Abstract
To better define the role of B cells in the control of pathogenic simian immunodeficiency virus (SIV) replication, six rhesus monkeys were depleted of B cells by intravenous infusion of rituximab (anti-CD20) 28 days and 7 days before intravaginal SIVmac239 inoculation and every 21 days thereafter until AIDS developed. Although the blood and tissues were similarly depleted of B cells, anti-SIV immunoglobulin G (IgG) antibody responses were completely blocked in only three of the six animals. In all six animals, levels of viral RNA (vRNA) in plasma peaked at 2 weeks and declined by 4 weeks postinoculation (PI). However, the three animals prevented from making an anti-SIV antibody response had significantly higher plasma vRNA levels through 12 weeks PI (P = 0.012). The remaining three B-cell-depleted animals made moderate anti-SIV IgG antibody responses, maintained moderate plasma SIV loads, and showed an expected rate of disease progression, surviving to 24 weeks PI without developing AIDS. In contrast, all three of the B-cell-depleted animals prevented from making anti-SIV IgG responses developed AIDS by 16 weeks PI (P = 0.0001). These observations indicate that antiviral antibody responses are critical in maintaining effective control of SIV replication at early time points postinfection.
Human immunodeficiency virus (HIV) infection typically results in an acute, 2- to 4-week period characterized by rampant viral replication in all lymphoid tissues and high plasma viral RNA (vRNA) levels. This acute phase is followed by a variable period of clinical latency, reduced viral replication, and readily detectable antiviral immune responses. Signs and symptoms of late-stage HIV disease then supervene as plasma vRNA levels rise and immunodeficiency develops (32, 33). This pattern of infection is also typical of rhesus monkeys infected with pathogenic simian immunodeficiency virus (SIV) (16).
CD8+ T cells apparently exert efficient control of virus replication during acute and chronic SIV (18, 43) and HIV (29) infections. The role of B-cell responses in controlling HIV and SIV replication, however, remains less clear. Some SIV-infected monkeys, for example, never develop anti-SIV immunoglobulin G (IgG) antibodies in plasma, rapidly develop uncontrolled viral replication, and progress to AIDS within a few months postinoculation (PI) (4, 16, 23, 34, 46), while most SIV-infected animals make strong antibody responses and develop AIDS at 10 to 24 months PI (8, 34). In these spontaneous rapid progressor monkeys, it is not clear if the lack of antibody response is the cause or the effect of the uncontrolled viral replication. Similarly, the passive transfer of high-titer SIV-specific gamma globulin or neutralizing anti-HIV antibodies inhibits SIV and simian-human immunodeficiency virus (SHIV) replication and retards the pace of disease progression (3, 14, 27, 39, 40). However, the relative importance of antibodies in the immune control of HIV and SIV infections may depend on individual host genetics. Thus, in most monkeys, CD20+ B cells play a major role in blunting the replication of a virulence-attenuated SHIV, but Mamu-A*01-positive monkeys can control the replication of this attenuated virus without the benefit of B cells or antiviral antibodies (26). Finally, the short-term (2- to 4-week) depletion of B cells by infusion of anti-CD20 monoclonal antibody (mAb) during the first 4 weeks after inoculation with SIVmac251 does not affect the resolution of peak viremia that typically occurs during the first 3 weeks after intravenous SIV inoculation (44). There was, however, an inverse correlation between neutralizing antibody levels and plasma virus levels during the post-acute phase of infection in this study, suggesting that humoral immune responses may contribute to the control of chronic SIV replication.
To better define the role of B cells in the control of pathogenic SIVmac239 infection, we delivered the anti-CD20 mAb rituximab to six rhesus macaques in a chronic, intermittent fashion designed to effect profound and long-term B-cell depletion (28 days and 7 days before intravaginal SIVmac239 inoculation and then every 21 days thereafter until the animals developed AIDS). Although the blood of all the animals was depleted of B cells, anti-SIV antibody responses were completely blocked in only three. Concomitantly, variable but similar levels of antiviral T-cell responses (as measured by proliferation and cytokine production after stimulation with viral epitopes) in all animals were detected. Monkeys that were prevented from making anti-SIV IgG responses could not control viral replication after 8 weeks PI, had significantly higher plasma vRNA levels through 12 weeks PI, and rapidly progressed to death from AIDS. B-cell-depleted animals that made anti-SIV antibody responses, by contrast, maintained moderate plasma vRNA levels and survived without AIDS until the study was ended at 24 weeks PI. Thus, effective control of viral replication and spread at early time points postinfection appears to require the generation of an antiviral antibody response.
MATERIALS AND METHODS
Animals.
The female, multiparous, regularly cycling rhesus macaques (Macaca mulatta) used in these studies were housed at the California National Primate Research Center (CNPRC), Davis, CA, in accordance with the regulations of the Association for Assessment and Accreditation of Laboratory Animal Care. The experiments were approved by the Institutional Animal Care and Use Committee of the University of California, Davis. All animals were negative for antibodies to HIV type 2, SIV, type D retrovirus, and simian T-cell lymphotropic virus type 1 at the time the study was initiated. In SIV-infected animals at the CNPRC, AIDS is defined as a weight loss of >15% in 2 weeks, opportunistic infections which do not respond to therapy, or persistent leucopenia (total leukocyte count of <3,000/μl of blood).
MHC genotyping.
Each animal in this study was screened by PCR with allele-specific primers for the major histocompatibility complex (MHC) allele Mamu-A*01 by personnel in two independent reference laboratories. In both labs, the results of the PCR were verified with nucleotide sequencing of the PCR product (2, 21, 31).
Administration of anti-CD20 recombinant mouse-human chimera mAbs (human IgG1) or human gamma globulin.
Six monkeys (three Mamu-A*01 positive and three Mamu-A*01 negative) were infused intravenously (50 mg/kg of body weight) with a depleting monoclonal anti-CD20 antibody (rituximab [Rituxan; Genentech, Inc., South San Francisco, CA]). The infusions occurred 28 days and 7 days before intravaginal SIVmac239 inoculation and every 21 days thereafter until the animals developed AIDS. Genentech Inc. supplied some of the rituximab as a gift, and the remainder was purchased. As a control for potential effects of human IgG infusion, two monkeys (one Mamu-A*01 positive and one Mamu-A*01 negative) were treated with 100 mg of human gamma globulin (Baygam; Bayer Biological Products, Triangle Park, NC) by intramuscular administration 28 days and 7 days before SIVmac239 inoculation and every 21 days thereafter for 6 months.
Intravaginal SIVmac239 inoculation.
The pathogenic SIVmac239 stock used in these studies was produced in rhesus macaque peripheral blood mononuclear cells (PBMC) as previously described (35) and contained approximately 105 50% tissue culture infective doses/ml. The virus challenge of the monkeys consisted of two intravaginal inoculations (separated by 4 h) with 1 ml of the undiluted SIVmac239 stock. Blood samples were collected regularly postinfection and at necropsy. When B-cell-depleted animals developed unequivocal signs of AIDS, they were euthanized by phenobarbital overdose, and blood and tissues were collected. Six months postinfection, the two human gamma globulin-treated monkeys were killed by phenobarbital overdose, and blood and tissues were collected.
PBMC isolation.
PBMC were isolated from heparinized blood by using lymphocyte separation medium (ICN Biomedicals, Aurora, OH). PBMC samples were frozen in 10% dimethyl sulfoxide (Sigma, St. Louis, MO)-90% fetal bovine serum (Gemini BioProducts, Calabasas, CA) and stored in liquid nitrogen until future analysis in immunological and virological assays (35).
Virus load measurement.
Plasma samples were analyzed for vRNA by a quantitative branched-DNA assay (5), and results are reported as vRNA copy numbers per milliliter of plasma. The detection limit of this assay is 125 vRNA copies/ml of plasma.
Measurement of total plasma IgG levels.
IgG levels in plasma were measured using a radial immunodiffusion assay designed to quantify human IgG levels (The Binding Site, San Diego CA) as described previously (25). The human IgG standard supplied with the kit was used to generate the standard curve. The results of the assay are reported as milligrams of IgG per milliliter of plasma.
Measurement of anti-SIV IgG antibody titers.
SIV-specific IgG binding antibody titers in plasma were measured using enzyme-linked immunosorbent assay (ELISA) plates coated with detergent-disrupted SIVmac251 as previously described (24). The results of the anti-SIV IgG ELISA are reported as the dilution of a sample that produced optical density values above the cutoff value.
Immunoblot analysis of anti-SIV IgG antibody responses.
The range of virion antigens targeted by the SIV-specific IgG binding antibodies was determined using SIV-specific immunoblot kits (Zeptometrix, Buffalo, NY). Nitrocellulose strips preblotted with whole, detergent-disrupted SIV virions were incubated overnight with a 1:10 dilution of the plasma sample and then developed according to the manufacturer's instructions. To increase the sensitivity of this method, a chemiluminescence detection system (Pierce Inc.) was used according to the manufacturer's instructions.
ADCVI assay.
The antibody-dependent cell-mediated virus inhibition (ADCVI) assay was based on methods described previously for measles virus and HIV (9, 10). CEM×174 target cells were infected with SIVmac239 at a multiplicity of infection of approximately 0.01. After adsorption for 1 h, cells were washed, incubated in medium at 5% CO2 and 37°C for 48 h, and washed again. Infected target cells (5 × 104) were next plated in 96-well round-bottom microtiter plates, and plasma (1:100 final concentration) was added along with fresh human PBMC effectors at an effector/target ratio of 10:1. Plasma in the absence of effector cells was also tested for antiviral activity. After 5 or 7 days of incubation at 37°C in 5% CO2, supernatant fluid was collected and assayed for p27 by ELISA (Zeptometrix). ADCVI was calculated as follows: % inhibition = 100{1 − ([p27p]/[p27n])}, where [p27p] and [p27n] are the concentrations of p27 in supernatant fluids from wells containing sources of SIV-positive and SIV-negative antibodies, respectively.
Neutralizing antibody assays.
Neutralization was measured in a luciferase reporter gene assay that utilized either 5.25.EGFP.Luc.M7 cells or TZM-bl cells, as described previously (36). 5.25.EGFP.Luc.M7 cells were obtained from Nathanial Landau. TZM-bl cells were obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. Briefly, cell-free virus was incubated with multiple dilutions of heat-inactivated (56°C; 1 h) EDTA-plasma samples for 1 h prior to the addition of cells. Neutralization titers are expressed as the reciprocals of the serum dilutions at which the numbers of relative luminescence units were reduced by 50% compared to those for virus control wells (no test sample). The cell-free stock of T-cell line-adapted SIVmac251 was generated in H9 cells and assayed in 5.25.EGFP.LucM7 cells. SIVmac239/nef-open was used as a molecularly cloned, full-length gp160-pseudotyped virus generated in 293T cells by using the pSG3ΔEnv backbone vector (National Institutes of Health AIDS Research and Reference Reagent Program; John Kappes and Xiaoyun Wu, contributors) and assayed in TZM-bl cells. Both viral stocks were made cell free by filtration through 0.45-micrometer pores and stored at −70°C until use.
IFN-γ enzyme-linked immunospot assay.
As described previously (1), the number of gamma interferon (IFN-γ)-secreting cells in frozen PBMC responding to a SIVmac239 Gag p27 peptide pool was determined using an IFN-γ enzyme-linked immunospot kit (U-CyTech; Utrecht University, Utrecht, The Netherlands).
SIV-specific T-cell proliferation assay.
Antigen-specific proliferation was measured using PBMC from fresh blood samples in a modification of a previously described assay (30).
Flow cytometric analysis of cell populations in PBMC and lymph nodes.
Blood and peripheral lymph node biopsy specimens (axillary or inguinal nodes) were collected at frequent intervals, and the percentages of CD3+ CD4+ T cells, CD3+ CD8+ T cells, and CD20+ CD79a+ B cells within the lymphocyte populations were determined by flow cytometric analysis using a FACSCalibur (Becton Dickinson Immunocytometry Systems, Milpitas, CA) and rhesus macaque-reactive antibodies from Pharmingen/Becton Dickinson (San Diego, CA; anti-CD3 clone no. SP34, anti-CD4 clone no. M-T477, and anti-CD8 clone no. SK1) and Becton Dickinson (San Jose, CA; anti-CD20 clone no. L27 and anti-CD79a clone no. HM47). Because rituximab can cross-block anti-CD20 antibodies used for detecting B cells, CD79a was included in the flow cytometry panel. The B-cell antigen receptor complex consists of membrane Ig associated with CD79a/CD79b, the “invariant” chain of the transducer-transporter complex heterodimer (reviewed in reference 41). In all blood samples analyzed in this study, all CD79a+ cells coexpressed CD20 and all CD20+ cells were also CD79a+. Thus, in the flow cytometry analysis for this study, we defined B cells as CD20+ CD79a+ cells.
Tetramer staining and memory phenotyping of CD8+ T cells.
Cryopreserved and thawed PBMC were rested overnight in AIM V medium (GIBCO Inc.) with 20% fetal calf serum. Then, 1 × 106 PBMC were suspended in a 200-μl volume of complete RPMI 1640 and stained with 5 μl (0.1 mg/ml) of a pretitrated stock of the Mamu-A*01 Gag tetramer CM9 (CTPYDINQM) conjugated to allophycocyanin (a generous gift from D. Watkins). After a 1-h incubation, cells were stained for an additional 45 min at room temperature with surface markers by using a cocktail containing pretitrated amounts of the following mAbs: anti-CD3-Pacific blue, anti-CD4-Cy5.5-peridin chlorophyll protein, anti-CD8-Cy7-allphycocyanin, anti-CD28-phycoerythrin, anti-CD45RA-Cy7-phycoerythrin, and a fluorescein isothiocyanate (FITC)-conjugated lineage cocktail (CD3-FITC, CD14-FITC, CD16-FITC, CD19-FITC, CD20-FITC, and CD56-FITC, as a “dump channel”; all mAbs were obtained from Becton Dickinson). The cells were washed with fluorescence-activated cell sorter buffer and stained with 2 μl of 7-amino-actinomycin D (0.1 mg/ml; Molecular Probes) to exclude dead cells from the analysis. Samples were incubated for 15 min at room temperature, followed by a wash with fluorescence-activated cell sorter buffer and fixation in 1% paraformaldehyde. Data were acquired using a FACSAria flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Treestar, Inc., Ashland, OR) and Macintosh G5 computers (Apple Inc., Cupertino, CA). A minimum of 200,000 small lymphocyte events were collected per sample. Samples were gated on the live (7-amino-actinomycin D-negative) CD3+-, CD8+-T-cell population for tetramer analysis and on the tetramer-positive CD8+ lymphocytes for phenotyping analysis. Phenotypes of CM9 tetramer-positive CD8+ T cells are defined as naïve (C45RA+, CD28+), central memory (C45RA−, CD28+), intermediate effector memory (CD45RA−, CCD28−), or late effector memory (C45RA+, CD28−).
Immunohistochemical analysis of cell populations in lymph tissues and colon samples.
Tissue was fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) and embedded in paraffin, or tissues were embedded in optimal cutting temperature medium and snap-frozen. All slides were stained using an autostainer (Dako Inc., Carpenteria, CA). Tris-buffered saline (TBS) with Tween 20 (Dako) was used for all washes, and antibody diluent (Dako Inc.; 5% bovine serum albumin-TBS or 2% horse serum-TBS) was used for all mAb dilutions. The primary antibodies used were anti-CD20 (clone no. L27; Becton Dickinson) and anti-CD79a (clone no. HM47; Becton Dickinson). Slides were subjected to an antigen retrieval step consisting of incubation in AR10 (Biogenex Inc., San Ramon, CA) for 2 min at 123°C in a digital decloaking chamber (Biocare Medical Inc., Concord, CA) followed by cooling to 90°C before rinsing in running water and then buffer. As a control, primary antibody was replaced with normal mouse IgG (Dako, Inc.) and negative control slides were included with each staining series. After antigen retrieval, nonspecific binding sites were blocked with 10% goat serum and Tween 20 in phosphate-buffered saline (Background Eraser; Biocare Medical Inc.) for 30 min prior to staining with anti-CD20 or anti-CD79a mAb. After primary antibody incubation for 1 h, the slides were incubated for 20 min with peroxidase blocking reagent (Dako Inc.). The binding of anti-CD20 and anti-CD79a mAbs was detected using the labeled-streptavidin-biotin method (Zymed Inc.), consisting of a 30-min incubation with biotinylated goat anti-mouse antibody, a 10-min incubation with horseradish peroxidase-streptavidin (Zymed Inc.), and then a 5-min incubation with diaminobenzidine (Dako Inc.). Slides were visualized with bright-field illumination by using an Axiovision 1 microscope (Carl Zeiss Inc., Thornwood, NY). Digital images were captured using a Zeiss Axiocam system, and the brightness and contrast of images were digitally adjusted using Photoshop CS2 software (Adobe Systems Inc., San Jose, CA) and a Macintosh G5 computer (Apple Inc., Cupertino, CA). All the control experiments gave appropriate results with minimal nonspecific staining (data not shown).
Statistical analysis.
A Kaplan-Meier survival analysis was performed to compare the rates of survival through 24 weeks PI of the three rituximab-treated monkeys that made SIV-specific antibody responses, the three rituximab-treated monkeys that did not make SIV-specific antibody responses, and 18 untreated monkeys intravaginally inoculated with SIVmac239. The 18 untreated monkeys were from a previous study (1), and they were inoculated intravaginally with the same dose and stock of SIVmac239 as the animals in the present study. A Kaplan-Meier survival analysis was also performed to compare the rates of survival through 24 weeks PI of the Mamu-A*01-positive monkeys that made SIV-specific antibody responses and the Mamu-A*01-positive monkey that was prevented from making an SIV-specific antibody response (28062). For these comparisons, the group of Mamu-A*01-positive monkeys that made SIV-specific antibody responses included the two B-cell-depleted monkeys that made anti-SIV antibodies (26679 and 29495), the one human gamma globulin-treated monkey that made antibodies (29591), and three “historical” control monkeys from a previous study (1). All seven of these Mamu-A*01-positive monkeys were inoculated intravaginally with the same dose and stock of SIVmac239.
To compare viral loads in the monkey groups over the first 12 weeks postchallenge, the vRNA level in each animal was transformed into an area under the curve (AUC). The mean vRNA AUC for each group was calculated, and then the AUCs of the groups were compared by using Student's t test. GraphPad Prism version 4.a for Apple OSX10.4 (GraphPad Software, San Diego, CA) and Macintosh G5 computers (Apple Inc., Cupertino, CA) were used for all analyses.
Nucleotide sequence accession numbers.
Nucleotide sequences corresponding to FCGR3A variants in the six rituximab-treated animals have been deposited in GenBank under accession numbers EF396932 to EF396940.
RESULTS
Effects of anti-CD20 mAb administration on B-cell frequency in blood and tissues and on plasma IgG levels.
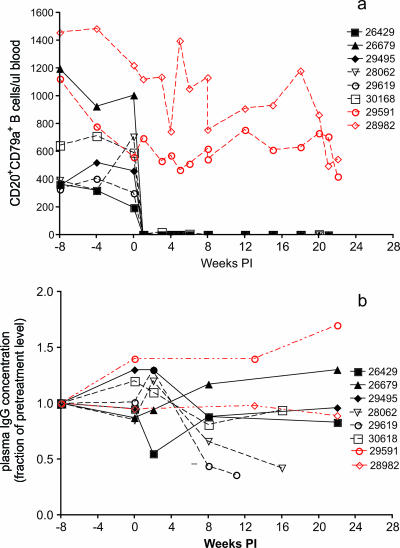
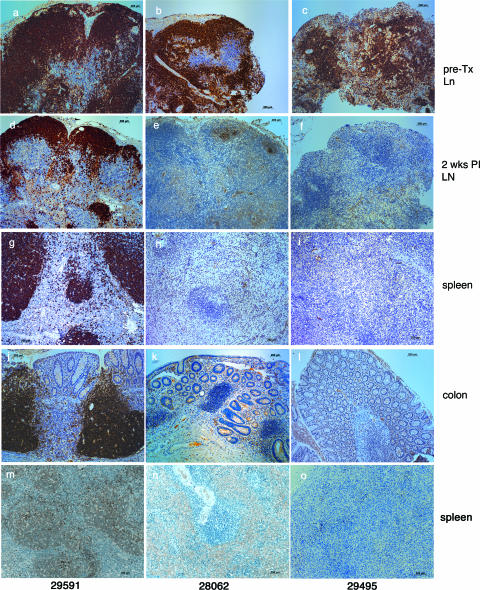
Rhesus macaques were infused with either a depleting monoclonal anti-CD20 antibody (rituximab; n = 6) or human gamma globulin (n = 2) every 21 days. The animals were infected intravaginally with the pathogenic SIVmac239 stock 7 days after the second rituximab infusion (see Materials and Methods). Seven days after the first infusion of rituximab, CD20+ CD79a+ B cells were undetectable in the blood of all six animals (Fig. 1a). Levels of circulating CD20+ CD79a+ B cells in five of the six animals remained undetectable until the animals were necropsied. A few CD20+ CD79a+ B cells were detected in one animal (30618) at very low levels (<25 cells/μl) 1 week prior to and 3 weeks after SIV inoculation, but from that point on, circulating B cells remained undetectable (Fig. 1a). As analyzed by flow cytometry, the peripheral lymph nodes of all six rituximab-treated animals were completely depleted of CD20+ CD79a+ B cells 6 weeks after the first infusion of rituximab (data not shown). By immunohistochemistry, however, variable numbers of CD20+ and CD79a+ B cells were found in lymph node biopsy samples (obtained 6 weeks after the first infusion of rituximab) and in necropsy samples of spleen and colon tissue from all six rituximab-treated animals (Fig. 2). Human gamma globulin infusion had no detectable effect on the frequency of CD20+ CD79a+ B cells in blood or the frequencies of CD79a+ or CD20+ B cells in the tissues examined (Fig. 2).
FIG. 1.
Effect of rituximab infusion on concentrations of CD20+ CD79a+ B cells in blood (a) and on plasma IgG concentrations (b). Black symbols and lines denote the rituximab-treated monkeys. The dashed black lines denote monkeys in group A (28062, 29619, and 30618) that survived AIDS free for a shorter period of time than those in group B (26429, 26679, and 29495; solid black lines) (see the text). The red lines and symbols indicate the gamma globulin-infused monkeys. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 4 weeks prior to SIV inoculation.
FIG. 2.
Effect of rituximab infusion on the frequencies of CD20+ and CD79a+ B cells in tissues. Panels a, d, g, j, and m correspond to human gamma globulin-infused monkey 29591; panels b, e, h, k, and n correspond to rituximab-infused monkey 28062; and panels c, f, i, l, and o correspond to rituximab-infused monkey 29495. (a to c) CD20+ B cells in pretreatment (pre-TX) lymph node (Ln) biopsy specimens. (d to f) CD20+ B cells in lymph node (LN) biopsy specimens collected 6 weeks after initial rituximab treatment (2 weeks PI with SIV). (g to i) CD20+ B cells in spleen necropsy samples. (j to l) CD20+ B cells in colon necropsy samples. (m to o) CD79a+ B cells in spleen necropsy samples.
In most (four of six) of the rituximab-treated monkeys, plasma IgG levels increased in the first 6 to 8 weeks after treatment initiation (Fig. 1b). After 16 weeks, the concentrations of IgG in plasma in four of six rituximab-treated monkeys returned to levels similar to or below those found pretreatment. In two of six animals, plasma IgG levels fell below 50% of pretreatment levels until the animals were necropsied. Human gamma globulin infusion had no apparent effect on plasma IgG levels in one animal, while IgG levels increased in the other animal.
B-cell depletion results in uncontrolled SIV replication and rapid loss of CD4+ lymphocytes in some monkeys.
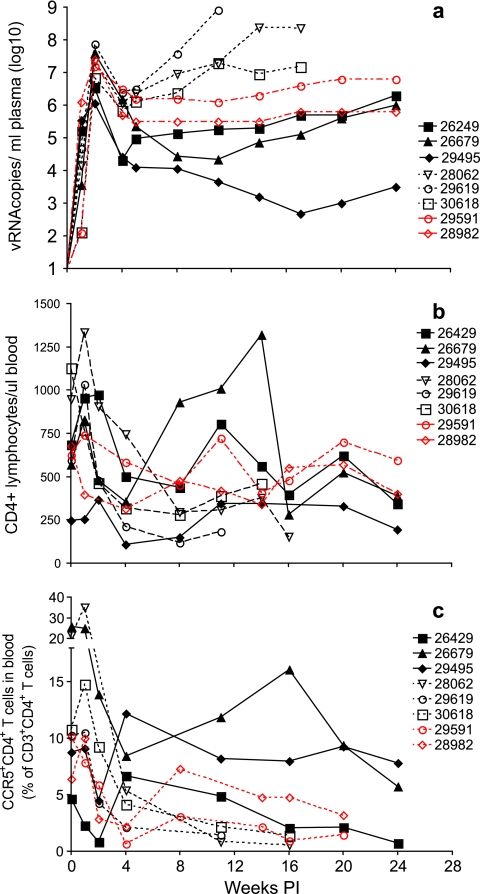
For all six rituximab-treated monkeys and both gamma globulin-treated monkeys, SIV RNA in plasma was detected at 1 week PI and the level peaked at 2 weeks PI and then declined by 1 to 2 orders of magnitude by 4 weeks PI (Fig. 3a). Thereafter, the course of disease progression split the B-cell-depleted monkey cohort into two distinct groups. In group A (monkeys 28062, 29619, and 30618), plasma vRNA levels rapidly increased by 8 weeks PI (Fig. 3a), levels of circulating CD4+ T cells and CCR5+ CD4+ T cells dramatically decreased (Fig. 3b and c, respectively), and death by AIDS occurred between 11 and 16 weeks PI (Fig. 3; Table 1). The other three rituximab-treated monkeys (group B: monkeys 26429, 26679, and 29495) survived for a longer period of time. In two of these three animals, plasma vRNA levels remained reasonably stable at low set-point levels from 6 weeks PI until 24 weeks PI, when the study was ended (Fig. 3; Table 1). In one group B monkey (29495), the amounts of vRNA in plasma decreased to very low levels during this same time frame. In all group B monkeys, the levels of circulating CD4+ and CCR5+ CD4+ T cells slowly declined during the 24-week observation period (Fig. 3b and c, respectively).
FIG. 3.
Plasma vRNA levels and concentrations of CD4+ T cells in blood. Shown are plasma SIV RNA levels (a), absolute numbers of CD3+ CD4+ T cells per microliter of blood (b), and frequencies of circulating CCR5+ CD3+ CD4+ T cells (c). Black symbols and lines denote the rituximab-treated monkeys; the solid black lines denote the group B monkeys. The red symbols and lines indicate the human gamma globulin-infused monkeys. Day 0 is the day of SIV inoculation. Rituximab was infused every 21 days beginning 4 weeks prior to SIV inoculation.
TABLE 1.
Summary of study animals, treatments, and anti-SIV immune responses
| Animal no. | Statusa of Mamu-A*01 allele | Treatmentb | End point binding antibody titerc | 50% Neutralizing antibody titerd | % ADCVIe | T-cell proliferation (SI)f at indicated time (wks) PI
|
Level of anti-Gag IFN-γ secretiong | Wks to necropsyh | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤4 | 4 to 6 | ≥8 | ||||||||
| 26249 | − | Rituximab | 6.4 × 106 | 118 | 96 | 3.6 | 4.9 | 3.8 | 50 | >24 |
| 26679 | + | Rituximab | 6.4 × 106 | 1,194 | 99.8 | <2 | 2 | 27 | 330 | >24 |
| 29495 | + | Rituximab | 8 × 104 | <20 | <50 | 2.6 | <2 | 9.1 | 350 | >24 |
| 28062 | + | Rituximab | <100 | <20 | <50 | 2.1 | <2 | <2 | 140 | 16 |
| 29619 | − | Rituximab | <100 | 30 | <50 | <2 | <2 | <2 | <50 | 11 |
| 30618 | − | Rituximab | 100 | <20 | <50 | 3.2 | 4.3 | <2 | <50 | 16 |
| 29591 | + | Human gamma globulin | 1 × 1010 | 43,740 | 91.8 | <2 | <2 | 13 | 100 | 24 |
| 28982 | − | Human gamma globulin | 1 × 1012 | 43,740 | 85.5 | <2 | 3.7 | 13 | <50 | 24 |
+, present; −, absent.
Beginning 28 days prior to SIV inoculation, rituximab (Rituxan; 50 mg/kg) was infused intravenously every 21 days until necropsy. Beginning 28 days prior to SIV inoculation, human gamma globulin (Baygam; 50 mg/kg) was infused intramuscularly every 21 days until necropsy.
Reciprocal of the highest plasma dilution that was positive in an ELISA using whole SIVmac251 as the coating antigen. The result shown is the highest level detected among plasma samples collected between SIV inoculation and necropsy.
Reciprocal of the highest plasma dilution at which relative luminescence units were reduced 50% compared to those for virus control wells (no plasma sample). Neutralization assays were performed with SIVmac251 in either M7-Luc or TZM-bl cells. The result shown is the highest level detected among plasma samples collected between SIV inoculation and necropsy.
Level of the decrease in replication in rhesus macaque PBMC-SIVmac239 cultures containing a 1:100 dilution of the plasma sample compared to that in control cultures (no plasma sample). The result shown is the highest level detected among plasma samples collected between SIV inoculation and necropsy.
Proliferation of T cells corresponding to whole AT-2-inactivated SIVmac239. Stimulation index (SI) = cpm for AT-2-inactivated-SIV-stimulated wells/cpm for medium-only wells × 100. The result shown is the highest level detected among the samples collected in the time periods indicated.
Number of anti-SIV Gag p27 IFN-γ-secreting T cells in 1 million PBMC. The result shown is the highest level detected among blood samples collected between SIV inoculation and necropsy.
Number of weeks between SIV inoculation and necropsy.
In contrast, both of the gamma globulin-treated monkeys had relatively stable set-point plasma vRNA levels and CD4+-T-cell levels until the animals were necropsied at 24 weeks PI, with mild weight loss but no other signs of retroviral infection (Fig. 3a to c). The postchallenge plasma vRNA levels in the two human gamma globulin-treated control monkeys fell within the range found among 18 historical SIVmac239-infected control monkeys (1) used for the survival analysis (see below).
SIV-specific T-cell responses following B-cell depletion.
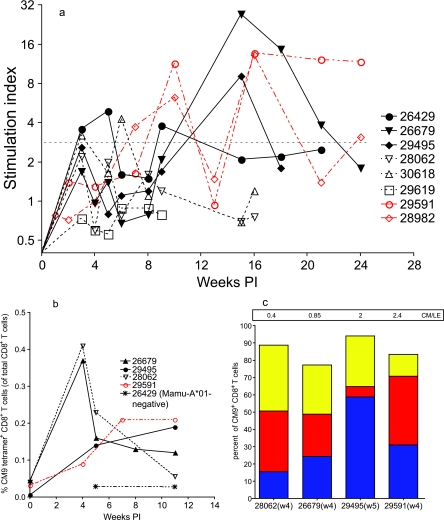
In an attempt to explain the strikingly different outcomes for the rapidly progressing animals (group A) and those surviving for a longer period of time (group B), anti-SIV T- and B-cell immune responses were characterized. T-cell responses to SIV were measured by proliferative capacity upon stimulation with aldrithiol-2 (AT-2)-inactivated SIVmac239 and by levels of cytokine production after stimulation with Gag p27 peptides (Table 1; Fig. 4). Both of the gamma globulin-treated monkeys developed and maintained consistently strong SIV-specific T-cell proliferative responses by 4 to 8 weeks PI, and Gag p27 peptide-induced IFN-γ production by T cells was detected in one of these two animals (29591) (Table 1; Fig. 4). Two of the three rapidly progressing monkeys in group A (28062 and 30618) developed similarly strong SIV-specific T-cell proliferative responses in the first 4 weeks PI, but these responses waned thereafter as the peripheral CD4+-T-cell counts declined (Table 1; Fig. 3b and c and 4a). At weeks 8 and 12 PI, T cells from monkey 28062 also produced IFN-γ in response to stimulation with a Gag p27 peptide pool (Table 1). In the group B monkeys, SIV-specific T-cell proliferative responses were as consistent, and of the same magnitude, as the responses detected in the gamma globulin-treated monkeys (Table 1; Fig. 4). T cells from all three of the group B animals also produced IFN-γ in response to stimulation with a Gag p27 peptide pool (Table 1; data not shown). Interestingly, the antiviral T-cell responses observed in group B monkey 29495 (with the lowest set-point plasma vRNA level) were not statistically different from those of the other two animals in group B but this animal consistently had the highest frequency of SIV Gag p27-specific IFN-γ-producing T cells (Table 1).
FIG. 4.
SIV-specific T-cell proliferation and the kinetics and phenotypes of Gag-specific CD8+-T-cell responses in Mamu-A*01-positive monkeys. (a) Levels of SIV-specific T-cell proliferation; (b) frequencies of CM9 tetramer-positive CD8+ T cells (percentages of total CD8+ T cells) in blood from four Mamu-A*01-positive monkeys and one Mamu-A*01-negative monkey (negative control) between 0 and 11 weeks PI; (c) frequencies of the CM9 tetramer-positive CD8+-T-cell phenotype in four Mamu-A*01-positive monkeys at 4 to 5 weeks PI. CM9 tetramer-positive, CD8+-T-cell phenotypes are defined as central memory (blue; C45RA−, CD28+), intermediate effector memory (red; C45RA−, CD28−), and late effector memory (yellow; C45RA+, CD28−). The ratios of central-memory to late-effector-memory (CM/LE) CM9-specific CD8+ T cells in the samples are indicated in the box at the top of the graph. w4, week 4; w5, week 5.
To determine if the timing or strength of SIV-specific CD8+-T-cell responses could explain the different levels of viral replication in the three B-cell-depleted Mamu-A*-01-positive monkeys in this study (Table 1), the frequencies of CD8+ T cells recognizing a major immunodominant Gag epitope (CM9) during the first 11 weeks after intravaginal SIV inoculation were determined using MHC class I tetramers and flow cytometry (Fig. 4b). Consistent with the results of our previous study (42), all four Mamu-A*01-positive monkeys had circulating CM9-specific CD8+ T cells by 4 or 5 weeks PI. Responses involving high frequencies of CM9-specific T cells were observed at 4 weeks PI in group A monkey 28062 and in group B monkey 26679, but these high frequencies declined in both animals by 5 weeks PI. In the rapidly progressing group A monkey 28062, CM9-specific T cells were undetectable by 11 weeks PI, but CM9-specific T-cell frequencies in the other Mamu-A*01-positive animals increased or remained relatively steady from 5 to 11 weeks PI. The frequency of CM9-specific T cells in the PBMC sample from the Mamu-A*01-positive animal with the lowest plasma vRNA levels, 29495, could not be determined at week 4 PI, but this animal had moderate levels of CM9-specific T cells at 5 and 11 weeks PI. Thus, there was no obvious relationship between the timing or strength of immunodominant Gag CD8+-T-cell responses in the first 8 weeks PI and the levels of viral replication in the Mamu-A*01-positive monkeys.
We also evaluated the phenotypes of circulating CM9-specific CD8+ T cells detected in the four Mamu-A*01-positive monkeys at 4 to 5 weeks PI. At 4 weeks PI, rapidly progressing group A monkey 28062 had a very low ratio (0.4) of central-memory to late-effector-memory CM9-specific CD8+ T cells (Fig. 4c). In contrast, the two Mamu-A*-01-positive monkeys of group B (26679 and 29495) had central-memory/late-effector-memory cell ratios that were near or above 1 (0.85 and 2, respectively) at 4 to 5 weeks PI (Fig. 4c). Although it is not possible to draw definitive conclusions from this small number of comparisons, it is interesting that the pool of central-memory antiviral T cells was not being adequately maintained in the rapidly progressing group A monkey 28062, while the Mamu-A*01-positive animal with the lowest set-point vRNA levels (29495) had the highest central-memory/late-effector-memory CM9-specific CD8+-T-cell ratio (Fig. 4c). It is also notable that, like all B-cell-depleted animals, rapidly progressing Mamu-A*-01-positive monkey 28062 was able to resolve acute peak SIV replication by 4 weeks PI, despite the maturational skewing of the CM9-specific CD8+-T-cell response.
B-cell depletion that eliminates anti-SIV antibody responses is associated with increased viral replication and decreased time to the development of AIDS.
In addition to monitoring the total plasma IgG level (Fig. 1b), we analyzed specific antiviral antibody responses by ELISA (for binding antibodies), by a neutralization assay, and by immunoblotting. As indicated by each of these measures, both of the gamma globulin-treated monkeys developed progressively increasing levels of anti-SIV antibodies (Fig. 5a to c). Both had neutralizing antibody responses by 6 weeks PI, and binding and neutralizing responses steadily strengthened through 24 weeks PI (Table 1; Fig. 5a and b). Plasma from these animals neutralized tissue culture-adapted SIVmac251 at a high dilution (1:43,740) and SIVmac239 at a low dilution (1:30) (data not shown). Further, blood from both of these animals exhibited strong ADCVI in SIV-infected CEM×174 cultures (Table 1).
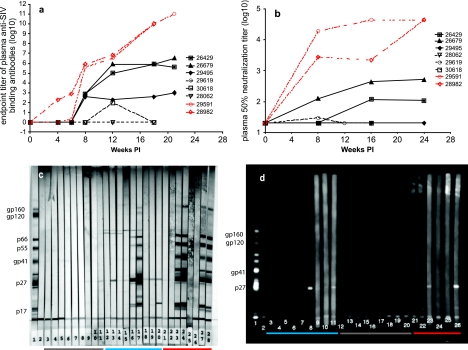
FIG. 5.
Effect of rituximab infusion on systemic antiviral antibody responses. (a) End point titers of anti-SIV binding antibodies in plasma. (b) 50% SIVmac251-neutralizing antibody titers in plasma. (c) SIVmac239 immunoblots. Lanes 3 to 20, plasma samples collected prior to inoculation, 8 weeks PI, and approximately 1 week prior to necropsy or at 20 weeks PI. Lanes 21 to 28, plasma samples collected prior to gamma globulin infusion, after gamma globulin infusion but prior to SIV inoculation, and at 8 and 20 weeks PI. Lanes: 1, positive control; 2, negative control; 3 to 5; monkey 28062; 6 to 8, monkey 29619; 9 to 11, monkey 30618; 12 to 14, monkey 26429; 15 to 17, monkey 26679; 18 to 20, monkey 29495; 21 to 24, monkey 29591; and 25 to 28, monkey 28982. (d) SIVmac 239 chemiluminescent immunoblots. Plasma samples collected from each animal prior to inoculation and 2 and 4 weeks PI were analyzed (lanes 3 to 26). Lanes: 1, positive control; 2, negative control; 3 to 5, monkey 26429; 6 to 8, monkey 26679; 9 to 11, monkey 29495; 12 to 14, monkey 28062; 15 to 17, monkey 29619; 18 to 20, monkey 30618; 21 to 23, monkey 29591; 24 to 26, monkey 28982.
Two of three group B monkeys developed anti-SIV Gag p27 binding IgG responses detected by chemiluminescence immunoblotting, but not by ELISA, by 4 weeks PI (Fig. 5d). In contrast, none of the group A animals demonstrated similar early anti-Gag antibody responses. Moderate-titer anti-SIV IgG binding antibody responses in the three group B monkeys (26429, 26679, and 29495) were detectable by ELISA beginning at 8 weeks PI, demonstrating a 2-week delay compared to the human gamma globulin-treated monkeys (Fig. 5a). Two of these group B animals (26429 and 26679) developed moderate neutralizing antibody titers that never reached the level observed in the gamma globulin-treated monkeys (Table 1; Fig. 5b). Further, blood samples from both of these group B animals exhibited ADCVI. Of the three group B monkeys, 29495 (the one with the lowest set-point plasma vRNA levels) had the weakest anti-SIV antibody responses (lowest IgG binding titer and no neutralizing or ADCVI activity) (Table 1; Fig. 5a and b).
In contrast, the three rapidly progressing monkeys of group A failed to show anti-SIV IgG antibody responses, as measured by ELISA (Fig. 5a), neutralization (Fig. 5b), and immunoblotting (Fig. 5c) (except in the cases of the week-12 PI sample from monkey 30168, which had very-low-titer SIV IgG binding antibodies, and one sample from monkey 29619, which had very-low-titer SIVmac251-neutralizing activity) (Table 1; Fig. 5a and b). Of note, monkey 30618 did not demonstrate the level of total plasma IgG suppression (<50% of baseline) that occurred in the other two monkeys of this group (28062 and 29619) (Fig. 1b).
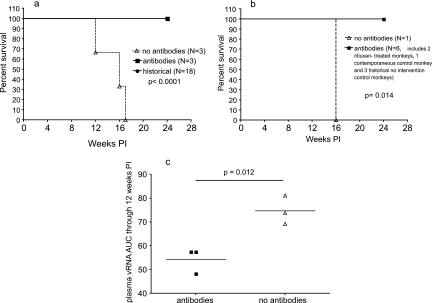
The above-described observations demonstrate that the B-cell-depleted monkeys that were prevented from making SIV-specific antibody responses (group A) rapidly progressed to AIDS (11 to 16 weeks PI) while those that could mount SIV-specific IgG antibody responses (group B) survived to 24 weeks PI without developing AIDS. The latter rate of disease progression was similar to that observed in 18 untreated monkeys intravaginally inoculated with SIVmac239 (1), and as shown in Fig. 6a, the difference in survival rates between groups A and B was highly significant (P < 0.0001). To clarify the role of antibodies in the survival of SIVmac239-infected Mamu-A*01-positive rhesus monkeys, the time to development of AIDS in the four Mamu-A*01-positive animals in the present study (Table 1) and those in three additional treatment-naïve Mamu-A-01-positive animals intravaginally inoculated with the same SIVmac239 stock as part of a previous study (1) were compared. The survival time from intravaginal SIV inoculation to the development of AIDS was significantly shorter (P < 0.014) for the Mamu-A*01-positive monkey (28062) that was prevented from making a SIV-specific IgG antibody response than for the six Mamu-A*01-positive monkeys (two B-cell-depleted and one human gamma globulin-treated monkey and three treatment-naïve monkeys from a previous study [1]) that made SIV-specific IgG antibodies by 8 weeks after intravaginal SIVmac239 inoculation (Fig. 6b). Finally, the three B-cell-depleted monkeys that made anti-SIV antibody responses had significantly higher plasma vRNA levels (AUCs) through 12 weeks PI (P = 0.012) than the three B-cell-depleted monkeys that did not make anti-SIV antibody responses (Fig. 6c).
FIG. 6.
Relationship between antiviral antibody responses, survival, and plasma SIV levels. RNA levels in rituximab-treated monkeys were determined. (a) Survival through 24 weeks PI. Based on a Kaplan-Meier analysis, significantly more rituximab-treated monkeys that made antiviral antibody responses (n = 3) and treatment-naïve monkeys (n = 18) than B-cell-depleted monkeys that were prevented from making antiviral antibody responses (n = 3) survived to 24 weeks PI (P < 0.0001). (b) Survival through 24 weeks PI of seven Mamu-A*01-positive monkeys. Based on a Kaplan-Meier analysis, the rituximab-treated Mamu-A*01-positive monkeys that made antiviral antibody responses (n = 2), the human gamma globulin-treated Mamu-A*01-positive monkey that made an antiviral antibody response (n = 1), and the historically treatment-naïve Mamu-A*01-positive monkeys that made antiviral antibody responses (n = 3) survived longer than the B-cell-depleted Mamu-A*-01-positive monkey that was prevented from making an antiviral antibody response (n = 1) (P = 0.014). (c) AUCs corresponding to the plasma vRNA levels in the six B-cell-depleted monkeys through 12 weeks PI. The mean plasma vRNA levels through 12 weeks PI (the time point when the first animal was necropsied) were transformed into AUCs. In a two-tailed paired t test, the mean plasma vRNA AUC for B-cell-depleted monkeys that made antiviral antibody responses (n = 3; left horizontal line) was significantly lower than that for B-cell-depleted monkeys that were prevented from making antiviral antibody responses (n = 3; right horizontal line; P = 0.012).
DISCUSSION
It is generally accepted that CD8 + T lymphocytes play a major role in immune control of SIV replication because the short-term depletion of CD8 + T lymphocytes in acute or chronic SIV infection is associated with uncontrolled replication until SIV-specific CD8+ T cells return (18, 43). Using anti-CD20 mAb infusion to achieve chronic B-cell depletion, we found that de novo antiviral antibody responses were also critical for maintaining the stable set-point viral replication levels that developed 6 to 8 weeks after intravaginal SIV inoculation. The effects of chronic anti-CD20 mAb infusion on SIV IgG antibody production varied among individual rhesus monkeys: half of the treated animals (three of six) were blocked from producing SIV-specific IgG antibodies, and the other half produced moderate-titer anti-SIV IgG antibody responses. The animals that were completely blocked from making de novo antiviral IgG antibody responses had significantly increased plasma SIV RNA levels and a significantly shortened interval from inoculation to the development of AIDS compared to the monkeys that had incomplete inhibition of the antiviral antibody response. Thus, even the relatively modest anti-SIV IgG antibody responses in these three B-cell-depleted monkeys were sufficient to prevent the uncontrolled viral replication that was seen in the three short-term survivor animals that could mount no antibody response. Significantly, and in contrast to the effect of B-cell depletion on the replication of a virulence-attenuated SHIV (26), these effects occurred in both Mamu-A*01-positive and Mamu-A*01-negative monkeys infected with the neutralization-resistant and highly virulent SIVmac239 variant.
In assessing the immunologic factors contributing to the differential outcomes for the B-cell-depleted monkeys, the first 8 weeks of infection deserves particular attention because the uncontrolled viral replication characteristic of group A monkeys was apparent by 8 weeks PI. Thus, although SIV-specific T-cell proliferation and CD8+-T-cell responses of similar strengths were detected in five of the six B-cell-depleted monkeys during the first 2 to 6 weeks PI, these CD8+-T-cell responses were not maintained in the animals that did not develop a SIV-specific IgG binding or neutralizing antibody response by 8 weeks PI. CD4+ T helper cells play a central role in the development and maintenance of cytotoxic T lymphocytes in HIV infection (19, 20). Significantly, HIV preferentially infects and destroys virus-specific CD4+ T helper cells (6, 7, 15) and SIV preferentially infects and kills memory CD4+ T cells (22, 28). Our results suggest that antibodies are critical for protecting SIV-specific memory CD4+ T helper cells from devastating destruction in the initial stages of infection. Thus, monkeys that produce anti-SIV antibodies by week 4 PI are able to live for extended periods because SIV-specific CD4+ T helper cells can persist and maintain effective antiviral CD8+-T-cell responses. However, the protective effect of the early antiviral antibody response on SIV-specific CD4+ T helper cells does not persist indefinitely, as there was a steady rise in plasma vRNA levels and a decline in CD4+ T cells over 6 months of observation in B-cell-intact monkeys and the long-term survivor B-cell-depleted monkeys that likely reflect decreasingly effective immune responses (Fig. 3). Significantly, chemiluminescence immunoblot analysis revealed SIV-specific IgG antibodies at 4 weeks PI in two of three of the group B monkeys that survived for a longer period of time (Fig. 5d). The timing of these early antibody responses suggests that they may play a critical role in protecting the CD4+ T cells from irreversible depletion in the acute PI period.
The reason for the different responses of the two groups of monkeys to the rituximab administration is unclear. One explanation is that there was a gradient of incomplete B-cell depletion in hematolymphoid organs. In animals of group A, for instance, such depletion was sufficiently complete to abolish all anti-SIV antibody responses, whereas in those of group B, sufficient B cells persisted to confer a protective anti-SIV antibody response. This possibility is consistent with observations made with humans treated with rituximab, in which differential responses are due to discrete underlying genetic polymorphisms of FCGR3A (reviewed in reference 12). In fact, upon the cloning and sequencing of FCGR3A cDNA (45) from the PMBC of the six rituximab-treated animals, we found that all three group A animals had a distinct allelic variant of FCGR3A while all three group B animals had a different allelic variant of FCGR3A. The FCGR3A polymorphism included two amino acids in the transmembrane and proximal cytoplasmic tail regions. Thus, Met229 and Iso233 were found in group B animals that made an antibody response and Val229 and Val233 were found in group A animals that were completely blocked from making anti-SIV antibodies (data not shown). Ongoing experiments are aimed at determining if this genetic polymorphism in the macaques underlies differential responses to rituximab treatment.
In this report, we demonstrate the importance of even moderate de novo SIV-specific antibody responses in controlling viral replication. An important caveat to this report is that the number of animals studied was relatively small, and larger studies will be needed to confirm these results. However, the results reported here are consistent with those of previous studies demonstrating that the passive transfer of high-titer SIV-specific gamma globulin or neutralizing anti-HIV antibodies can alter the courses of SIV and SHIV infections and disease progression (3, 14, 27, 39, 40). Further, the critical role of antibody responses in the long-term control of viral replication is consistent with the finding that neutralizing antibody responses drive the evolution of the HIV env gene in individuals that mount such responses (13). Of note, HIV infection in a patient with congenital hypogammaglobulinemia has been documented previously (17); careful analyses of HIV infections in patients with congenital immunodeficiencies may reveal the relative contributions of specific immune effector functions in controlling HIV replication in humans. The three group B monkeys had plasma anti-SIV binding antibody responses that were moderate and delayed compared to those of the two human gamma globulin-treated control monkeys. The plasma from two of these three B-cell-depleted monkeys neutralized the heterologous and neutralization-sensitive SIVmac251 but could not neutralize the autologous and neutralization-resistant SIVmac239. Further, in the three group B monkeys, much of the anti-SIV antibody response, including the acute-phase response, was directed to Gag, Pol, and Env gp41 antigens that would not be expected to be involved in classic viral neutralization (Fig. 4c). Nonneutralizing antibodies may bind defective Env glycoprotein spikes (38) and contribute to the control of viral replication through complement opsonization or Fc receptor-mediated functions (reviewed in reference 37). In particular, nonneutralizing antibodies in the presence of natural killer cells can block virus replication in vitro (10, 11), and in this regard, it is of note that levels of ADCVI activity directed at SIVmac239 were high in two of the B-cell-depleted monkeys that made anti-SIVmac251 neutralizing antibodies.
The results of this study demonstrate that an early antiviral antibody response is required to constrain SIV replication and maintain stable plasma vRNA levels after SIV infection. Thus, in chronic SIV infection, control of SIV replication is maintained in the presence of both cellular and humoral immune antiviral effector mechanisms. It seems likely that the ability to elicit a similarly balanced set of immune effector activities will be desirable in an HIV vaccine.
Acknowledgments
We thank David Ho and Joern Schmitz for helpful discussions and Joseph Dutra, Tracy Rourke, Linda Fritts, Paul Chohan, Katherine Lantz, Gary Landucci, the Immunology Core Laboratory, and the Primate Services Unit at the CNPRC for excellent technical assistance. We also thank the Veterinary Genetics Laboratory, University of California, Davis, and the D. Watkins Laboratory at the University of Wisconsin for Mamu-A01 haplotyping.
This work was supported by Public Health Services grants U51 RR00169 P01 AI066314 to C.J.M., R01 AI 52039 to D.F., R01 AI30034 to D.M., and DPI OD00329 to J.M.M., who is a recipient of the Burroughs Wellcome Fund Clinical Scientist Award in Translational Research and of the National Institutes of Health Director's Pioneer Award, part of the National Institutes of Health Roadmap for Medical Research. Genentech Inc. provided some of the rituximab as a gift under the auspices of a material transfer agreement with the University of California, Davis.
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Abel, K., L. Compton, T. Rourke, D. Montefiori, D. Lu, K. Rothaeusler, L. Fritts, K. Bost, and C. J. Miller. 2003. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J. Virol. 77:3099-3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Baskin, G. B., M. Murphey-Corb, E. A. Watson, and L. N. Martin. 1988. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 25:456-467. [DOI] [PubMed] [Google Scholar]
- 5.Dailey, P. J., M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea. 1995. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected rhesus macaques using a branched DNA (bDNA) signal amplification assay. J. Med. Primatol. 24:209. [Google Scholar]
- 6.Demoustier, A., B. Gubler, O. Lambotte, M. G. de Goer, C. Wallon, C. Goujard, J. F. Delfraissy, and Y. Taoufik. 2002. In patients on prolonged HAART, a significant pool of HIV infected CD4 T cells are HIV-specific. AIDS 16:1749-1754. [DOI] [PubMed] [Google Scholar]
- 7.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 8.Dykhuizen, M., J. L. Mitchen, D. C. Montefiori, J. Thomson, L. Acker, H. Lardy, and C. D. Pauza. 1998. Determinants of disease in the simian immunodeficiency virus-infected rhesus macaque: characterizing animals with low antibody responses and rapid progression. J. Gen. Virol. 79:2461-2467. [DOI] [PubMed] [Google Scholar]
- 9.Forthal, D. N., and G. Landucci. 1998. In vitro reduction of virus infectivity by antibody-dependent cell-mediated immunity. J. Immunol. Methods 220:129-138. [DOI] [PubMed] [Google Scholar]
- 10.Forthal, D. N., G. Landucci, and E. S. Daar. 2001. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J. Virol. 75:6953-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forthal, D. N., G. Landucci, T. B. Phan, and J. Becerra. 2005. Interactions between natural killer cells and antibody Fc result in enhanced antibody neutralization of human immunodeficiency virus type 1. J. Virol. 79:2042-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg, J. W. 2005. Unique toxicities and resistance mechanisms associated with monoclonal antibody therapy. Hematology Am. Soc. Hematol. Educ. Program 2005:329-334. [DOI] [PubMed] [Google Scholar]
- 13.Frost, S. D., T. Wrin, D. M. Smith, S. L. Kosakovsky Pond, Y. Liu, E. Paxinos, C. Chappey, J. Galovich, J. Beauchaine, C. J. Petropoulos, S. J. Little, and D. D. Richman. 2005. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc. Natl. Acad. Sci. USA 102:18514-18519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haigwood, N. L., A. Watson, W. F. Sutton, J. McClure, A. Lewis, J. Ranchalis, B. Travis, G. Voss, N. L. Letvin, S. L. Hu, V. M. Hirsch, and P. R. Johnson. 1996. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol. Lett. 51:107-114. [DOI] [PubMed] [Google Scholar]
- 15.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, D. D., T. Moudgil, H. S. Robin, M. Alam, B. J. Wallace, and Y. Mizrachi. 1989. Human immunodeficiency virus type 1 in a seronegative patient with visceral Kaposi's sarcoma and hypogammaglobulinemia. Am. J. Med. 86:349-351. [DOI] [PubMed] [Google Scholar]
- 18.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalams, S. A., and B. D. Walker. 1998. The critical need for CD4 help in maintaining effective cytotoxic T lymphocyte responses. J. Exp. Med. 188:2199-2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapp, L. A., E. Lehmann, M. S. Piekarczyk, J. A. Urvater, and D. I. Watkins. 1997. A high frequency of Mamu-A*01 in the rhesus macaque detected by polymerase chain reaction with sequence-specific primers and direct sequencing. Tissue Antigens 50:657-661. [DOI] [PubMed] [Google Scholar]
- 22.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 23.Lifson, J. D., M. A. Nowak, S. Goldstein, J. L. Rossio, A. Kinter, G. Vasquez, T. A. Wiltrout, C. Brown, D. Schneider, L. Wahl, A. L. Lloyd, J. Williams, W. R. Elkins, A. S. Fauci, and V. M. Hirsch. 1997. The extent of early viral replication is a critical determinant of the natural history of simian immunodeficiency virus infection. J. Virol. 71:9508-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lü, X. S., H. Kiyono, D. Lu, S. Kawabata, J. Torten, S. Srinivasan, P. J. Dailey, J. R. McGhee, T. Lehner, and C. J. Miller. 1998. Targeted lymph node immunization with whole-inactivated SIV or envelope and core subunit antigen vaccines does not reliably protect rhesus macaques from vaginal challenge with SIVmac251. AIDS 12:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lü, X. S., and C. J. Miller. 1996. Concentration of IgG in the sera of normal rhesus macaques as determined by a species-specific radial immunodiffusion assay. J. Immunol. Methods 197:193-196. [DOI] [PubMed] [Google Scholar]
- 26.Mao, H., B. A. Lafont, T. Igarashi, Y. Nishimura, C. Brown, V. Hirsch, A. Buckler-White, R. Sadjadpour, and M. A. Martin. 2005. CD8+ and CD20+ lymphocytes cooperate to control acute simian immunodeficiency virus/human immunodeficiency virus chimeric virus infections in rhesus monkeys: modulation by major histocompatibility complex genotype. J. Virol. 79:14887-14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 29.McChesney, M., F. Tanneau, A. Regnault, P. Sansonetti, L. Montagnier, M. P. Kieny, and Y. Riviere. 1990. Detection of primary cytotoxic T lymphocytes specific for the envelope glycoprotein of HIV-1 by deletion of the env amino-terminal signal sequence. Eur. J. Immunol. 20:215-220. [DOI] [PubMed] [Google Scholar]
- 30.McChesney, M. B., J. R. Collins, D. Lu, X. Lÿ, J. Torten, R. L. Ashley, M. W. Cloyd, and C. J. Miller. 1998. Occult systemic infection and persistent simian immunodeficiency virus (SIV)-specific CD4+-T-cell proliferative responses in rhesus macaques that were transiently viremic after intravaginal inoculation of SIV. J. Virol. 72:10029-10035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDermott, A. B., D. H. O'Connor, S. Fuenger, S. Piaskowski, S. Martin, J. Loffredo, M. Reynolds, J. Reed, J. Furlott, T. Jacoby, C. Riek, E. Dodds, K. Krebs, M. E. Davies, W. A. Schleif, D. R. Casimiro, J. W. Shiver, and D. I. Watkins. 2005. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J. Virol. 79:15556-15566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellors, J. W., L. A. Kingsley, C. R. Rinaldo, Jr., J. A. Todd, B. S. Hoo, R. P. Kokka, and P. Gupta. 1995. Quantitation of HIV-1 RNA in plasma predicts outcome after seroconversion. Ann. Intern. Med. 122:573-579. [DOI] [PubMed] [Google Scholar]
- 33.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 34.Miller, C. J., N. J. Alexander, S. Sutjipto, A. A. Lackner, A. G. Hendrickx, A. Gettie, L. J. Lowenstine, M. Jennings, and P. A. Marx. 1989. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J. Virol. 63:4277-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller, C. J., M. B. McChesney, X. S. Lÿ, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montefiori, D. C. 2004. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays, p. 12.11.1-12.11.15. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, W. Strober, and R. Coico (ed.), Current protocols in immunology. John Wiley & Sons, New York, NY. [DOI] [PubMed]
- 37.Montefiori, D. C. 1997. Role of complement and Fc receptors in the pathogenesis of HIV-1 infection. Springer Semin. Immunopathol. 18:371-390. [DOI] [PubMed] [Google Scholar]
- 38.Moore, P. L., E. T. Crooks, L. Porter, P. Zhu, C. S. Cayanan, H. Grise, P. Corcoran, M. B. Zwick, M. Franti, L. Morris, K. H. Roux, D. R. Burton, and J. M. Binley. 2006. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J. Virol. 80:2515-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishimura, Y., T. Igarashi, N. Haigwood, R. Sadjadpour, R. J. Plishka, A. Buckler-White, R. Shibata, and M. A. Martin. 2002. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J. Virol. 76:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reth, M. 1992. Antigen receptors on B lymphocytes. Annu. Rev. Immunol. 10:97-121. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z.-M. Ma, L. Compton, G. Napoe, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 79:9228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz, J. E., M. J. Kuroda, S. Santra, M. A. Simon, M. A. Lifton, W. Lin, R. Khunkhun, M. Piatak, J. D. Lifson, G. Grosschupff, R. S. Gelman, P. Racz, K. Tenner-Racz, K. A. Mansfield, N. L. Letvin, D. C. Montefiori, and K. A. Reimann. 2003. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 77:2165-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Treon, S. P., M. Hansen, A. R. Branagan, S. Verselis, C. Emmanouilides, E. Kimby, S. R. Frankel, N. Touroutoglou, B. Turnbull, K. C. Anderson, D. G. Maloney, and E. A. Fox. 2005. Polymorphisms in FcγRIIIA (CD16) receptor expression are associated with clinical response to rituximab in Waldenstrom's macroglobulinemia. J. Clin. Oncol. 23:474-481. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, J., L. N. Martin, E. A. Watson, R. C. Montelaro, M. West, L. Epstein, and M. Murphey-Corb. 1988. Simian immunodeficiency virus/delta-induced immunodeficiency disease in rhesus monkeys: relation of antibody response and antigenemia. J. Infect. Dis. 158:1277-1286. [DOI] [PubMed] [Google Scholar]