Abstract
Infection by human cytomegalovirus (HCMV) is associated with the development of vascular diseases and may cause severe brain damage in infected fetuses. Platelet-derived growth factor receptors alpha and beta (PDGFR-α and -β) control important cellular processes associated with atherosclerosis and fetal development. In the present investigation, our goal was to determine whether infection by HCMV can influence the expression of PDGFR-α and -β in human smooth muscle cells (SMCs). In connection with HCMV infection in vitro the levels of PDGFR-α and -β at the cell surface and in the total cellular protein of SMCs were reduced in parallel with decreases in the levels of the corresponding mRNAs. These effects were dependent on immediate-early (IE) or early (E) HCMV gene products, since inhibition of late genes did not prevent HCMV from affecting the expression of PDGFR-α and -β. The downregulation of PDGFR caused by HCMV was dose dependent. Furthermore, confocal microscopy revealed that the localization of PDGFR-β was altered in HCMV-infected cells, in which this protein colocalized with proteins associated with endosomes (Rab4 and -5) and lysosomes (Lamp1 and -2), indicating entrance into pathways for protein degradation. Altogether these observations indicate that an IE and/or E HCMV protein(s) downregulates the expression of PDGFR-α and -β in SMCs. This phenomenon may disrupt cellular processes of importance in connection with cellular differentiation, migration, and/or proliferation. These observations may explain why congenital infection with HCMV can cause fetal brain damage.
Platelet-derived growth factor (PDGF), an important mitogen and chemoattractant for a variety of different types of cells, is involved in embryonic development, wound healing, carcinogenesis, and atherosclerosis. PDGF is recognized by the PDGF receptor (PDGFR), belonging to the tyrosine kinase family of receptors. To date, two human forms of PDGFRs have been identified: PDGFR-α, which binds PDGF-A and -B isoforms of PDGF, and PDGFR-β, which shows affinity for the PDGF-B isoform (reviewed in references 6 and 9). PDGF-C has a binding pattern similar to that of PDGF-A/B as it binds PDGFR-α/α homodimers as well as PDGFR-α/β heterodimers (8, 15), whereas PDGF-D binds only to PDGFR-β (2).
PDGF is secreted by a variety of cells, including platelets, smooth muscle cells (SMCs), endothelial cells, and macrophages. PDGF has been found to be mitogenic and to serve as a chemotactic factor for mesenchymal cells such as SMCs, fibroblasts, neutrophils, and mononuclear cells (9). This factor can induce SMCs to differentiate, which involves alteration from a contractile to a synthetic phenotype, following which these cells can proliferate and migrate from the medium to the intima layer of the vessel wall (reviewed in reference 30). PDGFs also play important roles in connection with the migration, proliferation, differentiation, and deposition of extracellular matrix associated with the development of many other types of cells of mesenchymal origin.
Mice lacking PDGFR-β exhibit abnormal kidney glomeruli, thrombocytopenia, widespread edema, and hemorrhage, and they die during late gestation (25). Interestingly, mice expressing a nonfunctional mutant form of PDGF-B demonstrate similar defects but also display capillary microaneurysms, hypoplasia of arterial SMCs, and cardiac muscle hypotrophy, suggesting a loss of PDGFR-α signaling in response to PDGF-BB or -AB (14). The defects observed in mice lacking PDGFR-α include cleft face, subepidermal blistering, spina bifida, impaired formation of the neural crest, incomplete cephalic closure, cardiovascular and skeletal defects, and edemas resulting in embryonal death (26).
Differences in the expression of α and/or β receptors on different types of cells, as well as the variability in PDGF ligand binding, allow a wide range of potential biological responses to PDGF. For example, this signaling system has, on the basis of the elevated levels of PDGFs and PDGFRs detected in atherosclerotic in comparison to healthy vessels, been proposed to participate in the formation of atherosclerotic lesions (reviewed in reference 9). This participation might involve, e.g., induction of the migration of SMCs from the medium to the intima layer of the vessel wall and subsequent proliferation of these cells as well as enhancement of the production of components of the extracellular matrix.
Human cytomegalovirus (HCMV) belongs to the herpesvirus family, whose members remain in the body in a latent state following primary infection. HCMV may cause severe disease in immunocompromised patients, such as individuals with AIDS and patients taking immunosuppressive drugs following transplantation (5). HCMV is also clearly the most important congenital infection that may lead to developmental damage of the central nervous system, including hearing loss, mental retardation, and visual impairment. The prevalence of congenital HCMV infection is 0.2 to 2.3% of all live births, and 50 to 70% of these infected infants show symptoms at birth (1).
Moreover, a number of previous investigations have revealed the presence of HCMV in atherosclerotic lesions and vessels from patients with transplant vascular sclerosis (TVS) (16, 17). By the employment of in situ DNA hybridization and PCR techniques, nucleic acids characteristic for HCMV have been detected in atheromatous plaques collected from the abdominal aorta of HCMV-seropositive patients undergoing reconstructive vascular surgery (10, 11). In addition, the HCMV genome and HCMV immediate-early (IE) antigens (IEAs) have been found in different layers of the human aorta (20). Previous studies have also demonstrated that prophylaxis with ganciclovir, a potent inhibitor of HCMV replication, and HCMV hyperimmune globulin can reduce and delay the risk of TVS in heart transplant patients (32), which further suggests that HCMV may be a causative agent for TVS.
However, the mere presence of HCMV in vessel walls and in atherosclerotic lesions cannot be considered to provide direct evidence that HCMV causes atherosclerosis. At the same time, a number of different types of cells involved in the pathogenesis of atherosclerosis, such as SMCs, monocytes/macrophages, and endothelial cells, have been shown to be susceptible to infection by HCMV, both in vitro and in vivo (7, 24, 29, 31). Such infection may damage the endothelium, thereby altering the patterns of secretion of different growth factors, chemokines, and cytokines by different types of cells. This ability to change the local environment in the vessel wall makes HCMV a prime candidate for involvement in the pathogenesis of atherosclerosis.
In support of this hypothesis, our laboratory and others have previously presented evidence that chemokines and their receptors may participate in a significant manner in the development of vascular diseases (27, 28). US28, encoded by the HCMV genome, similar in structure to the human chemokine receptor CCR-2, and capable of binding many of the CC chemokines (including RANTES and monocyte chemoattractant protein 1 [MCP-1]), promotes migration of vascular SMCs in vitro. HCMV also changes the patterns of secretion of chemokines, such as RANTES and MCP-1, by infected cells (12, 18). Furthermore, in connection with heart transplant models, CMV-mediated upregulation of chemokine expression was associated with accelerated chronic rejection (27).
In this study, our goal was to determine whether infection of SMCs by HCMV alters the pattern of expression of PDGFRs by these cells. Interestingly, HCMV was found to downregulate the expression of both PDGFR-α and -β, a phenomenon which may have important biological implications in particular during fetal development.
MATERIALS AND METHODS
Cells.
Human pulmonary artery SMCs (HPASMC), human coronary artery SMCs (HCASMC) (Clonetics, BioWhittaker, Walkersville, MD), and human aortic SMCs isolated with an explant technique were maintained in cell culture flasks (Costar, Corning Incorporated, Corning, NY) in SmGm2 medium (Clonetics) containing growth factors (Clonetics), while human pulmonary artery endothelial cells (Clonetics) were maintained in EGM2 medium containing growth factors (Clonetics). HPASMC, HCASMC, and human pulmonary artery endothelial cells were used after 8 to 14 passages. Fibroblasts were maintained in Dulbecco's modified Eagle's medium with glutamine supplemented with 100 U penicillin and 100 μg streptomycin per ml (Gibco BRL, Grand Island, NY) and used following 20 to 23 passages.
Infection with HCMV.
At 80% confluence, cells were exposed to HCMV at various multiplicities of infection (MOIs; 0.01 to 10) at 37°C. The four different strains of HCMV utilized were AD169, Towne, the clinical isolate HA, and the endothelial cell-adapted clinical isolate VR1814 (kindly provided by Giuseppe Gerna, University of Pavia, Pavia, Italy). Experiments with UV-irradiated virus and virus-cleared supernatants collected at 4 h, 12 h, 24 h, or 72 h after infection and filtered through a 0.1-μm-pore-size filter in order to remove infectious virus particles were also performed, as was infection in the presence of 3 mM foscarnet (Foscavir; Astra, Södertälje, Sweden). Both filtration and UV irradiation of the virus reduced the extent of infection of human lung fibroblasts by >95%, confirming the effectiveness of these treatments. In some experiments supernatants from HCMV-infected and uninfected SMC cultures were collected at 4, 12, 24, and 72 h postinfection (hpi); cleared by centrifugation; and stored at −70°C until use. These supernatants were either filtered to remove virus particles, diluted 1:3, and used for infection or mock infection of SMC cultures or used in enzyme-linked immunosorbent assays for PDGF-AB and PDGF-BB (R&D Systems, Minneapolis, MN) according to the manufacturer's protocol.
As shown immunocytochemically, foscarnet inhibited the expression of the late HCMV proteins gB and SL20 by human lung fibroblasts infected with HCMV by >95% as well. Cell-free stocks of virus were prepared by centrifugation of the culture medium from infected fibroblasts and subsequently frozen and stored at −70°C until use. Virus titers were determined by plaque assays, as described previously (33).
Flow cytometric analyses.
A fluorescence-activated cell sorter (FACSort; Becton Dickinson, San Jose, CA) was employed to analyze the expression of PDGFR-α and -β, as well as for the expression of other cell markers by uninfected and HCMV-infected SMCs. For this purpose, the cells were harvested and incubated with antibodies recognizing PDGFR-α and -β (purified mouse monoclonal immunoglobulin G1 [IgG1] [R&D Systems, Minneapolis, MN]) and the cell surface markers CD13 and intercellular adhesion molecule (ICAM), as well as with control IgG1-isotype antibodies (Dakopatts, Glostrup, Denmark), followed by staining with the appropriate fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (Dakopatts). The levels of expression of these different surface antigens were determined as the mean channel fluorescence corrected for the control value. The histograms of the mean channel values for uninfected and HCMV-infected cells were compared on a linear scale, and a difference encompassing more than 10 channels was considered to reflect a change.
Monitoring of apoptosis.
Seven days after infection, HCMV-infected and mock-infected HPASMC were analyzed for apoptotic markers utilizing the TACS annexin V-FITC apoptosis detection kit (R&D Systems, Minneapolis, MN) and flow cytometric analysis in accordance with the protocol supplied by the manufacturer. As a positive control, apoptosis was induced in HPASMC with 1 mM NaN3 and 500 μM H2O2 in Krebs-Ringer phosphate buffer for 1.5 h at room temperature prior to the experiment.
Western blotting.
Cells were cultured in 75-cm2 cell culture flasks and subsequently exposed to HCMV at an MOI of 1.0 as described above. For PDGFR phosphorylation experiments uninfected and HCMV-infected SMCs were stimulated with 100 ng/ml of human recombinant PDGF-ββ (R&D Systems, Minneapolis, MN). Cells were disrupted in an immunoprecipitation lysis buffer, the protein concentration was determined by the bicinchoninic acid procedure, and samples were subjected to Western blotting employing antibodies directed against PDGFR-α or -β (both rabbit polyclonal IgG [Santa Cruz Biotechnology] or both mouse monoclonal antibodies [R&D Systems, Minneapolis, MN]) or mouse monoclonal anti-actin-β (Abcam, Cambridge, United Kingdom). For detection of phosphorylated PDGFR-α and -β samples were subjected to affinity-purified rabbit anti-phospho-PDGFR-α (Y762) or -β (Y751) (R&D Systems, Minneapolis, MN). For this latter purpose, a volume of sample containing 10 μg protein was loaded onto 4 to 20% precast Tris-glycine gels (PAGEr Gold precast gels; Cambrex Bio Science Rockland, Rockland, ME) and thereafter transferred to a polyvinylidene difluoride transfer membrane (Amersham Bioscience, Buckinghamshire, United Kingdom). Polyvinylidene difluoride transfer membranes were incubated together with diluted primary antibody overnight at 4°C. Protein bands were detected by the electrogenerated chemiluminescence method followed by development using high-performance chemiluminescence film (Amersham Pharmacia Biotech UK Limited) or by the diaminobenzidine peroxidase substrate kit (Vector Laboratories, Burlingame, CA) after a 2-h incubation of the blots with the secondary antibody, donkey anti-rabbit Ig linked with horseradish peroxidase (Amersham Life Science) or anti-mouse IgG horseradish peroxidase-linked whole antibody from sheep (Amersham Bioscience, Buckinghamshire, United Kingdom). In the case of quantification of Western blot bands, by using the software ImageJ, version 1.37, background intensity was subtracted for each band respectively, and the intensity was normalized to the corresponding β-actin loading control and presented as the relative level of intensity.
Real-time PCR.
Total cellular RNA was isolated from HPASMC or HCASMC. Cell pellets were dissociated in buffer-RLT (QIAGEN, Chatsworth, CA) containing β-mercaptoethanol and stored at −80°C prior to isolation of total cellular RNA with the RNeasy minikit (QIAGEN) in accordance with the manufacturer's instructions. The resulting RNA samples were analyzed with the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), utilizing the 6000 Nano LabChip kit and the Ambion RNA ladder. Reverse transcription-PCR was performed with the SuperScript First-Strand Synthesis System for reverse transcription-PCR with oligo(dT)20 primers (Invitrogen Life Technologies, Stockholm, Sweden), with a starting amount of 500 ng RNA, according to the manufacturer's protocol. The primers and probes for PDGFR-α and -β and for β-2 microglobulin were Assay-on-Demand products obtained commercially from Applied Biosystems, Foster City, CA (PDGFR-α, catalog no. Hs00183486_ml; PDGFR-β, Hs00182163_ml; β-2 microglobulin, Hs00187842_ml). The IEA was manufactured by Custom Taqman Gene Expression Assay Service (forward, TGACGAGGGCCCTTCCT; reporter, 6-carboxyfluorescein-AAGGTGCCACGGCCCG-5′; reverse, CCTTGGTCACGGGTGTCT). TaqMan semiquantitative PCR was performed according to the manufacturer's protocol using the ABI Prism 7700 Sequence Detection system (Perkin-Elmer Applied Biosystems). Differences in the quantity of cDNA loaded were compensated for by expressing the results relative to β-2 microglobulin cDNA employing the comparative threshold cycle method (Applied Biosystems).
Immunocytochemical staining and confocal microscopy of cells.
In the case of quantification of percentages of HCMV-infected SMCs at various MOIs, SMCs were cultured in 96-well plates in the presence of complete SmGm2 medium. When they had reached 80% confluence, the cells were infected with HCMV (AD169, Towne, or HA) at various MOIs (0.1 to 10) and fixed at 24 hpi with ice-cold methanol-acetone (1:1) for 10 min at room temperature. In the case of confocal microscopy HPASMC were cultured on eight-well sterile chamber glass slides in the presence of complete SmGm2 medium. When they had reached 80% confluence, the cells were infected with the HCMV laboratory strain AD169 at an MOI of 1. Cells were washed in phosphate-buffered saline (PBS) and then fixed at 1, 3, and 7 days postinfection (dpi) with ice-cold methanol-acetone (1:1) for 10 min at room temperature followed by three washes in PBS and later on incubated in protein block (Dakopatts) for 5 min. For detection of HCMV infection, the cells were incubated together with antibodies to the IEA or a late antigen (SL20) (both procured from Argene, Biosoft, Parc Technologique Delta Sud, Varilhes, France) or anti-HCMV gB (monoclonal antibody, produced and kindly provided by William B. Britt, University of Alabama at Birmingham, Birmingham, AL), followed by incubation with a secondary antibody conjugated with FITC (Dakopatts), and then examined by fluorescence microscopy.
In the case of confocal microscopy, the cells were exposed to primary antibodies specific for PDGFR-β (purified mouse monoclonal IgG1) (R&D Systems), Rab4 (rabbit polyclonal IgG), Rab5 (rabbit polyclonal IgG), Lamp1 (goat polyclonal IgG), and Lamp2 (goat polyclonal IgG) (all obtained from Santa Cruz Biotechnology, Santa Cruz, CA); histone deacetylase 1 Cy5 (rabbit polyclonal; Abcam); or rabbit polyclonal antibody to CMV (Abcam, Cambridge, United Kingdom), all at a dilution of 1/100 in PBS, for 60 min at 4°C followed by 10 min at room temperature. After three washes with PBS the cells were incubated with the appropriate secondary antibodies conjugated with FITC or Cy5, i.e., rabbit anti-mouse antibodies (FITC; Dakopatts) or goat anti-mouse IgG (Alexa Fluor 488), goat anti-rabbit IgG (Texas Red), goat anti-rabbit IgG (FITC), donkey anti-goat IgG (Cy5), donkey anti-rabbit IgG (Cy5), and donkey anti-mouse IgG (FITC) (all purchased from Jackson ImmunoResearch Laboratories, West Grove, PA, and all at a dilution of 1/100), together with 4′,6′-diamidino-2-phenylindole (DAPI) (diluted 1/100 in PBS) for 30 min at 4°C and then at room temperature for 5 min.
Fluorescence microscopy analysis was performed with a Leica DMRXA microscope (Leica Microsystems GmbH, Wetzlar, Germany) equipped with a cooled charge-coupled device camera (model S/N 370 KL 0565; Cooke Corporation, NY). Sets of filters for DAPI/Hoechst stain, FITC, Cy3, and Cy5 were obtained from Chroma Technology (Brattleboro, VT). The images were acquired using a 40× or 60× objective and further analyzed using the image processing software SlideBook 2.1.5 (Intelligent Imaging Innovations Inc., Denver, CO) and then postprocessed and mounted in Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA) or analyzed with a Leica TCS SP5 microscope. Images were acquired using a 63× objective and Leica Application Suite Advanced Fluorescence software.
RESULTS
Infection of SMCs by HCMV leads to downregulation of their expression of PDGFR-α and -β.
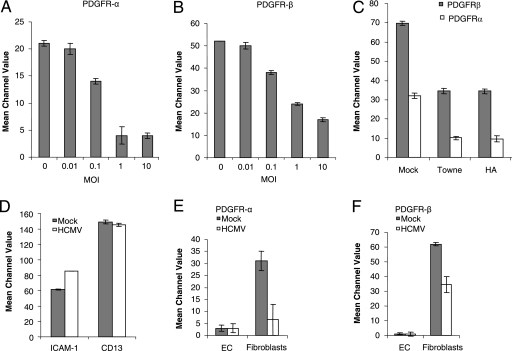
As demonstrated by fluorescence-activated cell sorting (FACS) analysis, the level of expression of PDGFR-α and -β on the surface of HPASMC was reduced by HCMV in a dose-dependent fashion at 3 dpi (Fig. 1A and B). At an MOI of 1, the three HCMV strains tested (i.e., AD169, Towne, and HA) all downregulated this expression to similar extents (Fig. 1C). We infected SMCs at three different passages (p10, p11, and p14) with different HCMV strains (AD169, Towne, and the clinical isolate HA) at MOIs of 0.1, 1, and 5 and stained these cells for IE proteins 24 hpi. At an MOI of 0.1, 30%, 45%, and 70% of the cells were infected using AD169, Towne, and HA, respectively. At an MOI of 1, 80%, >90%, and >90% of the cells were positive for IE proteins in AD169-, Towne-, and HA-infected cells, respectively. At an MOI of 5, >90% of the cells stained positive for IE proteins with all strains, AD169, Towne, and HA (data not shown). To exclude the possibility that the attenuated expression of PDGFR simply reflects a general shutdown of protein synthesis in HCMV-infected cells, we examined the expression of two other proteins, ICAM-1 and CD13, as well. Infection of HPASMC with HCMV was associated with upregulation of ICAM-1 expression (as previously reported [23]) but did not alter CD13 expression (Fig. 1D).
FIG. 1.
Infection with HCMV results in downregulation of the expression of PDGFR-α and -β on the surface of SMCs. (A and B) The dose-dependent effects of infection with AD169 (at an MOI of 0.01 to 10) on SMC expression of PDGFR-α (A) and -β (B) were determined by flow cytometric analysis. (C) Effects of infection with the Towne or clinical HA strain of HCMV at an MOI of 1 on PDGFR expression by SMCs. (D) Expression of the ICAM-1 and CD13 proteins in uninfected and HCMV (AD169)-infected SMCs, as determined by flow cytometric analysis. (E and F) Expression of PDGFR-α (E) and -β (F) on the surface of uninfected and HCMV-infected endothelial cells (EC) and fibroblasts 3 dpi as determined by flow cytometric analysis. The values presented are mean channel values ± standard errors of the means, obtained in at least three independent experiments.
Since PDGFR-α and -β are expressed by a variety of cells, we examined the influence of infection by HCMV on this expression by fibroblasts and endothelial cells as well. Fibroblasts were found to express PDGFR-α and -β at levels similar to those present on SMCs, and this expression was reduced by HCMV in a similar manner at 3 dpi (Fig. 1E and F). As expected, PDGFRs were not detected on endothelial cells and infection with HCMV did not elicit their expression (Fig. 1E and F).
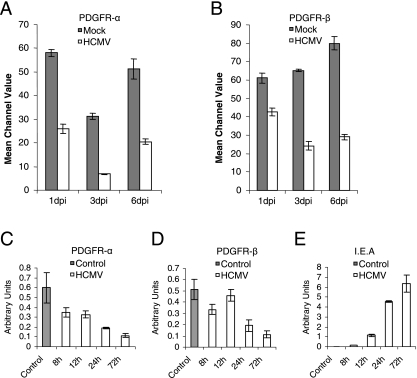
Next, we assessed the effect of viral infection on the expression of PDGFR-α and -β on the surface of SMCs at different time points following infection. FACS analysis revealed that expression of PDGFR-α was downregulated by 55%, 77%, and 61% at 1, 3, and 6 dpi, respectively, while the corresponding reductions in PDGFR-β were 30%, 63%, and 64%, respectively (Fig. 2A and B). Further analysis of PDGFR-α and -β and IEA mRNA expression 0, 8, 12, 24, and 72 hpi showed a downregulation of PDGFR-α and -β mRNA expression as early as 8 hpi (Fig. 2C and D). At 72 h the mRNA expression of PDGFR-α and -β had decreased by approximately 80% (Fig. 2C and D), and IEA mRNA levels were high at the same time point (Fig. 2E).
FIG. 2.
Time dependency of the reduction in PDGFR-α and -β expression in HCMV-infected SMCs. The expression of PDGFR-α (A) and PDGFR-β (B) on uninfected and HCMV-infected SMCs was analyzed at 1, 3, and 6 dpi by flow cytometry. PDGFR-α (C), PDGFR-β (D), and IEA (E) mRNA expression levels were analyzed at 0, 8, 12, 24, and 72 hpi. The values presented are mean channel values (A and B) or arbitrary units (C to E) ± standard errors of the means (n = 3).
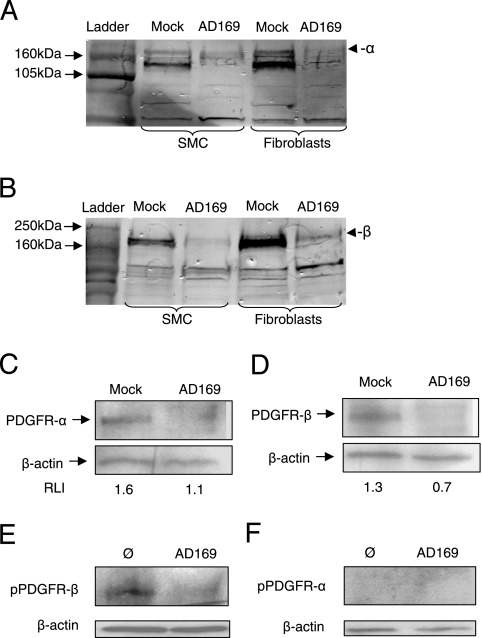
In order to determine if the total or only the surface levels of PDGFR-α and -β were reduced in HCMV-infected cells, total protein extracted from these cells was subjected to Western blotting for these proteins with both monoclonal and polyclonal antibodies. HCMV reduced the total levels of PDGFR-α and -β proteins in SMCs and fibroblasts, as demonstrated with polyclonal antibodies (Fig. 3A and B) as well as monoclonal antibodies (Fig. 3C and D). To assess if the functionality of PDGFR-α and -β was affected upon HCMV infection, experiments were performed in which uninfected and HCMV-infected SMCs were stimulated with recombinant human PDGF-ββ for 7 min and thereafter analyzed by Western blotting, for detection of phosphorylated PDGFR-β or PDGFR-α. The phosphorylation of PDGFR-β (tyrosine residue Y751) was lower in the infected SMCs than in the uninfected cells at 3 dpi (Fig. 3E), but phosphorylation was not detected at the PDGFR-α tyrosine residue Y762 in uninfected or infected cells after PDGF-BB stimulation at 3 dpi (Fig. 3F).
FIG. 3.
HCMV reduces the total levels of expression of PDGFR-α and -β by SMCs and fibroblasts. (A to D) Following infection with HCMV at an MOI of 1, total cellular protein was extracted and Western blotting was performed at 3 dpi with polyclonal antibodies directed against PDGFR-α (A) or PDGFR-β (B) or with monoclonal antibodies directed against PDGFR-α (C) or PDGFR-β (D) as well as with an antibody directed against β-actin as a loading control (C and D), and results were quantified by using the software ImageJ version 1.37. Data in panels C and D are presented as relative levels of intensity (RLI). (E and F) Western blotting results of uninfected (Ø) and HCMV-infected (AD169) SMCs (3 dpi) after 7 min of stimulation with recombinant human PDGF-ββ, for detection of phosphorylated PDGFR-β (Y751) (E) or phosphorylated PDGFR-α (Y762) (F).
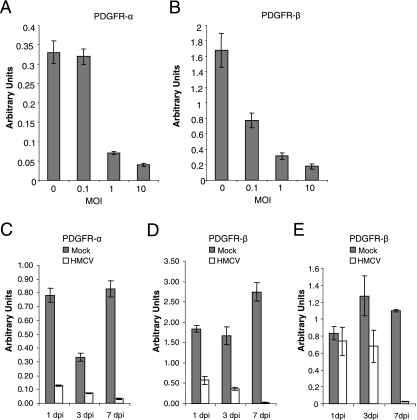
Furthermore, analysis of total cellular mRNA by real-time PCR demonstrated that infection of HPASMC by HCMV was associated with decreases in the levels of PDGFR-α and -β mRNA at early time points after infection and in a dose-dependent manner (Fig. 4A and B). Thus, the level of PDGFR-α mRNA was reduced by 84%, 78%, and 96% at 1, 3, and 7 dpi, respectively, after infection by HCMV, while the corresponding values for PDGFR-β mRNA were 69%, 78%, and 99%, respectively (Fig. 4C and D). Similarly, infection of HCASMC with the endothelial cell-adapted clinical isolate VR1814 decreased the level of PDGFR-β mRNA by 12%, 46%, and 98% at these same time points, respectively (Fig. 4E).
FIG. 4.
HCMV apparently inhibits transcription of the genes encoding PDGFR-α and -β in SMCs. PDGFR-α and PDGFR-β mRNA levels were determined by analysis of total cellular mRNA by real-time PCR, and the data are presented as means ± standard errors of the means in arbitrary units (n = 6 to 12). (A and B) Dose-dependent effects of HCMV (AD169; MOI, 0.1 to 10) infection for PDGFR-α mRNA (A) and PDGFR-β mRNA (B) levels (n = 6). (C and D) Time dependency of the effects of infection with this same strain of HCMV for the level of PDGFR-α mRNA (C) and PDGFR-β mRNA (D) in uninfected or infected HPASMC. (E) Time dependency of the effects of HCASMC infected by the endothelial cell-adapted clinical isolate VR1814 on PDGFR-β mRNA levels.
Downregulation of the expression of PDGFR-α and -β in HCMV-infected SMCs requires IE or early gene expression.
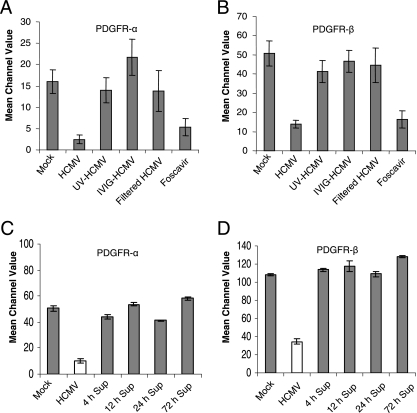
To exclude the possibility that the effect of HCMV on PDGFR-α and -β expression by SMCs was mediated by soluble factors present in the viral inoculums, we cleared viral preparations from virus particles by filtration. Virus-free supernatants from HCMV-infected fibroblasts did not affect PDGFR expression by SMCs. Thus, the downregulation was mediated by the virus itself, rather than by soluble factors released from the fibroblast during viral infection. Moreover, pretreatment of the virus with intravenous Ig (IVIG) completely blocked its ability to downregulate the expression of PDGFR, suggesting that early events associated with virus binding or fusion are necessary to obtain this effect.
In order to examine further whether active replication of HCMV was required for inhibition of PDGFR expression, SMCs were challenged with UV-inactivated nonreplicative HCMV. UV-inactivated virus did not affect PDGFR expression as demonstrated by FACS analysis (Fig. 5A and B). Furthermore, the drug foscarnet, which inhibits the expression of late HCMV genes, did not prevent the reduction in PDGFR-α and -β, indicating that IE and/or early viral gene products are responsible for this phenomenon (Fig. 5A and B). To rule out the possibility that the downregulation of PDGFR expression was dependent on an SMC-inducible factor upon HCMV infection of SMCs, experiments were also performed with supernatants from HCMV-infected SMCs collected at different time points after infection (4 h, 12 h, 24 h, and 72 h). The supernatants from SMCs infected with HCMV were cleared from viral particles by filtration, and these virus-free supernatants from HCMV-infected SMCs did not affect PDGFR expression in SMCs at 3 dpi as shown by FACS analysis (Fig. 5C and D). We further analyzed supernatants from SMCs at 24 hpi with enzyme-linked immunosorbent assay for PDGF-AB or PDGF-BB. There were no different levels of PDGF-AB and -BB in uninfected and HCMV-infected SMC supernatants (data not shown).
FIG. 5.
Downregulation of PDGFR expression in HCMV (AD169)-infected SMCs is dependent on the expression of IE and/or E viral genes. (A and B) Expression of PDGFR-α (A) and -β (B) following infection of SMCs with HCMV, HCMV inactivated by UV irradiation (UV-HCMV), infection of SMCs pretreated with IVIG (IVIG-HCMV), and virus-cleared supernatants (filtered HCMV) and in HCMV-infected SMCs treated with the anti-HCMV drug foscarnet as determined by flow cytometric analysis at 3 dpi. The values presented are mean channel values ± standard errors of the means (n = 7 to 10). (C and D) Expression of PDGFR-α (C) and -β (D) following infection of SMCs by virus-cleared supernatants collected at 4 h, 12 h, 24 h, and 72 h as determined by flow cytometric analysis at 3 dpi. Values presented are mean channel values ± standard errors of the means (n = 3).
PDGFR-β accumulates in late endosomes/lysosomes in HCMV-infected SMCs.
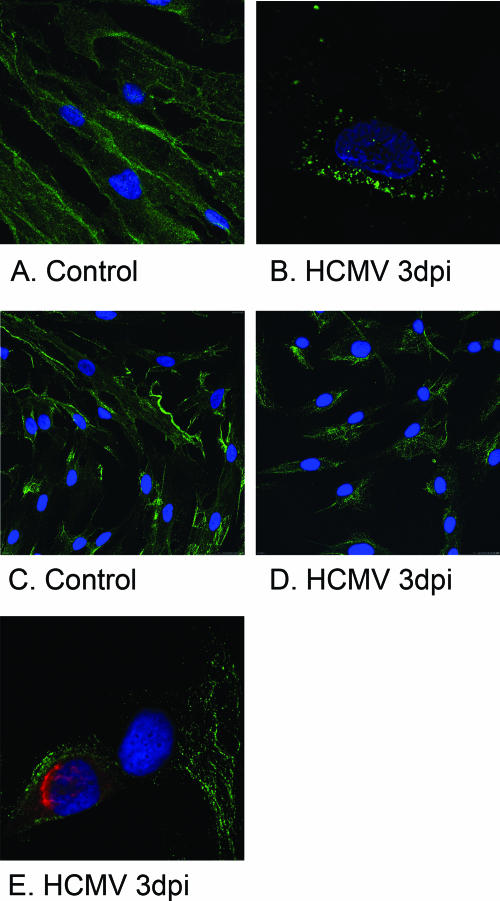
When confocal microscopy was employed to examine the intracellular distribution of PDGFR-β in uninfected and HCMV-infected SMCs, an interesting difference in PDGFR-β localization was observed 1 dpi, and this difference became even more pronounced at later time points. Whereas PDGFR-β was evenly distributed along the plasma membrane in uninfected cells, in HCMV-infected cells this protein was located in vacuoles close to the plasma membrane (Fig. 6). Under these conditions, HCMV infection did not affect cell viability, since more than 90% of the infected cells were nonapoptotic or necrotic, as demonstrated by FACS analysis (data not shown).
FIG. 6.
The pattern of PDGFR-β expression is altered in HCMV-infected SMCs. (A and B) Confocal microscopy images of uninfected and HCMV-infected SMCs acquired at 3 dpi using the exact same gain settings for uninfected and infected cells demonstrated that PDGFR-β was evenly distributed along the plasma membrane in uninfected cells (A), whereas in HCMV-infected cells (B) this protein was located in vacuoles situated in proximity to the plasma membrane. Magnifications, ×40 (A) and ×60 (B). (C and D) Confocal microscopy images are shown of uninfected (C) and HCMV-infected (MOI, 1) (D) SMCs at 3 dpi (63× oil-immersion objective plus 1.7× zoom). (E) An uninfected cell and an infected cell (63× oil-immersion objective plus 2.2× zoom).
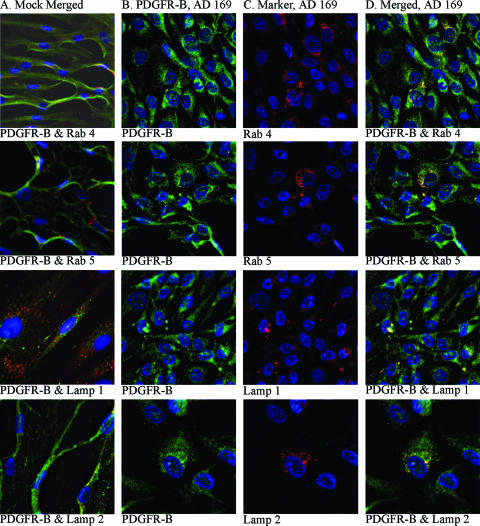
In order to identify the intracellular localization of PDGFR-β in HCMV-infected SMCs in more detail, the possible colocalization of this protein with markers for early endosomes (Rab4), late endosomes (Rab 5), late endosomes/early lysosomes (Lamp1), and late endosomes (Lamp2) was examined utilizing specific antibodies. PDGFR-β was found to be colocalized with Rab4 and Rab5, specific for endosomes that play key roles in the secretory and endocytic pathways (22). We also observed colocalization with Lamp1 and Lamp2, suggesting that in HCMV-infected cells PDGFR-β enters intracellular pathways for protein degradation (Fig. 7). However, PDGFR-β was not associated with histone deacetylase 1, a chromatin-remodeling factor (data not shown).
FIG. 7.
Confocal microscopy analyses of the intracellular localization of PDGFR-β in HCMV-infected SMCs. (A) Uninfected SMCs stained for PDGFR-β (FITC) together with one of the following markers: Rab4 (early endosomes), Rab5 (late endosomes), Lamp1 (early lysosomes/late endosomes), and Lamp2 (late lysosomes). (B) HCMV-infected SMCs stained for PDGFR-β. (C) The same cells stained for Rab4, Rab5, Lamp1, and Lamp2. (D) Overlay of the images presented in columns B and C. For all confocal microscopy images, magnifications were ×40 or ×60, and the gain settings were optimized for each marker for colocalization analyses.
DISCUSSION
In the present investigation, we demonstrate that infection of SMCs by HCMV downregulates the expression of PDGFR-α and -β on these cells. In connection with such infection the levels of PDGFR-α and -β on the surface and in the total protein of SMCs were reduced in parallel with attenuated mRNA levels, as shown by FACS analysis, Western blotting, and real-time PCR, respectively. This reduced expression of PDGFRs did not reflect a general downregulation of cell surface receptors since the expression of CD13 was unaffected and that of ICAM-1 was upregulated in the HCMV-infected cells. These findings may have important implications with respect to the influence of congenital HCMV infection on fetal development.
PDGFs have been reported to participate in the proliferation, migration, differentiation, and various other processes in a variety of specialized mesenchymal cells, glial progenitor cells, and migratory cells in connection with developmental processes involved in the formation of the cardiovascular system, epithelial organs, the central nervous system, and the skeleton. The observation that mice lacking PDGFR-β die during the late period of gestation from cardiovascular complications, exhibiting abnormal kidney glomeruli, capillary microaneurysms, hypoplasia of arterial SMCs, cardiac muscle hypotrophy, placental defects, and widespread edema and hemorrhage (reviewed in reference 3) clearly demonstrates the important roles played by PDGF-B/PDGFR-β signaling in connection with fetal development. Mice lacking PDGFR-α also die in utero, and these mice exhibit incomplete cephalic closure and skeletal abnormalities (26).
PDGFs were identified initially as mitogenic growth factors but have subsequently been shown to act as survival factors as well: e.g., mice lacking PDGFR-α exhibit elevated rates of apoptosis along the pathways followed by migrating neural crest cells (26). Moreover, PDGFR-α is expressed throughout the mesenchyme of the developing mouse kidney (15) and is required for lung development including the formation of alveoli (4). HCMV is considered to be the congenital infection that most often leads to disruption of development of the central nervous system. We have recently found that HCMV inhibits the differentiation of neural precursor cells into neurons (19). Similar observations have been described for the animal model (13). Our present finding that infection of SMCs and fibroblasts by HCMV causes downregulation of both PDGFR-α and -β may have important mechanistic implications in this context. However, most likely the virus effect on SMC PDGFR expression will not have any major implications for the development of vascular diseases in adults.
In vitro studies by Zhou et al. (34) have previously shown enhanced migration in response to PDGF and upregulation of PDGFR-β expression by HCMV-infected SMCs. However, these experiments involved rat, rather than human, SMCs infected by HCMV. The differences between our study and theirs may be explained by different effects of HCMV in human and in rodent cells, since CMV is known to be a highly species-specific virus. However, in direct disagreement with our observations, Reinhardt et al. (21) recently reported that infection of HCASMC with HCMV leads to enhanced expression of PDGFR-β. At present, the explanation for this discrepancy is unknown; however, there are multiple differences between the experimental setup in the study by Reinhardt et al. and the setup in ours. First, we analyzed the effect of HCMV on both PDGFR-α and -β and used both monoclonal and polyclonal antibodies. Furthermore, in our study we also analyzed the transcription of PDGFR-α and -β, which upon HCMV infection results in a clear downregulation. We obtained highly consistent results in observing downregulation with multiple techniques. However, no obvious explanation for the differences exists at this point.
In summary, we observed here that infection of both human SMCs and fibroblasts by HCMV is followed by a general downregulation of PDGFR expression and that apparently the virus has an ability to affect both transcriptional and posttranslational events. Although there are several possibilities concerning why HCMV exerts such an effect, the implications for viral pathogenesis and developmental biology require further elucidation.
Acknowledgments
This work was supported financially by grants from the Heart-Lung Foundation (199941305, 200241138, 20030055, and 20050266), King Gustaf V's 80-Year Anniversary Foundation, The Swedish Research Council (K2004-16X-12615-07A), Konung Gustaf V's och Drottning Victorias Foundation, Groschinsky's Foundation, Pfizer, Swedish Cancer Foundation (060253), and Children's Cancer Foundation (05/100). C.S.-N. is a fellow of the Royal Swedish Academy of Science, Sweden.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Alford, C. A., S. Stagno, R. F. Pass, and W. J. Britt. 1990. Congenital and perinatal cytomegalovirus infections. Rev. Infect. Dis. 12(Suppl. 7):S745-S753. [DOI] [PubMed] [Google Scholar]
- 2.Bergsten, E., M. Uutela, X. Li, K. Pietras, A. Ostman, C. H. Heldin, K. Alitalo, and U. Eriksson. 2001. PDGF-D is a specific, protease-activated ligand for the PDGF beta-receptor. Nat. Cell Biol. 3:512-516. [DOI] [PubMed] [Google Scholar]
- 3.Betsholtz, C., L. Karlsson, and P. Lindahl. 2001. Developmental roles of platelet-derived growth factors. Bioessays 23:494-507. [DOI] [PubMed] [Google Scholar]
- 4.Bostrom, H., A. Gritli-Linde, and C. Betsholtz. 2002. PDGF-A/PDGF alpha-receptor signaling is required for lung growth and the formation of alveoli but not for early lung branching morphogenesis. Dev. Dyn. 223:155-162. [DOI] [PubMed] [Google Scholar]
- 5.Britt, W. A., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 6.Claesson-Welsh, L. 1994. Signal transduction by the PDGF receptors. Prog. Growth Factor Res. 5:37-54. [DOI] [PubMed] [Google Scholar]
- 7.Fish, K. N., C. Soderberg-Naucler, L. K. Mills, S. Stenglein, and J. A. Nelson. 1998. Human cytomegalovirus persistently infects aortic endothelial cells. J. Virol. 72:5661-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbertson, D. G., M. E. Duff, J. W. West, J. D. Kelly, P. O. Sheppard, P. D. Hofstrand, Z. Gao, K. Shoemaker, T. R. Bukowski, M. Moore, A. L. Feldhaus, J. M. Humes, T. E. Palmer, and C. E. Hart. 2001. Platelet-derived growth factor C (PDGF-C), a novel growth factor that binds to PDGF alpha and beta receptor. J. Biol. Chem. 276:27406-27414. [DOI] [PubMed] [Google Scholar]
- 9.Heldin, C. H., and B. Westermark. 1999. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 79:1283-1316. [DOI] [PubMed] [Google Scholar]
- 10.Hendrix, M. G., P. H. Dormans, P. Kitslaar, F. Bosman, and C. A. Bruggeman. 1989. The presence of cytomegalovirus nucleic acids in arterial walls of atherosclerotic and nonatherosclerotic patients. Am. J. Pathol. 134:1151-1157. [PMC free article] [PubMed] [Google Scholar]
- 11.Hendrix, M. G., M. M. Salimans, C. P. van Boven, and C. A. Bruggeman. 1990. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am. J. Pathol. 136:23-28. [PMC free article] [PubMed] [Google Scholar]
- 12.Hirsch, A. J., and T. Shenk. 1999. Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene. J. Virol. 73:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosugi, I., Y. Shinmura, H. Kawasaki, Y. Arai, R. Y. Li, S. Baba, and Y. Tsutsui. 2000. Cytomegalovirus infection of the central nervous system stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Investig. 80:1373-1383. [DOI] [PubMed] [Google Scholar]
- 14.Leveen, P., M. Pekny, S. Gebre-Medhin, B. Swolin, E. Larsson, and C. Betsholtz. 1994. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 8:1875-1887. [DOI] [PubMed] [Google Scholar]
- 15.Li, X., A. Ponten, K. Aase, L. Karlsson, A. Abramsson, M. Uutela, G. Backstrom, M. Hellstrom, H. Bostrom, H. Li, P. Soriano, C. Betsholtz, C. H. Heldin, K. Alitalo, A. Ostman, and U. Eriksson. 2000. PDGF-C is a new protease-activated ligand for the PDGF alpha-receptor. Nat. Cell Biol. 2:302-309. [DOI] [PubMed] [Google Scholar]
- 16.Melnick, J. L., E. Adam, and M. E. Debakey. 1993. Cytomegalovirus and atherosclerosis. Eur. Heart J. 14(Suppl. K):30-38. [PubMed] [Google Scholar]
- 17.Melnick, J. L., C. Hu, J. Burek, E. Adam, and M. E. DeBakey. 1994. Cytomegalovirus DNA in arterial walls of patients with atherosclerosis. J. Med. Virol. 42:170-174. [DOI] [PubMed] [Google Scholar]
- 18.Michelson, S., P. Dal Monte, D. Zipeto, B. Bodaghi, L. Laurent, E. Oberlin, F. Arenzana-Seisdedos, J. L. Virelizier, and M. P. Landini. 1997. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J. Virol. 71:6495-6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odeberg, J., N. Wolmer, S. Falci, M. Westgren, A. Seiger, and C. Soderberg-Naucler. 2006. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J. Virol. 80:8929-8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pampou, S., S. N. Gnedoy, V. B. Bystrevskaya, V. N. Smirnov, E. I. Chazov, J. L. Melnick, and M. E. DeBakey. 2000. Cytomegalovirus genome and the immediate-early antigen in cells of different layers of human aorta. Virchows Arch. 436:539-552. [DOI] [PubMed] [Google Scholar]
- 21.Reinhardt, J., G. B. Smith, C. T. Himmelheber, J. Azizkhan-Clifford, and E. S. Mocarski. 2005. The carboxyl-terminal region of human cytomegalovirus IE1491aa contains an acidic domain that plays a regulatory role and a chromatin-tethering domain that is dispensable during viral replication. J. Virol. 79:225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schimmoller, F., I. Simon, and S. R. Pfeffer. 1998. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273:22161-22164. [DOI] [PubMed] [Google Scholar]
- 23.Sedmak, D. D., D. A. Knight, N. C. Vook, and J. W. Waldman. 1994. Divergent patterns of ELAM-1, ICAM-1, and VCAM-1 expression on cytomegalovirus-infected endothelial cells. Transplantation 58:1379-1385. [PubMed] [Google Scholar]
- 24.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1998. Growth of human cytomegalovirus in primary macrophages. Methods 16:126-138. [DOI] [PubMed] [Google Scholar]
- 25.Soriano, P. 1994. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 8:1888-1896. [DOI] [PubMed] [Google Scholar]
- 26.Soriano, P. 1997. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development 124:2691-2700. [DOI] [PubMed] [Google Scholar]
- 27.Streblow, D. N., C. Kreklywich, Q. Yin, V. T. De La Melena, C. L. Corless, P. A. Smith, C. Brakebill, J. W. Cook, C. Vink, C. A. Bruggeman, J. A. Nelson, and S. L. Orloff. 2003. Cytomegalovirus-mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J. Virol. 77:2182-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 29.Taylor-Wiedeman, J., P. Sissons, and J. Sinclair. 1994. Induction of endogenous human cytomegalovirus gene expression after differentiation of monocytes from healthy carriers. J. Virol. 68:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thyberg, J., U. Hedin, M. Sjolund, L. Palmberg, and B. A. Bottger. 1990. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis 10:966-990. [DOI] [PubMed] [Google Scholar]
- 31.Tumilowicz, J. J., M. E. Gawlik, B. B. Powell, and J. J. Trentin. 1985. Replication of cytomegalovirus in human arterial smooth muscle cells. J. Virol. 56:839-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valantine, H. A. 1999. Role of CMV in transplant coronary artery disease and survival after heart transplantation. Transplant. Infect. Dis. 1(Suppl. 1):25-30. [PubMed] [Google Scholar]
- 33.Wentworth, B. B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253-258. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, Y. F., Z. X. Yu, C. Wanishsawad, M. Shou, and S. E. Epstein. 1999. The immediate early gene products of human cytomegalovirus increase vascular smooth muscle cell migration, proliferation, and expression of PDGF beta-receptor. Biochem. Biophys. Res. Commun. 256:608-613. [DOI] [PubMed] [Google Scholar]