Abstract
Reverse transcriptase (RT) remains a primary target in therapies directed at human immunodeficiency virus type 1 (HIV-1). RNA aptamers that bind RT from HIV-1 subtype B have been shown to protect human cells from infection and to reduce viral infectivity, but little is known about the sensitivity of the inhibition to amino sequence variations of the RT target. Therefore, we assembled a panel of 10 recombinant RTs from phylogenetically diverse lentiviral isolates (including strains of HIV-1, simian immunodeficiency virus SIVcpz, and HIV-2). After validating the panel by measuring enzymatic activities and inhibition by small-molecule drugs, dose-response curves for each enzyme were established for four pseudoknot RNA aptamers representing two structural subfamilies. All four aptamers potently inhibited RTs from multiple HIV-1 subtypes. For aptamers carrying family 1 pseudoknots, natural resistance was essentially all-or-none and correlated with the identity of the amino acid at position 277. In contrast, natural resistance to aptamers carrying the family 2 pseudoknots was much more heterogeneous, both in degree (gradation of 50% inhibitory concentrations) and in distribution across clades. Site-directed and subunit-specific mutagenesis identified a common R/K polymorphism within the p66 subunit as a primary determinant of resistance to family 1, but not family 2, pseudoknot aptamers. RNA structural diversity therefore translates into a nonoverlapping spectrum of mutations that confer resistance, likely due to differences in atomic-level contacts with RT.
The reverse transcriptases (RTs) of the human and simian immunodeficiency viruses (HIVs and SIVs, respectively) are encoded by the viral pol gene and are expressed from viral mRNA as part of the multifunctional Gag-Pol polyprotein. Mature RT is the product of proteolytic processing of the polyprotein, first into an asymmetric homodimer and then into the mature heterodimer (21). Due to its central role in HIV type 1 (HIV-1) replication and the early clinical availability of anti-RT compounds, RT has long been an established therapeutic target. Of the 22 anti-HIV compounds currently approved by the U.S. Food and Drug Administration, 15 target the viral RT (60). The nucleoside analogue RT inhibitors (NRTIs) are deoxynucleoside triphosphate analogues that result in chain termination when incorporated by RT into a growing DNA strand. The nonnucleoside RT inhibitors (NNRTIs) bind RT near the active site and disrupt enzymatic activity by allosteric inhibition (38, 59). The search for new anti-RT compounds is fueled by cytotoxicity and clinically selected resistance—resulting from drug exclusion or from postincorporation excision (45)—which are persistent problems for both classes of RT inhibitors.
Nucleic acid aptamers, ribozymes, antisense RNA, and small interfering RNA exhibit potent antiviral effects and are under development as potential gene therapy adjuvants to small-molecule therapeutics (25, 34), and several of these have recently entered into clinical trials (1, 39, 42). Biochemical and crystallographic analyses of RNA aptamers to HIV-1 RT established that they bind in the same cleft normally occupied by the primer-template, where they competitively inhibit primer-template binding (26, 31, 66); hence, they are sometimes denoted “primer-template analog RT inhibitors” (16). Many of the RNA aptamers are capable of forming bent pseudoknot structures (5, 62), which are divided into two families (5). The family 1 pseudoknots conform closely to a well-defined sequence definition derived from analysis of the canonical T1.1 aptamer (5, 22), while the family 2 pseudoknots are defined by not conforming to that sequence definition. The most potent of the RNA aptamers from both families inhibit both DNA polymerase and RNase H activities in vitro, with IC50 values (concentration of inhibitor at which half-maximal inhibition is observed) in the low nanomolar to high picomolar range (5, 26, 37, 62). Importantly, when these RNA aptamers were expressed in cultured lymphocytes, they suppressed the replication of multiple viral isolates by 1 to 2 orders of magnitude (6, 33, 35), and at high multiplicities of infection they offered more antiviral protection than did expressed small interfering RNA (35).
The M group (main) of HIV-1 strains accounts for the vast majority of worldwide infections; the O group (outlier) is of some epidemiological relevance in Western Africa, and the N group (non-M, non-O) accounts for only a few known human infections. RT amino acid sequence identities are roughly 78 to 85% between HIV-1 groups and 87 to 97% between subtypes within the HIV-1 M group. RT sequence diversity among the eight HIV-2 groups (10) is comparable to that among the three HIV-1 groups; however, there is only ∼60% identity in amino acid sequence between RT proteins of HIV-1 group M and HIV-2 group A. Both HIV-1 and HIV-2 entered human populations through multiple cross-species transmissions by distinct groups of SIVs (18, 36, 57, 64). The 81% amino acid identity between RTs from HIV-1 group M and SIVcpzP.t.t. (Pan troglodytes troglodytes) and the 89% amino acid identity between RTs from HIV-2 group A and sooty mangabey SIV illustrate the more proximal relationships between the two HIV types and the SIV taxa from which they originated. The HIV-1 enzymes are, in general, slightly more active than their HIV-2 counterparts (14, 27, 49, 56), which has been proposed to contribute to the greater virulence of the HIV-1 strains than of the HIV-2 strains (19, 44). Many of the same factors that drive rapid viral evolution and diversification of the M group (7, 8, 17, 40, 43) have also contributed to the rapid appearance of drug resistance mutations in the RT and protease (PR) genes.
Although most HIV-1 isolates from drug-naïve patients are equivalently sensitive to NRTI and NNRTI therapies, their propensities for developing new drug resistance are nonequivalent due to polymorphisms and silent mutations within RT. Subtype C isolates are highly polymorphic at positions associated with NNRTI resistance, such as V106 and A98, in comparison to the variation found in subtype B isolates (23, 41), and exposure of subtype C strains to efavirenz rapidly selected multidrug NNRTI resistance following a V106M mutation not previously observed for subtype B strains (4, 41, 47). In cell culture models comparing the selection of drug-resistant subtype B and C viruses, two resistance mutations in subtype C RT (S98I and G190A) were reported that had not been observed in RT from nevirapine (NVP)-challenged subtype B virus (41). In other tissue culture experiments, the K65R tenofovir resistance mutation appeared in only 12 weeks among subtype C viruses, while the K65R mutation was not observed among subtype B viruses in those studies, even after 78 weeks of passaging (4). The same mutation is found more frequently among highly active antiretroviral therapy patients infected with subtype C virus than among those infected with subtype A or B virus (24). Many clinical group O isolates carry a Y181C mutation which confers complete resistance to NNRTI drugs (11), and HIV-2 isolates are naturally resistant to NNRTIs due to the presence of a Y181I mutation (29, 52, 53, 65). Finally, zidovudine (AZT) resistance mutations in HIV-2 (e.g., Q151M) augment a preexisting ability to exclude the drug from the active site, while AZT resistance mutations in HIV-1 enhance unblocking of the chain-terminated strand via excision of AZT monophosphate (3, 30).
The examples above highlight several ways in which natural variation influences the viral predisposition towards resistance to current therapies. The existing collections of anti-HIV aptamers were all selected to recognize proteins from subtype B viruses. It is therefore important to define how aptamer inhibition is affected by RT amino acid sequence variation. The present work describes a panel of recombinant RTs from 10 HIV-1, HIV-2, and SIVcpz isolates. The recombinant RT panel was validated through measurements of intrinsic enzymatic activities and their sensitivities to inhibition by two NRTI and two NNRTI compounds. Each member of the panel was then challenged with four previously described pseudoknot RNA aptamers, which showed distinct inhibition profiles according to their structural subfamilies. Aptamers with family 1 pseudoknots strongly inhibited the RTs from HIV-1 group M subtypes B and C and demonstrated significant potency against HIV-2 RT, but they were ineffective against the remaining seven RTs, correlating with the identity of the amino acid at position 277. In contrast, aptamers with family 2 pseudoknots were strongly inhibitory against the same three RTs and also inhibited four of the remaining RTs, with a spectrum of weakening potency in loose accordance with phylogenetic distance from the original aptamer selection target RT (HIV-1 M subtype B). Site-directed mutagenesis of each subunit revealed a single amino acid as a potent determinant of resistance to the aptamers with family 1 pseudoknots. The same position had little effect on aptamers with family 2 pseudoknots. We interpret these results in terms of differential molecular interactions between the RTs and the aptamers.
MATERIALS AND METHODS
Materials.
Cy3 fluorophore-labeled and unlabeled DNA and RNA oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). AZT triphosphate (AZTTP) was purchased from Calbiochem (San Diego, CA). Zalcitabine triphosphate (ddCTP) was purchased from Takara (Japan). NVP and efavirenz (EFV) were purchased from Toronto Research Chemicals, Inc. (Toronto, Canada). RNA aptamers 70.5, 70.8, and 80.55 were previously identified by in vitro selection from random pools (5), and their complete sequences are presented elsewhere (26). The RNA sequence of aptamer T1.1 (62) used here is identical to that of the variant used previously for cocrystallization with HIV-1 M/B RT (31).
Cloning and phylogenetic analysis of RT-encoding HIV and SIV pol gene fragments.
The RT-encoding pol gene fragments within HIV and SIV viral DNA or RNA templates (Table 1) were amplified by PCR or RT-PCR, using the corresponding primers for separate cloning of the large and small subunits into the pET-200/D-TOPO expression vector (Invitrogen, Carlsbad, CA). Viral RNA isolation was performed using a QIAamp Viral RNA Mini kit (QIAGEN) according to the manufacturer's instructions. The source molecular clone for EHO-287 was missing the 3′-terminal 107 nucleotides (nt) of the RT-encoding pol gene fragment. Therefore, this portion was reconstructed from overlapping synthetic oligonucleotides based on the GenBank reference sequence, using Vent DNA polymerase (New England Biolabs) for high-fidelity sequence replication.
TABLE 1.
HIV and SIV source material for recombinant RTs
| Virus and group/clade | Isolate (GenBank accession no.; ARRRP catalog no.)a | Source material | Source contributor(s) | Reference(s) | Differences from GenBank reference sequence |
|---|---|---|---|---|---|
| HIV-1 | |||||
| M:B | HXB2 (K03455; 1067) | pHXB2gpt fragment | Kathleen Page and Dan Littman | 15, 57, 67 | None |
| M:A/D | 92UG021 (AF009396; 1648) | Cell-free virus | The UNAIDS Network for HIV Isolation and Characterization and the DAIDS, NIAID | 8 | D121C, E169D, V293Ib |
| M:C | 98CN009 (AF286230; 4165) | Cell-free virus | The UNAIDS Network for HIV Isolation and Characterization and the DAIDS, NIAID | 73 | R102K, S554Tc |
| M:A | 94CY017.41 (AF286237; 6175) | Near-full-length molecular clone | Stanley A. Trask, Feng Gao, Beatrice H. Hahn, and the Aaron Diamond AIDS Research Center | 25 | S554Tc |
| M:A/E | 93TH253.3 (U51189; 3283) | Full-length molecular clone | Feng Gao and Beatrice H. Hahn | 24 | D6E,c I553S,c S554Ac |
| M:G | RU570 (DQ234256; 3508) | Cell-free virus | Aleksei Bobkov and Jonathon Weber | 2 | See noteb |
| O | MVP5180 (L20571; 2878) | Cell-free virus | Lutz Gürtler | 31 | M60V, I184M, V261I, V435I |
| SIVcpzp.t.t. | US (AF103818) | Full-length molecular clone | Beatrice H. Hahn | 22 | Y208H, E223K, H315R, R345Q, L392P, G498D, V518A |
| SIVcpzp.t.s. | TAN1B (AF447763) | Full-length molecular clone | Beatrice H. Hahn | 75 | None |
| HIV-2 B | EHO-287 (U27200) | Partial genome molecular clone | Shui-Lok Hu | 69 | E15G, K64R, K66R, N176S, K206R, A449V,d E520Kd |
ARRRP, NIH AIDS Research and Reference Reagent Program.
The reference sequence for 92UG021 contains only the first 297 amino acids, and RU570 has no reference database sequence for the RT-encoding segment.
In HIV-1 M clones, positions 1 to 9 and 553 to 560 are encoded by primer-derived sequences. Mutations at these positions were introduced during PCR prior to cloning. Position 554 is highly polymorphic (including threonine) among HIV-1 sequences compiled by the NIH Sequence Database, and it is located on the back side of the RNase H domain, away from the nucleic acid binding surface. Thus, the position 554 mutation is not expected to influence either the enzymatic activity or the aptamer susceptibility of the affected proteins.
Positions 346 and higher in HIV-2 are numbered n + 1, according to the HIV-1 sequence, because HIV-2 RT has a 3-nt deletion at codon position 346 (and thus positions 448 and 519 in EHO-287 are designated 449 and 520, respectively).
Position 277 point mutants.
To create site-specifically mutated RTs, p66- and p51-expressing plasmids were used as templates for PCRs to generate overlapping 5′-half and 3′-half RT-encoding amplicons. Both internal primers spanned RT nucleotide positions 817 to 854 for HXB2 and 817 to 853 for 93TH253. A single base change at RT nucleotide position 830 (codon 277) was introduced into the overlapping region of each amplicon such that overlap extension and PCR from the ends generated full-length, RT-encoding PCR products carrying an R277K or K277R mutation. These fragments were gel purified and cloned for expression. p66 and p51 subunits were expressed and purified as described above but were kept separate during purification to allow for later reconstitution of different combinations of heterodimeric RTs. Because reconstitution by this method yielded less active enzyme than colysis, the mutant RTs were used at a final concentration of 50 nM to achieve equivalent overall enzymatic activity for DNA-dependent DNA polymerization (DDDP) and RNA-dependent DNA polymerization (RDDP) assays.
Sequence analysis.
All plasmid clones were sequenced by Gene Gateway LLC (Hayward, CA). Each pair of p66- and p51-encoding clones encoded proteins with identical amino acid sequences over the length of the small subunit, including small, vector-derived, N-terminal affinity tags. Sequence alignment and analysis were performed using both DIALIGN 2.2.1 (46), available online at the Bielefeld University Bioinformatics Server (http://bibiserv.techfak.uni-bielefeld.de/dialign/), and CLUSTAL W (61), available online at the European Bioinformatics Institute (http://www.ebi.ac.uk/clustalw/). Phylogenetic relationships among the RT-encoding pol gene fragments were determined by maximum likelihood analysis (15) of all 1,680 nt, using Treefinder software (32).
Expression and purification of heterodimeric RTs.
Expression plasmids were transformed into BL21(DE3) Star One Shot chemically competent Escherichia coli (Invitrogen) for protein expression and purification. Bacterial cells were grown in 500 ml Luria broth supplemented with 50 μg/ml kanamycin in a shaking incubator at 37°C to an optical density at 600 nm of 0.4 to 0.6, at which time IPTG (isopropyl-β-d-thiogalactopyranoside) was added to a final concentration of 0.5 mM. Cells were harvested 2 to 3 h later by centrifugation, and the cell pellets were stored at −80°C for one to several days until protein purification was initiated. Frozen cell pellets containing p66- and p51-expressing plasmids were thawed on ice and resuspended together in a total of 40 ml of 10 mM imidazole, 500 mM NaCl, 40 mM MgCl2, 20 mM NaH2PO4 (pH 7.4), and 5% glycerol. The combined slurry of resuspended cells was sonicated on ice to disrupt cell membranes and centrifuged for 30 min at 14,000 rpm (Beckman JA-17 rotor) to remove insoluble cell debris. Clarified lysates containing soluble RT were passed over a Ni-nitrilotriacetic acid (Ni-NTA) agarose column (QIAGEN) (5-ml bed volume), washed with 150 to 200 ml wash buffer (20 mM imidazole, 500 mM NaCl, 20 mM NaH2PO4, pH 7.4, 0.01% Triton X-100), and eluted with 10 ml 500 mM imidazole, 500 mM NaCl, and 20 mM NaH2PO4 (pH 7.4). Imidazole-eluted RT was further purified on a Sephadex G-100 Superfine gel filtration column (Sigma) equilibrated with wash buffer, and the recovered RT was passed over a second Ni-NTA column, as described above. RT recovered from the second Ni-NTA column (6 to 8 ml) was dialyzed against 1 liter of 400 mM NaCl, 32 mM NaH2PO4 (pH 7.4), 20% glycerol, and 0.016% Triton X-100 at 4°C once overnight and again for 24 h, with one change of buffer. Following dialysis, 0.6 volume of 100% glycerol was added to the recovered sample to bring the final glycerol concentration to 50%. Enzyme aliquots were stored at −20°C. The absorbance at 280 nm (A280) was used to determine final protein concentrations, using molar extinction coefficients (ɛ280) corresponding to the recombinant heterodimer (including N-terminal fusion tags) and calculated as described previously (20).
Comparison of RT activities across the panel.
DDDP, RDDP, and RNase H activities were monitored as described previously (26), using an 18-nt Cy3-labeled DNA primer annealed to a 103-nt DNA or 106-nt RNA long terminal repeat template, or by annealing a Cy3-labeled 43-nt RNA oligonucleotide to a complementary 53-nt DNA oligonucleotide. To enable comparison across the panel, the active-site concentration was determined for each enzyme by pre-steady-state titrations of single-nucleotide incorporation reactions performed using a rapid-quench flow device (KinTek model RQF-3). All subsequent reactions were initiated by the addition of enzyme to a 0.4 nM final active-site concentration, quenched at various times with 2 volumes of 95% formamide, 50 mM EDTA, and then heated for 2 min at 90°C prior to electrophoresis on 15% denaturing (8 M urea) polyacrylamide gels. Gels were scanned for Cy-3 fluorescence by using a Fujifilm FLA5000 imaging system, and RT activity data were collected using Fujifilm Multi Gauge V2.3 image analysis software. Product formation for all RTs was normalized against that for the most active clone for each assay to define 100% relative extension. Normalized product formation curves were fit to the following equation to solve rate constants (k) for each experiment: Y = Ymax(1 − e−kt), where Y is product formation over time (t), which proceeds to a defined maximum (Ymax). Assays for inhibition of DNA polymerization and RNase H activities using the templates defined above were performed as described previously (26), and IC50 values were derived from activity inhibition assays performed in triplicate.
Additional supporting information.
Unpublished supplemental information (cloning primers, excluded RTs, baseline [uninhibited] enzymatic activities, and tabulated IC50 values for inhibition by RNA aptamers) is available via e-mail or at the DHB website (http://web.missouri.edu/∼mmiwww/burke/db.php). If information is requested via email, the subject line should contain the following: “JVIROL_Held_Additional_Info_Request.”
RESULTS
Diversity panel of RTs from representative HIV-1, HIV-2, and SIVcpz strains.
We constructed a panel of recombinant RTs that reflect worldwide epidemiology and a phylogeny encompassing both the HIV-1 and HIV-2 branches (Fig. 1A). The panel includes all five of the major subtypes of HIV-1 group M (47% of recent HIV-1 infections worldwide are subtype C, 27% are subtype A, 12% are subtype B, 5% are subtype D, and 3% are subtype A/E) (48). Subtype A is represented in the panel by isolates 94CY017.41 and 93TH253.3. The former is a “pure” clade A strain, while the latter is a Thai A/E recombinant (CRF01_AE group) for which the pol gene is of subtype A. HXB2 is a standard laboratory strain that has been used widely as representative of the B subtype, the predominate HIV-1 clade in Western Europe and North America. 98CN009 is a subtype C isolate from China which is representative of the spreading epidemic in Asia, southern and eastern Africa, and South America. Subtype D is represented by isolate 92UG021, an A/D recombinant (gag of subtype A and pol of subtype D) from Uganda that was originally described as part of a collection of pol gene sequences spanning all of the PR-encoding region and part of the RT-encoding region (9). Although there is no pol gene reference sequence for the Russian isolate RU570, a BLAST query using the cloned RU570 RT-encoding pol gene fragment returned numerous G subtype HIV-1 isolates as the closest relatives, consistent with the assignment of this strain to subtype G based on gp120 sequences (2). The HIV-1 group O isolate MVP5180 is a phylogenetic neighbor of the HIV-1 group M cluster and is flanked evolutionarily by SIV strains infecting chimpanzee subspecies Pan troglodytes troglodytes (represented by the US clone) and Pan troglodytes schweinfurthii (represented by clone TAN1B). HIV-2 group B is represented in our RT panel by EHO-287. Throughout the panel, minor amino acid sequence differences relative to the GenBank reference sequence (Table 1) likely reflect the sequence diversity within viral quasispecies populations.
FIG. 1.
RT diversity panel. (A) Evolutionary relationships among viral isolates represented in the RT diversity panel. The phylogeny was generated using the maximum likelihood approach (15, 32) for analysis of the 1,680 nt of the pol gene, which encodes (the full p66) RT (1,677 nt for HIV-2). Approximate bootstrap values (LRSH-RELL) (58) were derived by analysis of 50,000 replicates. E543 from sooty mangabey SIV (GenBank accession no. U72748) was included in the phylogeny as the SIV strain most closely related to the HIV-2 clades. The colobus monkey SIV RT gene sequence (GenBank accession no. AF301156) was used for the outgroup. (B) Relative DNA polymerization and RNase H activities among recombinant RTs. Product formation for DDDP (top), RDDP (middle), and RNase H (bottom) activities was measured for 10 minutes with 0.4 nM active RT. Relative activities were determined by normalization against the most active RT in each assay.
Comparative enzymatic activity.
Purified RTs were diluted to equal active-site concentrations (0.4 nM), and their DDDP, RDDP, and RNase H activities were assessed in the absence of inhibitor, using a 75-fold excess of nucleic acid substrate to ensure multiple turnover conditions (Fig. 1B). For each activity, at least half of the panel displayed activities that were within a factor of 2 of that displayed by RT from the subtype B isolate HXB2, and in general the DDDP and RDDP activities tracked together. RTs from four strains (92UG021, 98CN009, RU570, and EHO-287) displayed especially low DNA polymerization activities on both RNA and DNA templates. The viral strains from which the first two of these RTs were derived previously exhibited especially low infectivities in cell-based growth assays (33). RNase H proficiency did not track with DNA polymerization activity. For example, the DNA polymerase activities of HXB2 RT were among the most active in the panel (highest RDDP activity and second-highest DDDP activity), but its RNase H activity was only the seventh-most active (along with that of MVP5180). RTs from strains RU570 and 93TH253 shared the highest relative RNase H rates (5.5-fold greater than that of HXB2), but while 93TH253 RT exhibited one of the most vigorous DNA polymerization activities, RU570 RT was among the weakest for DNA polymerization activity. RT from 98CN009 was the least efficient enzyme in the panel for all three activities. Consistent with previous reports (14, 27, 49, 56), the DNA polymerase and RNase H activities of RT from the HIV-2 strain EHO-287 were significantly less than those of most of the HIV-1 RTs.
Inhibition of HIV and SIVcpz RTs by NRTI and NNRTI compounds.
To establish that the diversity panel responds as expected to small-molecule inhibitors, DNA polymerization was monitored on a 103-nt DNA template for each of the 10 recombinant RTs in the presence of two NRTIs (AZTTP and ddCTP) and two NNRTIs (EFV and NVP) (Table 2). Nineteen of the 20 measured IC50 values for the NRTIs were within a twofold difference of the IC50 value for HXB2. For AZTTP, these values ranged from 0.40 μM (98CN009 RT) to 1.02 μM (SIVcpz US RT), while for ddCTP they ranged from 0.43 μM (98CN009) to 2.12 μM (RU570). Inhibition by one compound is predictive of inhibition by the other compound, and the values we obtained are comparable to those obtained previously from biochemical studies using recombinant subtype B RT (13, 50).
TABLE 2.
Inhibition of HIV and SIV RTs by small-molecule RT inhibitors
| Virus and group/clade | Viral isolate | IC50 (μM) (mean ± SD)a
|
|||
|---|---|---|---|---|---|
| AZTTP | ddCTP | EFV | NVP | ||
| HIV-1 | |||||
| M:B | HXB2 | 0.66 ± 0.09 | 1.13 ± 0.13 | 0.06 ± 0.01 | 1.29 ± 0.15 |
| M:A/D | 92UG021 | 0.49 ± 0.04 | 0.72 ± 0.11 | 0.14 ± 0.05 | 1.43 ± 0.21 |
| M:C | 98CN009 | 0.40 ± 0.06 | 0.43 ± 0.04 | 0.10 ± 0.02 | 3.13 ± 0.52 |
| M:A | 94CY017.41 | 0.72 ± 0.10 | 1.18 ± 0.15 | 0.06 ± 0.02 | 2.45 ± 0.77 |
| M:A/E | 93TH253.3 | 0.62 ± 0.09 | 0.98 ± 0.13 | 0.16 ± 0.05 | 1.92 ± 0.22 |
| M:G | RU570 | 0.81 ± 0.12 | 2.12 ± 0.37 | 0.02 ± 0.01 | 3.54 ± 0.59 |
| O | MVP5180 | 1.01 ± 0.11 | 1.76 ± 0.17 | 0.58 ± 0.19 | 11.4 ± 1.9 |
| SIVcpzp.t.t. | US | 1.02 ± 0.24 | 0.88 ± 0.10 | 0.06 ± 0.02 | 0.64 ± 0.12 |
| SIVcpzp.t.s. | TAN1B | 0.55 ± 0.05 | 0.80 ± 0.11 | 2.09 ± 0.62 | ≫100 |
| HIV-2 B | EHO-287 | 0.71 ± 0.10 | 0.73 ± 0.09 | ≫10 | ≫100 |
| Medianb | 0.69 | 0.93 | 0.08 | 2.19 | |
Inhibitors were assessed over concentration ranges of 0.1 to 30 μM for AZTTP, 0.1 to 30 μM for ddCTP, 0.01 to 10 μM for EFV, and 0.1 to 100 μM for NVP.
Median IC50 values are for all 10 panel members for AZTTP and ddCTP and for group M only for EFV and NVP.
For both EFV and NVP, at least half of the six RTs from HIV-1 group M were inhibited, with IC50 values that were within a twofold difference of that of HXB2, and IC50 values for the remaining group M RTs were within a factor of 5. IC50 values for these RTs ranged from 0.02 μM (RU570) to 0.16 μM (93TH253) for EFV and from 1.29 μM (HXB2) to 3.54 μM (RU570) for NVP. The median value for HIV-1 group M inhibition by EFV was approximately 27 times lower than that for inhibition by NVP, and there was no significant correlation between the inhibitory potencies of the two NNRTIs. Interestingly, the most closely related RT from outside the M group (SIVcpzP.t.t. US isolate) was inhibited at least as well as most of the group M RTs by both EFV and NVP (IC50 = 0.06 and 0.64 μM, respectively). For the remaining taxa, NNRTI potency weakened progressively and rapidly with increasing distance from the M group. Broad resistance to NNRTIs by HIV-2 has been well documented experimentally (65), and the HIV-2 member of the panel was not inhibited by these compounds. Thus, the RTs in this panel are well behaved in terms of both enzymatic activity and small-molecule drug inhibition.
Cross-clade inhibition of HIV-1 RTs by RNA aptamers.
We and others have previously described RNA aptamers selected for binding to the RT from subtype B strain BH10 (5, 62). Most of these aptamers are capable of forming pseudoknots representing one of two distinct structural subfamilies (Fig. 2, top diagrams). The cross-clade inhibitory potencies of four such aptamers were assessed by measuring dose-response curves for each aptamer against the 10 panel RTs in assays for inhibition of DDDP, RDDP, and RNase H activities.
FIG. 2.
RT inhibition by RNA aptamers with family 1 or family 2 pseudoknots. RT enzymatic activities, indicated on the left, were assayed for each member of the diversity panel in the presence of the RNA aptamers indicated above. Each aptamer-RT combination was assayed in triplicate to calculate IC50 values.
The two aptamers with family 1 pseudoknots (T1.1 and 70.5) consistently and potently inhibited all three RT enzymatic activities (DDDP, RDDP, and RNase H) for RTs from subtype B isolate HXB2 and subtype C isolate 93CN009 (Fig. 2), with IC50 values ranging from 1.3 to 4.3 nM for DNA polymerization and 7 to 20 nM for RNase H inhibition. RT from HIV-2 isolate EHO-287 was also significantly inhibited in RDDP (IC50 ≈ 20 nM) and RNase H (IC50 ≈ 10 nM) activity assays. Aptamer 70.5 weakly inhibited the RDDP activities of the A/D recombinant 92UG021 and the subtype A isolate 94CY017.41, with IC50s of 72.3 and 78.0 nM, respectively. RTs from the other isolates were either poorly inhibited or resistant to the two pseudoknot family 1 aptamers. The two aptamers capable of forming family 2 pseudoknots (70.8 and 80.55) exhibited broader cross-clade inhibition than the family 1 pseudoknots, registering measurable IC50 values for up to 7 of the 10 RTs in the panel for inhibition of DNA polymerization. Only the RTs from the group O strain and the two SIVcpz strains were fully resistant (Fig. 2). For all four aptamers, the IC50RDDP and IC50DDDP values were within an order of magnitude of each other, and most were within a factor of 5. For RT from subtype C strain 98CN009, DDDP activity was significantly more sensitive than RDDP activity, while for the HIV-2 strain EHO-287, RDDP activity was significantly more sensitive than DDDP activity. These differences in aptamer sensitivities likely reflect differences in processivity on DNA versus RNA templates for these RTs.
Lys277 confers resistance to family 1, but not family 2, pseudoknot RNA aptamers.
In a cocrystal structure of HIV-1 RT with aptamer T1.1, 28 amino acids (15 in p66 and 13 in p51) lie within 5 Å of the aptamer (31). Thirteen of these residues also contact double-stranded nucleic acids in other cocrystal structures. Alignment of these 28 amino acids across the panel identified a single amino acid that correlates with the observed pattern of inhibition for the family 1 pseudoknot aptamers (Table 3). Position 277, located in the thumb domain of the p66 subunit, is polymorphic for Arg and Lys in the 10 panel RTs. The three RTs that are strongly inhibited by aptamers with family 1 pseudoknots (HXB2, 98CN009, and EHO-287) all carry R277, while the other seven RTs carry K277, suggesting that amino acid 277 may be an important determinant of resistance to RNA aptamers with family 1 pseudoknots, with R277 rendering RTs susceptible to aptamer inhibition and K277 conferring aptamer resistance.
TABLE 3.
RT polymorphisms across HIV/SIV clades at positions that contact aptamer T1.1a
| Virus and clade | Isolate | Polymorphism at p66 position
|
Polymorphism at p51 position
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 13 | 82 | 88 | 89 | 154 | 261 | 262 | 265 | 275 | 277 | 281 | 284 | 448 | 500 | 539 | 20 | 21 | 22 | 78 | 82 | 324 | 388 | 395 | 413 | 415 | 416 | 417 | 418 | ||
| HIV-1 | |||||||||||||||||||||||||||||
| M:B | HXB2 | K | K | W | E | K | V | G | N | K | R | K | R | R | Q | H | K | V | K | R | K | D | K | K | E | E | F | V | N |
| M:A/D | 92UG021 | R | K | E | D | ||||||||||||||||||||||||
| M:C | 98CN009 | ||||||||||||||||||||||||||||
| M:A | 94CY017.41 | K | |||||||||||||||||||||||||||
| M:A/E | 93TH253.3 | K | R | ||||||||||||||||||||||||||
| M:G | RU570 | K | |||||||||||||||||||||||||||
| O | MVP5180 | I | R | K | R | S | |||||||||||||||||||||||
| SIVcpzp.t.t. | US | K | K | R | E | D | |||||||||||||||||||||||
| SIVcpzp.t.s. | TAN1B | K | P | I | |||||||||||||||||||||||||
| HIV-2 B | EHO-287 | T | K | I | R | P | M | R | D | S | |||||||||||||||||||
To test the importance of position 277 for aptamer inhibition, point mutations were engineered into each subunit of HXB2 (R277K) and 93TH253 (K277R). The four mutated subunits and the four wild-type subunits were expressed and purified separately for reconstitution in the four possible combinations of p66/p51, i.e., wild type/wild type, wild type/mutant, mutant/wild type, and mutant/mutant. The dose-response curves in DDDP and RDDP assays supported the prediction that the potency of the class I aptamers (particularly T1.1) is heavily dependent upon the identity of amino acid 277 in the p66 subunit (Fig. 3). In every case, heterodimers containing R277 in the p66 subunit (singly or in combination with R277 in p51) were strongly inhibited by all of the aptamers, while heterodimers containing K277 in the p66 subunit (singly or in combination with K277 in p51) were at least 100-fold resistant to the aptamers carrying family 1 pseudoknots. For aptamer T1.1, the switch was essentially all-or-none in both directions. In contrast, the aptamers carrying family 2 pseudoknots were significantly less sensitive to the identity of the amino acid at position 277. The IC50 values for aptamer 80.55 were shifted approximately 10- to 30-fold higher when the K277 mutation was introduced into the HXB2 RT, but for the other six assays involving aptamers 80.55 and 70.8, inhibition was shifted only 2- to 10-fold by changing the identity of position 277.
FIG. 3.
Effects of mutations at RT position 277 on inhibition of DDDP and RDDP activities by aptamers with family 1 and family 2 pseudoknots. The top two rows are data for Arg277Lys point mutants of HXB2 RT (subtype B). The bottom two rows are data for Lys277Arg mutants of 93TH253 RT (subtype E). In all panels, symbols indicate the locations (not identities) of mutations, as follows: blue squares, both subunits; red triangles, p66 only; green inverted triangles, p51 only; magenta diamonds, no mutation.
DISCUSSION
This work represents the first comparative biochemical analysis of HIV and SIV RT susceptibility to RNA aptamers. All currently available RT aptamers were selected for their affinity for subtype B RTs (strain BH10 for the four aptamers studied here), with no requirement for recognition of RTs from other subtypes. Although the four aptamers tested here disrupted the function of the subtype B RT (HXB2) with comparable efficacies, their potencies against RTs from other clades varied according to the structural subfamily of the RNA. Aptamers with family 2 pseudoknots (70.8 and 80.55) inhibited the DNA polymerization activities of up to 7 of the 10 RTs in the panel, with a spectrum of decreasing potency that correlates with evolutionary distance from subtype B, suggesting that weakly unfavorable interactions at many sites may perturb aptamer binding. HIV-2 RT (EHO-287) was the exception to this rule, as its RDDP and RNase H activities were robustly inhibited by all four aptamers (although 70.8 failed to inhibit EHO-287's DNA polymerase activity). The only three RTs (those of US, MVP5180, and TAN1B) that were uniformly resistant to the family 2 pseudoknot aptamers represent viral clades that currently are of little (HIV-1 O) or no (SIVcpz) global epidemiological significance. We recently observed very different patterns of cross-clade inhibition for single-stranded DNA aptamers RT1t49 and RT8. The former inhibited every RT in the RT diversity panel described here, while the latter was strongly specific for only the HXB2 strain (37a).
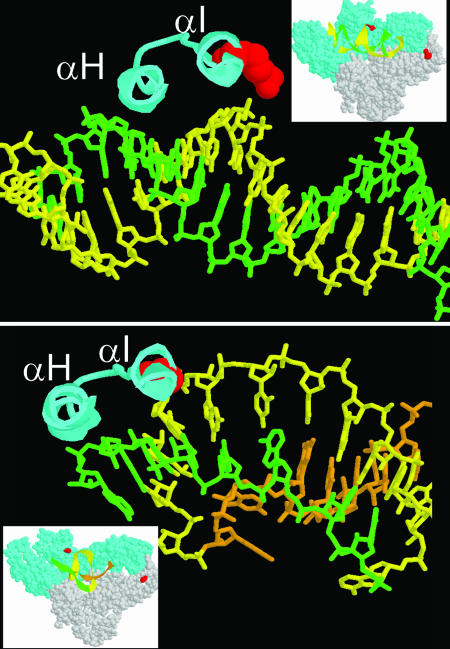
Position 277 is at the base of helix αI in the p66 subunit thumb domain, and it plays a key role in determining resistance to aptamers with family 1 pseudoknots. The R277 side chain interacts with template-strand phosphates in two cocomplexes of RT with double-stranded nucleic acids (double-stranded DNA [dsDNA] with incoming tenofovir-PP and an RNA-RNA duplex of the polypurine tract [54, 63]) (Fig. 4, top panel), and it swings away from the template in two other structures with dsDNA (12, 28). Although the resolution of the structure of the RT in complex with pseudoknot aptamer T1.1 is too low to discern side chains, the R277 alpha carbon is within 4 Å of several phosphates (A17 to C19) in stem 2 (31) (Fig. 4, bottom panel). In addition, R277 is more exposed in the aptamer-bound complex (finger and thumb domains touching) than in the dsDNA and DNA-RNA-bound complexes (finger and thumb domains separated). It remains to be determined why aptamers carrying family 1 pseudoknots are exquisitely sensitive to Lys at position 277 or why aptamers carrying family 2 pseudoknots are only marginally sensitive, although it is likely that the two families of pseudoknot aptamers make different contacts with RT.
FIG. 4.
Relation of Arg277 (red) to bound nucleic acids in two RT-nucleic acid complexes. Both structures show amino acids 258 to 282 (alpha helices H and I) of subunit p66 in approximately the same orientation. Views in close-up images are rotated approximately 30° forward relative to those in the insets. (Top) Structure 1T05, showing complex of RT with dsDNA and tenofovir (63) in an “open” configuration. The template strand is shown in yellow, and the primer strand is shown in green. (Bottom) Structure 1HVU, showing RT in a “closed” configuration in complex with aptamer T1.1 (31). For clarity, nt 4 to 14 are shown in orange, nt 14 to 25 are shown in yellow, and nt 26 to 33 are shown in green (nt 1 to 3 were unresolved in the structure). This structure does not include side chains.
Several pseudoknot RNA aptamers inhibit the propagation of diverse HIV-1 strains in cultured lymphocytes (6, 33, 35; reviewed in reference 25), with various degrees of protection that depend on the viral strain (33). Although no aptamer-resistant viruses emerged during continuous passage over short periods (up to 35 days) (6, 33), little is known about the virus's capacity to evolve resistance to aptamers during longer challenges. We speculate that the differential susceptibility to RT amino acid sequence variation noted here will translate into differential susceptibility to the evolution of de novo resistance during cell-based viral challenge or clinical gene therapy application. R277 and K277 appear with nearly equal prevalence in the genomic sequences catalogued in the Los Alamos HIV Sequence Database (http://hiv.lanl.gov/). To the extent that resistance to aptamers with family 1 pseudoknots arises more readily than does resistance to aptamers with family 2 pseudoknots, the latter become more attractive for developing anti-HIV gene therapy agents.
There is a growing appreciation that evaluation of the utility of novel antiviral compounds must include an assessment of their efficacies against RTs from phylogenetically diverse HIV strains. Although some inroads have been made in establishing the drug resistance profiles of non-B subtypes, RT expression constructs are not readily available for many viral strains, making head-to-head biochemical comparisons difficult. The RT panel we describe here could be useful in evaluations of new antiviral compounds. The panel RTs behaved as expected for small-molecule inhibitors and revealed new insights into aptamer inhibition when challenged with structurally related RNA aptamers. Six of the clones in the panel (94CY017.41, 92UG021, RU570, US, TAN1B, and EHO-287) represent the first cloning, expression, and purification of active RT enzymes from viral isolates of the corresponding clades. Where enzymatic activities of these isolates or close relatives have been reported separately (functional comparisons involving RTs from HIV-1 O [51] and HIV-2 group A [14, 27, 49, 55]), our results are consistent with previous observations. Although one or two individual clones from within a given clade cannot represent the full range of enzymatic activities or drug sensitivities across the entire clade, the recombinant RT panel described here provides a useful cross section of natural lentiviral genetic diversity.
Acknowledgments
We are grateful to Nirmala Bardiya and Linda Landon for careful readings of early drafts of the manuscript.
This work was supported by NIH grant AI62513 to D.H.B. and by the Milton Taylor Graduate Fellowship in Virology to D.M.H.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Amado, R., R. Mitsuyasu, J. Rosenblatt, F. Ngok, A. Bakker, S. Cole, N. Chorn, L. Lin, G. Bristol, M. Boyd, J. MacPherson, G. Fanning, A. Todd, J. Ely, J. Zack, and G. Symonds. 2004. Anti-human immunodeficiency virus hematopoietic progenitor cell-delivered ribozyme in a phase I study: myeloid and lymphoid reconstitution in human immunodeficiency virus type-1-infected patients. Hum. Gene Ther. 15:251-262. [DOI] [PubMed] [Google Scholar]
- 2.Bobkov, A., R. Cheingsong-Popov, M. Garaev, A. Rzhaninova, P. Kaleebu, S. Beddows, M. H. Bachmann, J. I. Mullins, J. Louwagie, W. Janssens, et al. 1994. Identification of an env G subtype and heterogeneity of HIV-1 strains in the Russian Federation and Belarus. AIDS 8:1649-1655. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, P., S. Sarafianos, P. Clark, E. Arnold, and S. Hughes. 2006. Why do HIV-1 and HIV-2 use different pathways to develop AZT resistance? PLoS Pathog. 2:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenner, B., D. Turner, M. Oliveira, D. Moisi, M. Detorio, M. Carobene, R. G. Marlink, J. Schapiro, M. Roger, and M. A. Wainberg. 2003. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS 17:F1-F5. [DOI] [PubMed] [Google Scholar]
- 5.Burke, D. H., L. A. Scates, K. Andrews, and L. Gold. 1996. Bent pseudoknots and novel RNA inhibitors of type 1 human immunodeficiency virus (HIV-1) reverse transcriptase. J. Mol. Biol. 264:650-666. [DOI] [PubMed] [Google Scholar]
- 6.Chaloin, L., M. J. Lehmann, G. Sczakiel, and T. Restle. 2002. Endogenous expression of a high-affinity pseudoknot RNA aptamer suppresses replication of HIV-1. Nucleic Acids Res. 30:4001-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L., A. Perlina, and C. J. Lee. 2004. Positive selection detection in 40,000 human immunodeficiency virus (HIV) type 1 sequences automatically identifies drug resistance and positive fitness mutations in HIV protease and reverse transcriptase. J. Virol. 78:3722-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins, J. A., M. G. Thompson, E. Paintsil, M. Ricketts, J. Gedzior, and L. Alexander. 2004. Competitive fitness of nevirapine-resistant human immunodeficiency virus type 1 mutants. J. Virol. 78:603-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cornelissen, M., R. van den Burg, F. Zorgdrager, V. Lukashov, and J. Goudsmit. 1997. pol gene diversity of five human immunodeficiency virus type 1 subtypes: evidence for naturally occurring mutations that contribute to drug resistance, limited recombination patterns, and common ancestry for subtypes B and D. J. Virol. 71:6348-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damond, F., M. Worobey, P. Campa, I. Farfara, G. Colin, S. Matheron, F. Brun-Vezinet, D. L. Robertson, and F. Simon. 2004. Identification of a highly divergent HIV type 2 and proposal for a change in HIV type 2 classification. AIDS Res. Hum. Retrovir. 20:666-672. [DOI] [PubMed] [Google Scholar]
- 11.Descamps, D., G. Collin, F. Letourneur, C. Apetrei, F. Damond, I. Loussert-Ajaka, F. Simon, S. Saragosti, and F. Brun-Vezinet. 1997. Susceptibility of human immunodeficiency virus type 1 group O isolates to antiretroviral agents: in vitro phenotypic and genotypic analyses. J. Virol. 71:8893-8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding, J., K. Das, Y. Hsiou, S. Sarafianos, A. Clark, Jr., A. Jacobo-Molina, C. Tantillo, S. Hughes, and E. Arnold. 1998. Structure and functional implications of the polymerase active site region in a complex of HIV-1 RT with a double-stranded DNA template-primer and an antibody Fab fragment at 2.8 A resolution. J. Mol. Biol. 284:1095-1111. [DOI] [PubMed] [Google Scholar]
- 13.Eron, J. J., Y. K. Chow, A. M. Caliendo, J. Videler, K. M. Devore, T. P. Cooley, H. A. Liebman, J. C. Kaplan, M. S. Hirsch, and R. T. D'Aquila. 1993. pol mutations conferring zidovudine and didanosine resistance with different effects in vitro yield multiply resistant human immunodeficiency virus type 1 isolates in vivo. Antimicrob. Agents Chemother. 37:1480-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, N., K. Rank, S. Poppe, W. Tarpley, and S. Sharma. 1996. Characterization of the p68/p58 heterodimer of human immunodeficiency virus type 2 reverse transcriptase. Biochemistry 35:1911-1917. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1981. Evolutionary trees from DNA sequences: a maximum likelihood approach. J. Mol. Evol. 17:368-376. [DOI] [PubMed] [Google Scholar]
- 16.Fisher, T. S., P. Joshi, and V. R. Prasad. 2002. Mutations that confer resistance to template-analog inhibitors of human immunodeficiency virus (HIV) type 1 reverse transcriptase lead to severe defects in HIV replication. J. Virol. 76:4068-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost, S. D., M. Nijhuis, R. Schuurman, C. A. Boucher, and A. J. Brown. 2000. Evolution of lamivudine resistance in human immunodeficiency virus type 1-infected individuals: the relative roles of drift and selection. J. Virol. 74:6262-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, F., E. Bailes, D. Robertson, Y. Chen, C. Rodenburg, S. Michael, L. Cummins, L. Arthur, M. Peeters, G. Shaw, P. Sharp, and B. Hahn. 1999. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397:385-386. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert, P. B., I. W. McKeague, G. Eisen, C. Mullins, N. A. Gueye, S. Mboup, and P. J. Kanki. 2003. Comparison of HIV-1 and HIV-2 infectivity from a prospective cohort study in Senegal. Stat. Med. 22:573-593. [DOI] [PubMed] [Google Scholar]
- 20.Gill, S. C., and P. H. von Hippel. 1989. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182:319-326. [DOI] [PubMed] [Google Scholar]
- 21.Graves, M. C., M. C. Meidel, Y. C. Pan, M. Manneberg, H. W. Lahm, and F. Gruninger-Leitch. 1990. Identification of a human immunodeficiency virus-1 protease cleavage site within the 66,000 dalton subunit of reverse transcriptase. Biochem. Biophys. Res. Commun. 168:30-36. [DOI] [PubMed] [Google Scholar]
- 22.Green, L., S. Waugh, J. P. Binkley, Z. Hostomska, Z. Hostomsky, and C. Tuerk. 1995. Comprehensive chemical modification interference and nucleotide substitution analysis of an RNA pseudoknot inhibitor to HIV-1 reverse transcriptase. J. Mol. Biol. 247:60-68. [DOI] [PubMed] [Google Scholar]
- 23.Grossman, Z., V. Istomin, D. Averbuch, M. Lorber, K. Risenberg, I. Levi, M. Chowers, M. Burke, N. Bar Yaacov, and J. M. Schapiro. 2004. Genetic variation at NNRTI resistance-associated positions in patients infected with HIV-1 subtype C. AIDS 18:909-915. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, R., I. Chrystie, S. O'Shea, J. Mullen, R. Kulasegaram, and C. Tong. 2005. K65R and Y181C are less prevalent in HAART-experienced HIV-1 subtype A patients. AIDS 19:1916-1919. [DOI] [PubMed] [Google Scholar]
- 25.Held, D., J. Kissel, J. Patterson, D. Nickens, and D. Burke. 2006. HIV-1 inactivation by nucleic acid aptamers. Front. Biosci. 11:89-112. [DOI] [PubMed] [Google Scholar]
- 26.Held, D., J. Kissel, D. Saran, D. Michalowski, and D. Burke. 2006. Differential susceptibility of HIV-1 reverse transcriptase to inhibition by RNA aptamers in enzymatic reactions monitoring specific steps during genome replication. J. Biol. Chem. 281:25712-25722. [DOI] [PubMed] [Google Scholar]
- 27.Hizi, A., R. Tal, M. Shaharabany, and S. Loya. 1991. Catalytic properties of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2. J. Biol. Chem. 266:6230-6239. [PubMed] [Google Scholar]
- 28.Huang, H., R. Chopra, G. Verdine, and S. Harrison. 1998. Structure of a covalently trapped catalytic complex of HIV-1 reverse transcriptase: implications for drug resistance. Science 282:1669-1675. [DOI] [PubMed] [Google Scholar]
- 29.Isaka, Y., S. Miki, S. Kawauchi, A. Suyama, H. Sugimoto, A. Adachi, T. Miura, M. Hayami, O. Yoshie, T. Fujiwara, and A. Sato. 2001. A single amino acid change at Leu-188 in the reverse transcriptase of HIV-2 and SIV renders them sensitive to non-nucleoside reverse transcriptase inhibitors. Arch. Virol. 146:743-755. [DOI] [PubMed] [Google Scholar]
- 30.Isel, C., C. Ehresmann, P. Walter, B. Ehresmann, and R. Marquet. 2001. The emergence of different resistance mechanisms toward nucleoside inhibitors is explained by the properties of the wild type HIV-1 reverse transcriptase. J. Biol. Chem. 276:48725-48732. [DOI] [PubMed] [Google Scholar]
- 31.Jaeger, J., T. Restle, and T. A. Steitz. 1998. The structure of HIV-1 reverse transcriptase complexed with an RNA pseudoknot inhibitor. EMBO J. 17:4535-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobb, G., A. von Haeseler, and K. Strimmer. 2004. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4:18. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Joshi, P., and V. R. Prasad. 2002. Potent inhibition of human immunodeficiency virus type 1 replication by template analog reverse transcriptase inhibitors derived by SELEX (systematic evolution of ligands by exponential enrichment). J. Virol. 76:6545-6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi, P. J., T. S. Fisher, and V. R. Prasad. 2003. Anti-HIV inhibitors based on nucleic acids: emergence of aptamers as potent antivirals. Curr. Drug Targets Infect. Disord. 3:383-400. [DOI] [PubMed] [Google Scholar]
- 35.Joshi, P. J., T. W. North, and V. R. Prasad. 2005. Aptamers directed to HIV-1 reverse transcriptase display greater efficacy over small hairpin RNAs targeted to viral RNA in blocking HIV-1 replication. Mol. Ther. 11:677-686. [DOI] [PubMed] [Google Scholar]
- 36.Keele, B., F. Van Heuverswyn, Y. Li, E. Bailes, J. Takehisa, M. Santiago, F. Bibollet-Ruche, Y. Chen, L. Wain, F. Liegeois, S. Loul, E. Ngole, Y. Bienvenue, E. Delaporte, J. Brookfield, P. Sharp, G. Shaw, M. Peeters, and B. Hahn. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kensch, O., B. Connolly, H. Steinhoff, A. McGregor, R. Goody, and T. Restle. 2000. HIV-1 reverse transcriptase-pseudoknot RNA aptamer interaction has a binding affinity in the low picomolar range coupled with high specificity. J. Biol. Chem. 275:18271-18278. [DOI] [PubMed] [Google Scholar]
- 37a.Kissel, J. D., D. M. Held, R. W. Hardy, and D. H. Burke. Single-stranded DNA aptamer RT1t49 inhibits RT polymerase and RNase H functions of HIV-1, HIV-2, and SIVcpz RTs. AIDS Res. Hum. Retrovir., in press. [DOI] [PubMed]
- 38.Kohlstaedt, L., and T. Steitz. 1992. Reverse transcriptase of human immunodeficiency virus can use either human tRNA(3Lys) or Escherichia coli tRNA(2Gln) as a primer in an in vitro primer-utilization assay. Proc. Natl. Acad. Sci. USA 89:9652-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levine, B., L. Humeau, J. Boyer, R. Macgregor, T. Rebello, X. Lu, G. Binder, V. Slepushkin, F. Lemiale, J. Mascola, F. Bushman, B. Dropulic, and C. June. 2006. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. USA 103:17372-17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Little, S., S. Holte, J. Routy, E. Daar, M. Markowitz, A. Collier, R. Koup, J. Mellors, E. Connick, B. Conway, M. Kilby, L. Wang, J. Whitcomb, N. Hellmann, and D. Richman. 2002. Antiretroviral-drug resistance among patients recently infected with HIV. N. Engl. J. Med. 347:385-394. [DOI] [PubMed] [Google Scholar]
- 41.Loemba, H., B. Brenner, M. A. Parniak, S. Ma'ayan, B. Spira, D. Moisi, M. Oliveira, M. Detorio, and M. A. Wainberg. 2002. Genetic divergence of human immunodeficiency virus type 1 Ethiopian clade C reverse transcriptase (RT) and rapid development of resistance against nonnucleoside inhibitors of RT. Antimicrob. Agents Chemother. 46:2087-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macpherson, J., M. Boyd, A. Arndt, A. Todd, G. Fanning, J. Ely, F. Elliott, A. Knop, M. Raponi, J. Murray, W. Gerlach, L. Sun, R. Penny, G. Symonds, A. Carr, and D. Cooper. 2005. Long-term survival and concomitant gene expression of ribozyme-transduced CD4+ T-lymphocytes in HIV-infected patients. J. Gene Med. 7:552-564. [DOI] [PubMed] [Google Scholar]
- 43.Mansky, L. M., D. K. Pearl, and L. C. Gajary. 2002. Combination of drugs and drug-resistant reverse transcriptase results in a multiplicative increase of human immunodeficiency virus type 1 mutant frequencies. J. Virol. 76:9253-9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marlink, R., P. Kanki, I. Thior, K. Travers, G. Eisen, T. Siby, I. Traore, C. C. Hsieh, M. C. Dia, E. H. Gueye, et al. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587-1590. [DOI] [PubMed] [Google Scholar]
- 45.Meyer, P., S. Matsuura, A. Mian, A. So, and W. Scott. 1999. A mechanism of AZT resistance: an increase in nucleotide-dependent primer unblocking by mutant HIV-1 reverse transcriptase. Mol. Cell 4:35-43. [DOI] [PubMed] [Google Scholar]
- 46.Morgenstern, B. 1999. DIALIGN 2: improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics 15:211-218. [DOI] [PubMed] [Google Scholar]
- 47.Morris, L., C. Pillay, C. Chezzi, P. Lupondwana, M. Ntsala, L. Levin, F. Venter, N. Martinson, G. Gray, and J. McIntyre. 2003. Low frequency of the V106M mutation among HIV-1 subtype C-infected pregnant women exposed to nevirapine. AIDS 17:1698-1700. [DOI] [PubMed] [Google Scholar]
- 48.Osmanov, S., C. Pattou, N. Walker, B. Schwardlander, and J. Esparza. 2002. Estimated global distribution and regional spread of HIV-1 genetic subtypes in the year 2000. J. Acquir. Immune Defic. Syndr. 29:184-190. [DOI] [PubMed] [Google Scholar]
- 49.Post, K., J. Guo, K. Howard, M. Powell, J. Miller, A. Hizi, S. Le Grice, and J. Levin. 2003. Human immunodeficiency virus type 2 reverse transcriptase activity in model systems that mimic steps in reverse transcription. J. Virol. 77:7623-7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quan, Y., B. G. Brenner, R. G. Marlink, M. Essex, T. Kurimura, and M. A. Wainberg. 2003. Drug resistance profiles of recombinant reverse transcriptases from human immunodeficiency virus type 1 subtypes A/E, B, and C. AIDS Res. Hum. Retrovir. 19:743-753. [DOI] [PubMed] [Google Scholar]
- 51.Quinones-Mateu, M., V. Soriano, E. Domingo, and L. Menendez-Arias. 1997. Characterization of the reverse transcriptase of a human immunodeficiency virus type 1 group O isolate. Virology 236:364-373. [DOI] [PubMed] [Google Scholar]
- 52.Ren, J., C. Nichols, L. Bird, P. Chamberlain, K. Weaver, S. Short, D. Stuart, and D. Stammers. 2001. Structural mechanisms of drug resistance for mutations at codons 181 and 188 in HIV-1 reverse transcriptase and the improved resilience of second generation non-nucleoside inhibitors. J. Mol. Biol. 312:795-805. [DOI] [PubMed] [Google Scholar]
- 53.Rodes, B., A. Holguin, V. Soriano, M. Dourana, K. Mansinho, F. Antunes, and J. Gonzalez-Lahoz. 2000. Emergence of drug resistance mutations in human immunodeficiency virus type 2-infected subjects undergoing antiretroviral therapy. J. Clin. Microbiol. 38:1370-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sarafianos, S. G., K. Das, T. Tantillo, A. D. J. Clark, J. Ding, J. M. Whitcomb, P. L. Boyer, S. H. Hughes, and E. T. Arnold. 2001. Crystal structure of HIV-1 reverse transcriptase in complex with a polypurine tract RNA:DNA. EMBO J. 20:1449-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sevilya, Z., S. Loya, N. Adir, and A. Hizi. 2003. The ribonuclease H activity of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2 is modulated by residue 294 of the small subunit. Nucleic Acids Res. 31:1481-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sevilya, Z., S. Loya, S. H. Hughes, and A. Hizi. 2001. The ribonuclease H activity of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2 is affected by the thumb subdomain of the small protein subunits. J. Mol. Biol. 311:957-971. [DOI] [PubMed] [Google Scholar]
- 57.Sharp, P. M., E. Bailes, D. L. Robertson, F. Gao, and B. H. Hahn. 1999. Origins and evolution of AIDS viruses. Biol. Bull. 196:338-342. [DOI] [PubMed] [Google Scholar]
- 58.Shimodaira, H., and M. Hasegawa. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol. Biol. Evol. 16:1114-1116. [Google Scholar]
- 59.Spence, R. A., W. M. Kati, K. S. Anderson, and K. A. Johnson. 1995. Mechanism of inhibition of HIV-1 reverse transcriptase by nonnucleoside inhibitors. Science 267:988-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Temesgen, Z., D. Warnke, and M. Kasten. 2006. Current status of antiretroviral therapy. Expert Opin. Pharmacother. 7:1541-1554. [DOI] [PubMed] [Google Scholar]
- 61.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuerk, C., S. MacDougal, and L. Gold. 1992. RNA pseudoknots that inhibit human immunodeficiency virus type 1 reverse transcriptase. Proc. Natl. Acad. Sci. USA 89:6988-6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuske, S., S. Sarafianos, A. J. Clark, J. Ding, L. Naeger, K. White, M. Miller, C. Gibbs, P. Boyer, P. Clark, G. Wang, B. Gaffney, R. Jones, D. Jerina, S. Hughes, and E. Arnold. 2004. Structures of HIV-1 RT-DNA complexes before and after incorporation of the anti-AIDS drug tenofovir. Nat. Struct. Mol. Biol. 11:469-474. [DOI] [PubMed] [Google Scholar]
- 64.Van Heuverswyn, F., Y. Li, C. Neel, E. Bailes, B. F. Keele, W. Liu, S. Loul, C. Butel, F. Liegeois, Y. Bienvenue, E. M. Ngolle, P. M. Sharp, G. M. Shaw, E. Delaporte, B. H. Hahn, and M. Peeters. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. [DOI] [PubMed] [Google Scholar]
- 65.Witvrouw, M., C. Pannecouque, K. Van Laethem, J. Desmyter, E. De Clercq, and A. M. Vandamme. 1999. Activity of non-nucleoside reverse transcriptase inhibitors against HIV-2 and SIV. AIDS 13:1477-1483. [DOI] [PubMed] [Google Scholar]
- 66.Wohrl, B. M., R. Krebs, R. S. Goody, and T. Restle. 1999. Refined model for primer/template binding by HIV-1 reverse transcriptase: pre-steady-state kinetic analyses of primer/template binding and nucleotide incorporation events distinguish between different binding modes depending on the nature of the nucleic acid substrate. J. Mol. Biol. 292:333-344. [DOI] [PubMed] [Google Scholar]