removed up to the number indicated. The first mutant, gH2Δ29 (deletion of residues 19 to 28), like wild-type (WT) gH, required gL for both transport and function. To our surprise, two other mutants (gH2Δ64 and gH2Δ72) were transported to the cell surface independent of gL but were nonfunctional, even when complexed with gL. Importantly, a fourth mutant (gH2Δ48) was transported independent of gL but was functional only when complexed with gL. Using a panel of monoclonal antibodies against gH2, we found that when gH2Δ48 was expressed alone, its antigenic structure differed from that of gH2Δ48/gL or gH2-WT/gL. Mutation of gH2 residue R39, Y41, W42, or D44 allowed gL-independent transport of gH. Our results also show that gL is not merely required for gH transport but is also necessary for the folding and function of the complex. Since gH2Δ64/gL and gH2Δ72/gL were nonfunctional, we hypothesized that residues critical for gH/gL function lie within this deleted region. Additional mutagenesis identified L66 and L72 as important for function. Together, our results highlight several key gH residues: R39, Y41, W42, and D44 for gH transport and L66 and L72 for gH/gL structure and function.
Glycoprotein H (gH) is conserved among all herpesviruses and is essential for virus entry. The herpes simplex virus (HSV) gH/gL heterodimer represents the functional form of these proteins (22, 35, 39). This heterodimer is an essential component of the core fusion machinery that also includes gB (41). Four glycoproteins (gB, gD, gH, and gL) as well as a gD receptor are required for entry into most alphaherpesviruses, including HSV (42). The current hypothesis is that the gD-receptor complex is required to stabilize the virus-cell interaction and also acts as a trigger for fusion that requires gB and/or gH/gL. The crystal structures of gD (5, 26) and gB (21) have been solved. The structure of gB closely resembles those of other known fusion proteins, most notably the vesicular stomatitis virus G protein (38), leaving open the question of the precise role of gH/gL.
In HSV, the formation of the gH/gL complex is necessary for protein localization both in the virion envelope and on the cell surface (22, 35, 39). When gH is expressed in the absence of gL, it remains in the endoplasmic reticulum (ER) (22, 25). When gL, which lacks a transmembrane domain, is expressed in the absence of gH, it is secreted from cells (8, 22, 35). It is hypothesized that gL harbors a chaperone-like activity necessary for the folding and transport of gH. The amino acids involved in the binding of gH to gL are located between residues 19 and 323 of HSV-1 gH (2, 35, 45) and residues 20 and 147 of gL (25, 35).
To date, most of the important functional residues have mapped downstream of the gL binding site on gH. For instance, Galdiero et al. (11) determined that residues near the C terminus of gH1 are important for its role in cell fusion and virus infectivity. Two cysteines at residues 652 and 706 are also important for gH1 function, although this does not appear to be the case for gH2 (3). Others (1, 20, 46) have shown that the transmembrane region and cytoplasmic tail of gH1 are critical for the fusion activity of gH1/gL1. Lopper and Compton (28) identified a region in gH1 (residues 445 to 465) as a possible coiled-coil through its similarity to a region in human cytomegalovirus gH. Gianni et al. (16, 17) expanded upon this idea and, through algorithmic analysis, detected a heptad repeat (HR-1) between amino acids 445 and 465 and a coiled-coil domain within amino acids 377 to 398 (Fig. 1A). Synthetic peptides corresponding to these regions blocked cell-cell fusion and virus infectivity (15) and also promoted the fusion of liposomes in vitro (12).
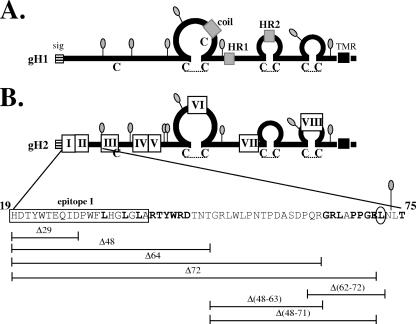
FIG. 1.
(A) Diagram of gH1, with the signal sequence (sig) (stripes), potential fusion domains (16, 17) (gray), and transmembrane region (TMR) (black) indicated with boxes. Cysteines are designated with a “C,” and hypothetical disulfide bonds (3) are represented with a dotted line. N-linked glycosylation sites (lollipop structures) are also shown. (B) Diagram of gH2. Epitopes I through VIII (4) are indicated with white boxes. Individual amino acids are shown in expanded form below gH2 for residues 19 to 75. Amino acid deletions are represented by horizontal lines below the gH2 diagram. Amino acids that were mutated to alanine are shown in bold, and residue L72 is circled. HR1, heptad repeat 1; HR2, heptad repeat 2; coil, hypothetical coiled-coil/fusion peptide.
We recently generated an epitope map for gH2 (Fig. 1), identifying eight separate linear antigenic regions (I to VIII) that span the entire molecule. We identified two gH2-specific monoclonal antibodies (MAbs), CHL17 and CHL32, that bind to region I (residues 19 to 38) and block cell-cell fusion (4). These same MAbs have virus-neutralizing activity, and MAb CHL17 also inhibits the cell-cell spread of HSV-2, prompting us to take a closer look at the gH2 N terminus. The deletion of the region containing the CHL17 and CHL32 epitopes had no effect on cell-cell fusion or gH-null virus complementation. To our surprise, the deletion of these N-terminal residues enabled the transport of gH to the cell surface without the need for the coexpression of gL. However, gL was still required for proper folding of the gH/gL complex, and in the case of a mutant lacking residues 19 to 47, it was also required for function. Expanding upon these results, we found that gH2 mutants missing residues 19 to 63 (gH2Δ64) or 19 to 71 (gH2Δ72) also exhibited the permissive gH transport phenotype but were unable to function when coexpressed with gL. Further analysis revealed that a point mutation at gH2 residue 72 (L72A) was sufficient to render gH/gL nonfunctional and another at gH2 residue 66 (L66A) impaired function. Our results highlight the importance of the gH2 N terminus in gH/gL transport, structure, and function.
MATERIALS AND METHODS
Plasmid DNAs.
Plasmids pTC510 (gH2-WT), pTC579 (gL2-WT), pTC578 (gD2-WT), and pTC580 (gB2-WT) were previously described (3). The plasmids used in the fusion assay, pCAGGS/MCS, pLUC, and pT7-pol, were gifts of P. G. Spear (33, 36). Plasmids pTC642 (gH2Δ48), pTC684 (gH2Δ64), pTC643 (gH2Δ72), pTC661 (gH2Δ48-71), pTC685 (gH2Δ48-63), and pLF663 (gH2Δ62-72) were created using a QuikChange XL site-directed mutagenesis kit (Stratagene Cloning Systems) as described previously (6). To create these deletion mutants, primers were designed to “loop out” unwanted residues during amplification and were as follows (deletion sites underlined): pTC642, 5′-GTGGGCGTTGCCGGGGGCGGGCGTCTGTGGTTGCCC and 5′-GGGCAACCACAGACGCCCGCCCCCGGCAACGCCCAC; pTC684, 5′-GTG GGC GTT GCC GGG GGC GGA CGC TTG GCG CCC CCG and 5′-CGG GGG CGC CAA GCG TCC GCC CCC GGC AAC GCC CAC; pTC643, 5′-GTGGGCGTTGCCGGGGGCCTCAACCTGACTACGGCATC and 5′-GATGCCGTAGTCAGGTTGAGGCCCCCGGCAACGCCCAC; pTC661, 5′-TGGCGCGACACAAACACCCTCAACCTGACTACGGCATC and 5′-GATGCCGTAGTCAGGTTGAGGGTGTTTGTGTCGCGCCA; pTC685, 5′-TGG CGC GAC ACA AAC ACC GGA CGC TTG GCG CCC CCG and 5′-CGG GGG CGC CAA GCG TCC GGT GTT TGT GTC GCG CCA; and pLF663, 5′-CCCGACGCCAGCGACCCCCTCAACCTGACTACGGCATC and 5′-GATGCCGTAGTCAGGTTGAGGGGGTCGCTGGCGTCGGG. The QuikChange XL kit was also used to generate plasmids from template pTC510 (gH2-WT) that contain single-residue point mutations: pTC682 (gH2-L32A), pTC683 (gH2-L35A), pTC710 (gH2-L37A), pTC673 (gH2-R39A), pTC755 (gH2-T40A), pLF681 (gH2-Y41A), pTC711 (gH2-W42A), pTC712 (gH2-R43A), pTC756 (gH2-D44A), pTC665 (gH2-G64A), pTC666 (gH2-R65A), pTC669 (gH2-L66A), pTC667 (gH2-P68A), pTC668 (gH2-P69A), pTC670 (gH2-G70A), pTC671 (gH2-E71A), pTC672 (gH2-L72A), and pTC657 (T75A). The gH gene in each plasmid was sequenced to screen out PCR errors.
Viruses and cells.
293T cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). Mouse melanoma cells expressing nectin-1 (designated C10) were grown in 10% FBS-DMEM containing 500 μg/ml G418 (30). CHO-K1 cells were grown in Ham's F-12 medium containing 10% FBS. The CHO cell line CHO-HVEM12, expressing the HSV receptor herpesvirus entry mediator HveA (HVEM) (44), and CHO-R3A, expressing nectin-1 (14), were grown in 10% FBS-F-12 containing 250 μg/ml G418. CHO-K1 and CHO-HVEM12 cells were kindly provided by P. G. Spear. African green monkey kidney (Vero) cells were grown in 5% FBS-DMEM. Propagation of the gH-null virus SCgH-z on F6 cells (gifts of A. Minson) was as previously described (10, 27).
Antibodies.
The polyclonal antibodies (PAbs) used in this study were prepared as follows. Rabbit serum R137 was prepared against purified gH1t/gL1 (35), and R176 was prepared against gH2t/gL2 (3). The CHL series of MAbs were characterized and described by Cairns et al. (4). The MAbs that recognize linear gH2 epitopes were as follows: CHL32 (group I, residues 19 to 38), CHL25 (group III, residues 73 to 92), CHL41 (group IV, residues 136 to 146), CHL35 (group Va, residues 145 to 155), CHL36 (group Vb, residues 145 to 155), CHL38 (group VI, residues 352 to 371), CHL43 (group VII, residues 352 to 371), and CHL29 (group VIII, residues 676 to 686). The MAb CHL2 recognizes a discontinuous, conformation-dependent epitope of gH2/gL2. MAbs CHL28, CHL34, and CHL39 recognize linear epitopes within the C terminus of gL2.
Immunoprecipitation.
293T cells were transfected with the desired plasmids according to the GenePORTER protocol (Gene Therapy Systems, Inc.). At 48 h posttransfection, cells were lysed in 10 mM Tris, pH 8, 150 mM NaCl, 10 mM EDTA, 1% NP-40, 0.5% deoxycholic acid, and 1 mM phenylmethylsulfonyl fluoride. Thirty microliters of lysate was mixed with 70 μl of lysis buffer and then incubated with the appropriate antibody overnight at 4°C. Proteins were precipitated with protein A-agarose beads (Gibco BRL) for 2 h at 4°C, separated by electrophoresis on a 12% sodium dodecyl sulfate-polyacrylamide gel, and detected by Western blotting with the appropriate counter antibody.
Immunofluorescence.
Immunofluorescence was performed as outlined by Cairns et al. (3) with some modifications. C10 cells were transfected using GenePORTER with plasmids encoding gL and either wild-type (WT) or mutant gH2. Cells were then seeded onto glass coverslips in 24-well plates at 1 × 105 cells/well and incubated overnight at 37°C. The following steps were carried out at room temperature. To test for surface expression, cells were first fixed with 3% paraformaldehyde (PFA) for 30 min (or overnight), then quenched with 50 mM NH4Cl2 for 10 min, and blocked for 30 min with 10% goat serum-phosphate-buffered saline (PBS). Cells were then incubated with PAb R176 (diluted 1:100 in blocking solution) and/or 5 to 10 μg/ml of MAb for 30 min, washed with PBS, and then incubated with a fluorescent-tagged secondary antibody (diluted 1:1,000 in blocking solution) for 30 min. Coverslips were washed three times with PBS and once with distilled water and finally adhered to the slides in mounting solution containing DAPI (4′,6′-diamidino-2-phenylindole; Molecular Probes, Inc., Eugene, OR). When the binding of the MAb CHL2 was being tested, cells on a second set of coverslips were first incubated with 2 μg/ml of MAb and then fixed. For internal staining, an additional step (a 10-min incubation with 0.1% Triton X-100-PBS) was carried out after the quenching step. For CHO-K1 cells, plasmids were transfected using Lipofectamine (Invitrogen) following the manufacturer's protocol; all other steps were performed as described above. Cells were observed under a Nikon Eclipse E600 microscope at a magnification of ×20.
Cell-cell fusion and complementation assays.
A luciferase reporter gene activation assay (33, 36) was performed in a 96-well format to quantitate cell-cell fusion as described by Cairns et al. (3). To test for syncytium formation, 24-well plates containing C10 cells were transfected and stained with Giemsa (2). C10 cells were used in place of CHO cells for this assay because they form more-extensive syncytia under WT conditions and therefore were easier to observe and photograph. The complementation protocol was as described previously (2). Briefly, Vero cells were transfected overnight at 37°C using GenePORTER and then infected with 106 PFU of SCgH-z. After 2 h, the virus was acid inactivated, fresh medium was added, and the cells were incubated overnight. At 24 h postinfection, cells were freeze-thawed and virus was collected from the culture medium. Virus-containing supernatants were serially diluted and titered on F6 cells. Cells were fixed with 5% formaldehyde-PBS and stained with crystal violet, and plaques were scored.
CELISA.
To detect gH/gL binding and cell surface expression, we used a cellular enzyme-linked immunosorbent assay (CELISA) (13, 19). C10 cells growing in 6-well plates were transfected with the appropriate plasmid via the GenePORTER protocol and then reseeded 24 h later onto 96-well plates (pretreated with 0.2% gelatin-PBS). At 48 h posttransfection, cells were fixed with 1% PFA for 20 min, quenched with 50 mM NH4Cl for 10 min, and then incubated for 2 h with various concentrations of an anti-gL MAb cocktail (CHL28, CHL34, and CHL39) diluted in 3% bovine serum albumin-PBS, all at room temperature. Secondary antibody goat anti-mouse coupled to horseradish peroxidase was added, and bound antibody was detected with ABTS [2,2′-azinobis(2-ethylbenzthiazolinesulfonic acid)] peroxidase substrate (Moss, Inc.).
RESULTS
Previously, we demonstrated that gH2-specific group I MAbs block gH/gL function (4), suggesting that region I (residues 19 to 38) is an important functional domain. To test this possibility, we constructed a series of deletion mutants within the gH2 N terminus that removed all or part of epitope I: gH2Δ29 (deleting residues 19 to 28), gH2Δ48 (deleting residues 19 to 47), gH2Δ64 (deleting residues 19 to 63), and gH2Δ72 (deleting residues 19 to 71) (Fig. 1B). All four deletion mutants were expressed as well as or better than WT gH2 and were immunoprecipitated with the anti-gH MAb CHL29 (group VIII, residues 676 to 686) (Fig. 2, top). As expected, the mutants were not immunoprecipitated by the group I-specific MAb CHL32 (Fig. 2, bottom).
FIG. 2.
Immunoprecipitation of gH2 deletion mutants. Lysates of C10 cells transfected with plasmids expressing gL2 and either WT gH2 or a gH2 deletion mutant were immunoprecipitated with either MAb CHL29 (group VIII, residues 676 to 686) or CHL32 (group I, residues 19 to 38). Immunoprecipitated proteins were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blotting. Blots were probed with the PAb R176.
Surface expression of the mutant proteins was determined on nectin-bearing B78-C10 cells (hereafter referred to as C10 cells) by using indirect immunofluorescence (Fig. 3). As expected, surface expression of gH2-WT required coexpression of gL2, confirming previous observations with HSV-1 that gL is required for the transport of gH out of the ER (8, 9, 19, 22, 25, 35, 37). When the four deletion mutants were coexpressed with gL, all were detected on the cell surface. The smallest deletion mutant, gH2Δ29, was like the WT in that it was not found on the cell surface in the absence of gL. Surprisingly, this was not the case for mutants gH2Δ48, gH2Δ64, and gH2Δ72; these three mutants were present on the cell surface even when expressed in the absence of gL. The phenotype of these three mutants suggested that residues within this region of gH normally block its independent transport and that coexpression of gL overcomes this block. Moreover, this unusual phenotype appeared to reside mainly within amino acids between residues 29 and 48, though we could not rule out some contribution of residues as far downstream as position 72. Identical results were seen when the mutants were expressed in CHO cells (data not shown).
FIG. 3.

Cell surface expression of WT and mutant forms of gH2 by an immunofluorescence assay. C10 cells were transfected with plasmids encoding gH in the absence or presence of gL2. Cells were fixed with 3% PFA and then incubated with the PAb R176. The gH2/gL2 complex was detected using a fluorescent-tagged goat anti-rabbit secondary antibody. Cell nuclei were stained blue with DAPI. Permeabilized C10 cells expressing gH-WT with and without gL are shown in the first two panels as a control for protein expression.
The transport phenotypes of gH2Δ48, gH2Δ64, and gH2Δ72 gave us the opportunity to study gH2 with and without gL. We therefore asked whether gH2Δ48, gH2Δ64, and gH2Δ72 could function in cell-cell fusion without gL. Using a luciferase reporter assay system (3, 33, 36), we tested the mutants for their fusogenic properties on receptor-expressing CHO target cells that were transfected with a plasmid encoding the luciferase protein under the control of the T7 promoter. Effector cells were prepared by cotransfection of CHO cells with plasmids containing the genes for T7 polymerase, gB, gD, and either the WT or mutant form of gH. In Fig. 4A, the plasmid for gL was not part of the transfection mixture (with the exception of the positive control, which included WT gB, gD, gH, and gL). In Fig. 4B, the plasmid for WT gL was added to each effector cell transfection.
FIG. 4.
Examination of gH function. (A) Results of a cell-cell fusion assay in the absence of gL. Target CHO-K1 cells (expressing the luciferase protein and the HSV receptor HVEM) were cocultivated with effector CHO cells (expressing T7 polymerase, gB1, and gD1 plus WT gH2, mutant gH2, or empty vector DNA) and tested for light production 20 h later. No difference was seen in fusion between target cells expressing either nectin-1 (CHO-R3A) or HVEM (CHO-HVEM12), so only CHO-HVEM12 data are presented. The percent WT was calculated as follows: (relative light units [RLU] of test sample/RLU of WT) × 100. (B) Results of a cell-cell fusion assay in the presence of gL. The quantitative fusion assay was performed as described for panel A, except the plasmid encoding gL2 was included in the transfection mix. Each sample was assayed in at least three separate experiments, and the average values (with error bars representing standard errors) are plotted on the bar graph. (C) Table representing the three distinct phenotypes seen upon assaying for gH/gL function.
Target and effector cells were cocultured for 20 h, lysed, and then assayed for luciferase activity (i.e., fusion). As expected from the results shown in Fig. 3, cells transfected with gH2-WT or gH2Δ29 were unable to fuse target cells when gL was absent (Fig. 4A). However, this was also the case for mutants that were expressed on the surface in the absence of gL (Fig. 4A), showing that gL is necessary for fusion even if gH is present on the cell surface. We next examined the functional activity of the gH mutants when gL was present. Mutants gH2Δ29 and gH2Δ48 fused cells at WT levels, but gH2Δ64 and gH2Δ72 were nonfunctional (Fig. 4B).
Similar results were seen when each mutant was tested in a virus complementation assay (Table 1). The infectivity of gH-null viruses complemented with gH2Δ29 and gH2Δ48 was greater than 50% of that achieved by gH2-WT, whereas the infectivity of gH-null viruses complemented with gH2Δ64 and gH2Δ72 was at least 3 logs below WT levels. Our results identify three phenotypic categories of gH proteins: (i) the WT phenotype of gL-dependent transport and function (gH2Δ29), (ii) gL-independent transport but gL-dependent function (gH2Δ48), and (iii) gL-independent transport of a nonfunctional protein, with or without gL (gH2Δ64 and gH2Δ72) (Fig. 4C).
TABLE 1.
Properties of WT and mutant gH2 forms
| Deletion or mutation | Surface expression
|
gL2 binding | CHL2 binding | Cell-cell fusion (%) | Complementation (%) | |
|---|---|---|---|---|---|---|
| Without gL | With gL | |||||
| None (WT) | − | + | + | + | 100 | 100.0 |
| Δ29 | − | + | + | + | 107 | 55.5 |
| Δ48 | + | + | + | + | 94 | 58.1 |
| Δ64 | + | + | + | +a | 3 | 0.2 |
| Δ72 | + | + | + | +a | 10 | 0.1 |
| Δ48-71 | + | + | + | +a | 7 | 1.5 |
| Δ48-63 | + | + | + | +a | 4 | 2.3 |
| Δ62-72 | − | + | + | +a | 5 | 0 |
| G64A | NDb | + | + | + | 71 | 54.5 |
| R65A | ND | + | + | + | 60 | 51.6 |
| L66A | − | + | + | +a | 29 | 9.6 |
| P68A | ND | + | + | + | 77 | 65.4 |
| P69A | ND | + | + | + | 43 | 30.0 |
| G70A | ND | + | + | + | 93 | 67.1 |
| E71A | − | + | + | + | 89 | 81.0 |
| L72A | − | Reduced | −c | − | 6 | 0.8 |
| T75A | ND | + | + | + | 60 | 33.8 |
Bound only if CHL2 was added prior to the fixation step (see Materials and Methods).
ND, not determined.
gL binding was undetectable by CELISA and immunofluorescence assay; however, gL coexpression was required for cell surface expression of gH2-L72A.
Analysis of gH2Δ48 and the trafficking phenotype.
Clearly, gH2Δ48 has very different properties when it is expressed without gL (i.e., nonfunctional) compared to when it is coexpressed with gL (i.e., functional). One possibility is that the conformation of gH2Δ48 is the same in both cases and that the missing function resides entirely in gL. The second possibility is that the structure of gH2Δ48 is altered by coexpression with gL. Since gL binding is thought to be a prerequisite for the folding of a functional gH/gL molecule, we hypothesized that gH2Δ48 alone never achieves this “functional conformation.”
To investigate this possibility, we tested the reactivity of surface-expressed gH2-WT/gL, gH2Δ48/gL, and gH2Δ48 with our panel of gH2 MAbs (Fig. 5 and Table 2). The MAb CHL2, which recognizes an epitope that is present only on the full gH2/gL2 complex, recognized gH2Δ48/gL but not gH2Δ48 alone (Fig. 5A). The group III MAb CHL25 had a recognition pattern similar to that of CHL2. The results obtained with CHL2 and CHL25 demonstrate that gL binding has an effect on the structure of gH. Group V MAbs (exemplified by CHL36) recognized all three forms of gH, while the group IV MAb CHL41 recognized none of them (Fig. 5A). Therefore, group IV and V MAbs were unable to distinguish between WT and mutant gH2 forms (Fig. 5B).
FIG. 5.
Characterization of the gH2Δ48 mutant. (A) C10 cells were transfected and processed for an immunofluorescence assay (IFA) as described for Fig. 3. The primary antibodies used for detection were as follows: PAb (R176), CHL2 (conformational; group IX), CHL36 (group V), CHL25 (group III), CHL41 (group IV), and CHL29 (group VIII). Those MAbs that were tested but not shown include CHL35 (group V), CHL38 (group VI), and CHL43 (group VII). (B) Schematic of the gH2Δ48 mutant depicting its antigenic regions. Epitopes III through VIII are shown as boxes. Shaded boxes (III, VI, VII, and VIII) indicate epitopes that are presented differently from the WT gH/gL complex, as determined by the IFA used for panel A; white boxes (IV and V) indicate epitopes that are presented in the same way as WT gH/gL. The gH signal sequence (sig) is depicted as a striped box and the transmembrane region (TMR) as a black box. The Δ48 deletion is shown as a gap in the line model.
TABLE 2.
MAb recognition of cell surface proteins, as determined by immunofluorescence assay
| Protein | Detection of protein by indicated gH2 MAb group (amino acids in epitope)
|
||||||
|---|---|---|---|---|---|---|---|
| III (73-92) | IV (136-146) | V (145-155) | VI (352-371) | VII (532-542) | VIII (676-686) | IXa | |
| gH2-WT/gL | + | − | + | − | − | − | + |
| gH2Δ48/gL | + | − | + | + | + | + | + |
| gH2Δ48 | − | − | + | + | + | + | − |
Group IX, represented by the MAb CHL2, recognizes a conformation-dependent, gL-dependent epitope that has not been mapped to a specific sequence of amino acids.
A particularly striking result was obtained with MAb CHL29 (group VIII) (Fig. 5A). This MAb, which recognizes gH residues 676 to 686, did not recognize gH2-WT/gL but did bind cells expressing gH2Δ48 or gH2Δ48/gL. We observed similar MAb recognition patterns with CHL38 (group VI) and CHL43 (group VII) (Table 2). We cannot rule out the possibility that cells expressing gH2Δ48 and gL may contain a mixed population of proteins (gH2Δ48 and gH2Δ48/gL) and that CHL29 binding occurs only in gH2Δ48 lacking gL. Another possibility is that epitopes normally hidden in the gH/gL complex become exposed in gH2Δ48. Taken together, our observations suggest that gH2Δ48 is structurally different from either gH2-WT/gL or gH2Δ48/gL (Table 2 and Fig. 5B), in turn implying that gL coexpression and complex formation are important for gH2 folding and function.
One way to explain the gL-independent transport of mutant gH2Δ48 is to postulate the presence of an ER retention signal at the N terminus of gH2-WT. According to this hypothesis, we would expect gH-WT to be retained in the ER unless gL is present. Upon gL binding, this signal would be covered/obscured and gH would then be transported out of the ER. Perusal of the amino acid sequence of the gH N terminus did not reveal any known ER retention signals, although it does contain residues commonly found in such signals, such as leucine, arginine, and tyrosine (7, 29, 40). We therefore targeted these residues (L32, L35, L37, R39, Y41, and R43) as well as several nearby residues (T40, W42, and D44) and used site-directed mutagenesis to change them individually to alanine. Each mutant was assayed for gH cell surface expression in the presence or absence of gL. Four of the point mutants (the R39A, Y41A, W42A, and D44 mutants) exhibited some gL-independent gH2 cell surface expression (Fig. 6 and data not shown), although the number of cells expressing gH2 always increased when gL was present. However, the results suggest that amino acids R39, Y41, W42, and D44 are important in modulating the trafficking of gH2.
FIG. 6.
Cell surface expression of gH2 N-terminal point mutants. C10 cells were transfected and processed for an immunofluorescence assay as described for Fig. 3. The gH2 amino acid sequence (residues 19 to 47) is shown, with a box highlighting those residues (in red) important for gH trafficking.
Further mutagenesis of gH2 between residues 48 and 75.
Deletion mutants gH2Δ64 and gH2Δ72 were unable to support either cell-cell fusion or gH-null virus complementation (Fig. 4B and Table 1), suggesting that the region from residues 48 to 71 is important for function. To further investigate this region, we constructed the internal deletion mutants gH2Δ48-71, gH2Δ48-63, and gH2Δ62-72 and tested them for function. None of these mutants were able to support complementation or cell-cell fusion (Fig. 7A). Interestingly, the mutant containing the smallest internal deletion, gH2Δ62-72, fared the worst and its titer in complementation was at least 5 logs lower than that achieved by WT gH/gL (Table 1). In order to more narrowly define the residues responsible for this phenotype, we made and tested the following point mutants: G64A, R65A, L66A, P68A, P69A, G70A, E71A, and L72A. Since it was conceivable that the N-linked glycosylation site immediately following our region of interest (residues 73 to 75) was important for function, we also constructed the T75A mutant to remove this glycosylation site (11, 35). Of these point mutants, most were only modestly altered in function. However, two mutants (the L66A and L72A mutants) showed major defects in complementation. Virus titers for these mutants were less than 10% of that achieved by gH2-WT (Fig. 7A, top). Both of these mutants also displayed markedly reduced activities in the cell-cell fusion assay, with levels of 29% and 6% of that of the WT, respectively (Fig. 7A, bottom, and Table 1). Although the L66A mutant was still able to generate syncytia on C10 cells when coexpressed with gB, gD, and gL, the L72A mutant was not (Fig. 7B).
FIG. 7.
Mutants spanning gH2 residues 48 to 72 are tested for function. (A) gH-null virus complementation (top) was performed as described in Materials and Methods. The percent WT was calculated as follows: (sample titer/WT titer) × 100. The quantitative cell-cell fusion assay (bottom) was performed as described for Fig. 4. Each sample was assayed in at least three separate experiments, and the average values (with error bars representing standard errors) are plotted on the graph. A black horizontal line denotes 20% WT levels of fusion, the threshold for observing syncytia. (B) Observation of syncytium formation. C10 cells were transfected with plasmids expressing gB2, gD2, and gL2 plus gH2-WT, mutant gH2, or empty vector DNA. Cells were then fixed with methanol and stained with Giemsa. Prominent syncytia are denoted by white arrows.
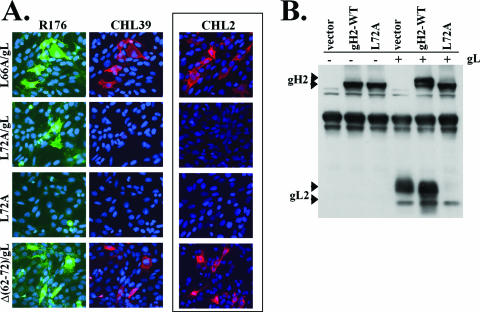
There are several possible reasons that the L66A and L72A mutants were functionally impaired, such as defects in surface expression, gL binding, and/or protein folding. gH2-L72A/gL was detected on the cell surface by immunofluorescence with an anti-gH2/gL2 polyclonal antibody (R176) (Fig. 8A), albeit at reduced levels compared to gH2-WT/gL (data not shown). That gH2-L72A/gL was unable to bind the conformation-dependent, gL-dependent MAb CHL2 (Fig. 8A) even though CHL2 can recognize gH2 with L72 deleted (the mutant gH2Δ62-72) most likely means that gH2-L72A/gL is misfolded due to the change of leucine to alanine. CHL2 readily bound the other point mutants when they were complexed with gL, including gH2-L66A/gL (Table 1). However, unlike gH2-WT/gL, gH2-L66A/gL was able to bind CHL2 only when the MAb was added before the fixation step (Fig. 8A and data not shown), suggesting that the structure of this mutant is somewhat unstable.
FIG. 8.
Mutant gH2-L72A/gL is structurally altered compared to gH-WT/gL. (A) C10 cells were transfected and processed for a cell surface immunofluorescence assay as described for Fig. 3. Cells were stained with both the PAb R176 (green) (left) and the anti-gL2 MAb CHL39 (red) (middle). For detection by the conformation-dependent MAb CHL2 (red) (right), the cells were first incubated with the MAb and then subjected to fixation with 3% PFA. (B) Western blot analysis of gH2-WT and the L72A mutant with or without gL2 by using cell lysates of transfected C10 cells. Blots were probed with the anti-gH2/gL2 PAb R176. gH, when coexpressed with gL and then separated on a denaturing gel, runs as a higher-molecular-weight species (top arrowhead) than gH expressed alone (8, 22, 39).
Since gH2-L72A/gL was not recognized by CHL2, whose epitope requires the gH/gL complex, we wondered whether the L72A mutant was able to bind gL. Curiously, anti-gL MAbs failed to recognize the L72A mutant when it was coexpressed with gL, as detected either by immunofluorescence (CHL39) (Fig. 8A) or by CELISA (data not shown). Also, the mature, higher-molecular-weight forms of gH and gL (2, 8, 22, 39) were not detected for gH2-L72A/gL when analyzed by Western blotting (Fig. 8B). Although we were unable to directly assess gL binding to the L72A mutant, we hypothesize that the L72A mutant might bind gL, since the L72A mutant is expressed on the cell surface only when coexpressed with gL (Fig. 8A). These observations may indicate that a complex of gH2-L72A/gL has a structure radically different from that of gH2-WT/gL; the possibility exists that epitopes for the gL MAbs and also glycosylation sites might be hidden or that gL might disassociate from the L72A mutant after reaching the cell surface.
DISCUSSION
We began this study to determine whether the N terminus of gH2 (amino acids 19 to 38) contained a functional domain, as our previous work showed that the binding of MAbs to this region inhibited function in terms of entry, fusion, and cell-cell spread (4). A most interesting result was the discovery that the removal of amino acids 19 to 47 allowed gH2 to traffic to the cell surface without the need for gL coexpression and binding. However, this protein did not support cell-cell fusion or virus entry unless coexpressed with gL, thereby separating the transport of gH from its function. Four specific amino acids within this region, R39A, Y41A, W42A, and D44, seem to be important in modulating gH transport. Interestingly, gH2Δ48 differs structurally from the complex of gH2Δ48 with gL, as detected by a panel of gH2 MAbs. Some epitopes that are normally hidden in WT gH2/gL or gH2Δ48/gL become exposed on the mutant protein expressed in the absence of gL, and others that are normally exposed in the functional complexes are hidden. Thus, our data suggest that gL does more than escort gH to the surface; it also plays a role in determining a functional gH structure.
gL-independent gH trafficking.
It is well established that for HSV and most other herpesviruses, transport, cell surface expression, and incorporation of gH into the virion envelope require the formation of a complex with gL (8, 22, 34, 35). gH expressed in the absence of gL remains trapped in the ER (9, 19, 25, 37). However, there have been several instances reported in the literature in which mutant forms of gH or gL form a complex yet are still not transported to the cell surface (2, 3, 25). Therefore, it appears that gH transport requires more than transport signals provided by gL.
We hypothesize that WT gH2 contains a unique ER retention signal that is exposed when gH2 is expressed alone but is hidden or deactivated upon gL binding; this in turn allows the transport of the complex through the Golgi and to the maturing virion and cell surface. The ER retention signals of both the γ-aminobutyric acid neurotransmitter receptor and the asialoglycoprotein receptor are deactivated in this manner (29, 40). The region of gH2 between residues 29 and 47 does not resemble any known ER retention signals. However, several residues in this region of gH (leucine, arginine, and tyrosine) are commonly found in such signals (7, 29, 40). The phenotypes of several gH2 mutants highlighted potential key residues (R39, Y41, W42, and D44) that may be part of a unique ER retention signal. These single amino acid changes allowed for gH2 surface expression in the absence of gL (Fig. 6), although expression levels were not as high as when gL was present or the full region (residues 19 to 47) was deleted (Fig. 3).
We started this study with four N-terminal deletion mutants: the gH2Δ29 (removing residues 19 to 28), gH2Δ48 (removing residues 19 to 47), gH2Δ64 (removing residues 19 to 63), and gH2Δ72 (removing residues 19 to 71) mutants. Since the gH2Δ29 mutant required gL for trafficking but the gH2Δ48 mutant did not, we deduced that the region encompassing residues 29 to 47 contained at least part of a putative ER retention signal (this region is also deleted in the gH2Δ64 and gH2Δ72 mutants). The fact that gH2 mutants containing internal deletions of residues 48 to 71 and 48 to 63 permitted gL-independent gH trafficking, while a mutant deleting residues 62 to 72 did not (Table 1), suggested that residues between positions 48 and 61 could also be important for trafficking. Alternatively, the phenotypes of the gH2Δ48-71 and gH2Δ48-63 mutants could be explained by a secondary effect of introducing a deletion so close to the residues we found to be important for trafficking (between amino acids 39 and 44) (Fig. 6).
To determine whether this region contained a retention signal that would work for other proteins, we appended residues 1 to 66 of gH2 (containing the potential signal) to the N terminus of gD. However, this gHgD chimeric protein was expressed on the cell surface just as efficiently as gD-WT (data not shown). One possibility is that the signal, as attached to the N terminus of gD, was not in the proper position to function. Several of the known ER retention signals are position specific (32, 40, 43). Another possibility is that WT gD contains a dominant “export” signal that the N-terminal region of gH2 is not able to overcome. Even so, our results provide a plausible explanation for how gL functions as a chaperone to help escort the gH/gL complex to its proper location.
Would an N-terminal truncation also enable gH1 to be transported without gL coexpression and binding? We found that the equivalent HSV-1 mutant (gH1Δ48) did not behave the same way as gH2Δ48; gH1Δ48, like WT gH1, still required gL for transport to the cell surface (data not shown). Perhaps the residues of gH1 that play the retention role are different from those we have uncovered for gH2. Among herpesviruses, the N terminus of gH is less conserved than the C terminus and MAbs generated to this region in HSV gH are type specific (4). The assumption is often made that gH1and gH2, being 80% identical, would be functionally and structurally the same. Indeed, gH1-WT is able to bind and form a functional complex with gL2 and vice versa (3, 31). However, several regions of gH1 and gH2 appear to be functionally and structurally distinct (3, 4). gH2 does not have a predicted coiled-coil in the region corresponding to the putative coiled-coil region of gH1 (16, 28). Furthermore, we found structural differences between gH1 and gH2 between cysteines 2 and 4 (residues 258 to 429) (3) in spite of the overall homology of the two proteins.
Role of gL in entry/fusion.
gL has positional and functional homologues among herpesviruses, but the various forms of gL have little sequence homology. Whether other gL proteins function merely as gH chaperones or whether they have their own function in virus entry and fusion is not clear. In the case of pseudorabies virus (PrV), it appears that gL normally functions only as a gH chaperone, since a gDgH chimera functions independent of gL (24). However, the WT gH protein of PrV, which can be transported in the absence of gL, is nonfunctional without gL coexpression (23). The phenotype of WT PrV gH resembles that of gH2Δ48 but not that of gH2-WT.
Although gH2Δ48 was functional when coexpressed with gL, it did not function if gL was provided in trans by a transient transfection of target cells (data not shown). Since gL is thought to be necessary for the folding of a functional gH/gL molecule, we hypothesized that gH2Δ48 alone never achieves this “functional conformation.” To test this possibility, we probed the structure of surface-expressed gH2-WT/gL, gH2Δ48/gL, and gH2Δ48 with MAbs and found that the three molecules presented sites III (residues 73 to 92), VI (residues 352 to 371), VII (residues 532 to 542), and VIII (residues 676 to 686) differently (Fig. 5B). These data suggest that gH2Δ48 is structurally different from either gH2-WT/gL or gH2Δ48/gL, in turn implying that gL coexpression and binding are important for gH2 folding and function.
L66 and L72 are key residues within gH2.
MAbs that bind residues 19 to 38 block gH/gL function, but deletion of this region has no discernible effect on function, as tested by fusion and complementation assays. It could be that binding of the MAbs results in steric hindrance to an important nearby functional domain, although those proposed for gH1 lie well downstream of residues 19 to 38 in the linear sequence (Fig. 1). Another possibility is that MAb binding prevents a conformational change needed for complex function. Structural data are needed to resolve this puzzle.
We found that deletions between residues 48 and 72, just downstream of the group I MAb site, were detrimental to gH/gL function. Two mutants in this region stand out: the L66A mutant, which was reduced in function, and the L72A mutant, which was nonfunctional (Table 1). It is interesting that the residue directly neighboring L72 (E71) could be mutated to alanine (a more drastic change than the L72A mutation) without inhibiting protein function or structure. Also, the N-linked glycosylation site that borders L72 is not important for gH2 function, as the T75A mutant showed only a modest reduction in function.
Both L66 and L72 are located outside predicted gH1 functional domains (Fig. 1), which include a hypothetical fusion peptide at amino acids 377 to 397 and heptad repeat regions HR-1 and HR-2 at residues 443 to 471 and 556 to 585, respectively (16-18). Fusogenic peptides (12, 15) also fall well downstream of residues 64 to 75. To date, the gH N terminus has not been very well characterized, but gL seems to bind within residues 19 to 323 (2, 35, 45).
Mutant gH2-L66A, which was reduced in function, bound the conformation-dependent MAb CHL2 only under certain conditions. For example, if cells were fixed with PFA prior to CHL2 binding, gH2-L66A/gL was no longer recognized by the antibody (data not shown). In contrast, when gH2-L66A/gL was incubated with CHL2 prior to fixation, the MAb reacted with the mutant complex (Fig. 8A); fixation had no effect on CHL2 binding to gH2-WT/gL. In fact, all of the nonfunctional deletion mutants exhibited this sensitivity to fixation (Table 1), suggesting an inherent structural instability in these proteins.
Mutant gH2-L72A was the only point mutant in our collection that had no detectable fusogenic activity, failed to complement the gH-null virus, and was not recognized by CHL2 (Fig. 7 to 8 and Table 1). What is the role of L72 in the gH2 molecule? That gH2-L72A/gL was unable to bind MAb CHL2 suggests a structural alteration, as does the lack of mature gH/gL proteins when this mutant was analyzed by Western blotting (Fig. 8). Moreover, we were unable to detect gL in complex with gH2-L72A. It is unlikely that L72 is involved directly in gL binding since this residue can be deleted in the context of the mutant gH2Δ62-72 with no effect on gL binding. Alternatively, the L72A mutant might be directly involved in gH2 function, although overlapping synthetic peptides corresponding to the region surrounding the L72A mutation were unable to block HSV-2 entry (data not shown). Further studies need to be done to understand the role played by L72 and L66 in the structure and function of gH.
Acknowledgments
Funding for this project was through NIH grant NS36731 to R.J.E. from the National Institute of Neurological Disorders and Stroke and grants AI-18289 and AI-05645 to G.H.C. and R.J.E., respectively, from the National Institute of Allergy and Infectious Diseases.
We are grateful to P. G. Spear and A. Minson for providing reagents. We also thank E. Lazear and T. Moser for technical assistance and our lab colleagues for helpful advice and discussions.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Browne, H. M., B. C. Bruun, and A. C. Minson. 1996. Characterization of herpes simplex virus type 1 recombinants with mutations in the cytoplasmic tail of glycoprotein H. J. Gen. Virol. 77:2569-2573. [DOI] [PubMed] [Google Scholar]
- 2.Cairns, T. M., R. S. B. Milne, M. Ponce-de-Leon, D. K. Tobin, G. H. Cohen, and R. J. Eisenberg. 2003. Structure-function analysis of herpes simplex virus type 1 gD and gH/gL: clues from gDgH chimeras. J. Virol. 77:6731-6742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairns, T. M., D. J. Landsburg, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Contribution of cysteine residues to the structure and function of herpes simplex virus gH/gL. Virology 332:550-562. [DOI] [PubMed] [Google Scholar]
- 4.Cairns, T. M., M. S. Shaner, Y. Zuo, M. Ponce-de-Leon, I. Baribaud, R. J. Eisenberg, G. H. Cohen, and J. C. Whitbeck. 2006. Epitope mapping of herpes simplex virus type 2 gH/gL defines distinct antigenic sites, including some associated with biological function. J. Virol. 80:2596-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carfí, A., S. H. Willis, J. C. Whitbeck, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and D. C. Wiley. 2001. Herpes simplex virus glycoprotein D bound to the human receptor HveA. Mol. Cell 8:169-179. [DOI] [PubMed] [Google Scholar]
- 6.Connolly, S. A., D. J. Landsburg, A. Carfi, D. C. Wiley, R. J. Eisenberg, and G. H. Cohen. 2002. Structure-based analysis of the herpes simplex virus glycoprotein D binding site present on herpesvirus entry mediator HveA (HVEM). J. Virol. 76:10894-10904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado, P., and B. Alarcon. 2005. An orderly inactivation of intracellular retention signals controls surface expression of the T cell antigen receptor. J. Exp. Med. 201:555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubin, G., and H. Jiang. 1995. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J. Virol. 69:4564-4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foà-Tomasi, L., E. Avitabile, A. Boscaro, R. Brandimarti, R. Gualandri, R. Manservigi, F. Dall'Olio, F. Serafini-Cessi, and G. Campadelli-Fiume. 1991. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology 180:474-482. [DOI] [PubMed] [Google Scholar]
- 10.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galdiero, M., A. Whiteley, B. Bruun, S. Bell, T. Minson, and H. Browne. 1997. Site-directed and linker insertion mutagenesis of herpes simplex virus type 1 glycoprotein H. J. Virol. 71:2163-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galdiero, S., A. Falanga, M. Vitiello, H. Browne, C. Pedone, and M. Galdiero. 2005. Fusogenic domains in herpes simplex virus type 1 glycoprotein H. J. Biol. Chem. 280:28632-28643. [DOI] [PubMed] [Google Scholar]
- 13.Geraghty, R. J., C. R. Jogger, and P. G. Spear. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268:147-158. [DOI] [PubMed] [Google Scholar]
- 14.Geraghty, R. J., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 15.Gianni, T., R. Fato, C. Bergamini, G. Lenaz, and G. Campadelli-Fiume. 2006. Hydrophobic α-helices 1 and 2 of herpes simplex virus gH interact with lipids, and their mimetic peptides enhance virus infection and fusion. J. Virol. 80:8190-8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane α-helix with attributes of an internal fusion peptide, positionally conserved in the Herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gianni, T., L. Menotti, and G. Campadelli-Fiume. 2005. A heptad repeat in herpes simplex virus 1 gH, located downstream of the α-helix with attributes of a fusion peptide, is critical for virus entry and fusion. J. Virol. 79:7042-7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianni, T., A. Piccoli, C. Bertucci, and G. Campadelli-Fiume. 2006. Heptad repeat 2 in herpes simplex virus 1 gH interacts with heptad repeat 1 and is critical for virus entry and fusion. J. Virol. 80:2216-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gompels, U. A., and A. C. Minson. 1989. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J. Virol. 63:4744-4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harman, A., H. Browne, and T. Minson. 2002. The transmembrane domain and cytoplasmic tail of herpes simplex virus type 1 glycoprotein H play a role in membrane fusion. J. Virol. 76:10708-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson, L., H. Browne, V. Wargent, N. Davis-Poynter, S. Primorac, K. Goldsmith, A. C. Minson, and D. C. Johnson. 1992. A novel herpes simplex virus glycoprotein, gL, forms a complex with glycoprotein H (gH) and affects normal folding and surface expression of gH. J. Virol. 66:2240-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klupp, B. G., W. Fuchs, E. Weiland, and T. C. Mettenleiter. 1997. Pseudorabies virus glycoprotein L is necessary for virus infectivity but dispensable for virion localization of glycoprotein H. J. Virol. 71:7687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klupp, B. G., and T. C. Mettenleiter. 1999. Glycoprotein gL-independent infectivity of pseudorabies virus is mediated by a gD-gH fusion protein. J. Virol. 73:3014-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klyachkin, Y. M., K. D. Stoops, and R. J. Geraghty. 2006. Herpes simplex virus type 1 glycoprotein L mutants that fail to promote trafficking of glycoprotein H and fail to function in fusion can induce binding of glycoprotein L-dependent anti-glycoprotein H antibodies. J. Gen. Virol. 87:759-767. [DOI] [PubMed] [Google Scholar]
- 26.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4144-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 78:8333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michelsen, K., H. Yuan, and B. Schwappach. 2005. Arginine-based endoplasmic-reticulum-sorting motifs in the assembly of heteromultimeric membrane proteins. EMBO Rep. 6:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 31.Muggeridge, M. I. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 81:2017-2027. [DOI] [PubMed] [Google Scholar]
- 32.Munro, S., and H. R. B. Pelham. 1987. A C-terminal signal prevents secretion of luminal ER proteins. Cell 48:899-907. [DOI] [PubMed] [Google Scholar]
- 33.Okuma, K., M. Nakamura, S. Nakano, Y. Niho, and Y. Matsuura. 1999. Host range of human T-cell leukemia virus type I analyzed by a cell fusion-dependent reporter gene activation assay. Virology 254:235-244. [DOI] [PubMed] [Google Scholar]
- 34.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus glycoprotein gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 37.Roberts, S. R., M. Ponce-de-Leon, G. H. Cohen, and R. J. Eisenberg. 1991. Analysis of the intracellular maturation of the herpes simplex virus type 1 glycoprotein gH in infected and transfected cells. Virology 184:609-624. [DOI] [PubMed] [Google Scholar]
- 38.Roche, S., S. Bressanelli, F. A. Rey, and Y. Gaudin. 2006. Crystal structure of the low-pH form of the vesicular stomatitis virus glycoprotein G. Science 313:187-191. [DOI] [PubMed] [Google Scholar]
- 39.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenkman, M., M. Ehrlich, and G. Z. Lederkremer. 2000. Masking of an endoplasmic reticulum retention signal by its presence in the two subunits of the asialoglycoprotein receptor. J. Biol. Chem. 275:2845-2851. [DOI] [PubMed] [Google Scholar]
- 41.Spear, P. G. 1993. Entry of alphaherpesviruses into cells. Semin. Virol. 4:167-180. [Google Scholar]
- 42.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 43.Szczesna-Skorupa, E., and B. Kemper. 2001. The juxtamembrane sequence of cytochrome P-450 2C1 contains an endoplasmic reticulum retention signal. J. Biol. Chem. 276:45009-45014. [DOI] [PubMed] [Google Scholar]
- 44.Terry-Allison, T., R. I. Montgomery, J. C. Whitbeck, R. Xu, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. HveA (herpesvirus entry mediator A), a coreceptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J. Virol. 72:5802-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westra, D. F., H. B. Kuiperij, G. W. Welling, A. J. Scheffer, T. H. The, and S. Welling-Wester. 1999. Domains of glycoprotein H of herpes simplex virus type 1 involved in complex formation with glycoprotein L. Virology 261:96-105. [DOI] [PubMed] [Google Scholar]
- 46.Wilson, D. W., N. Davis-Poynter, and A. C. Minson. 1994. Mutations in the cytoplasmic tail of herpes simplex virus glycoprotein H suppress cell fusion by a syncytial strain. J. Virol. 68:6985-6993. [DOI] [PMC free article] [PubMed] [Google Scholar]