Abstract
Induction of virus-specific CD8+ cytotoxic T-lymphocyte (CTL) responses is a promising strategy for AIDS vaccine development. However, it has remained unclear if or how long-term viral containment and disease control are attainable by CTL-based nonsterile protection. Here, we present three rhesus macaques that successfully maintained Env-independent vaccine-based control of simian immunodeficiency virus (SIV) mac239 replication without disease progression for more than 3 years. SIV-specific neutralizing antibody induction was inefficient in these controllers. Vaccine-induced Gag-specific CTLs were crucial for the chronic as well as the primary viral control in one of them, whereas those Gag-specific CTL responses became undetectable and CTLs specific for SIV antigens other than Gag, instead, became predominant in the chronic phase in the other two controllers. A transient CD8+ cell depletion experiment 3 years postinfection resulted in transient reappearance of plasma viremia in these two animals, suggesting involvement of the SIV non-Gag-specific CTLs in the chronic SIV control. This sustained, neutralizing antibody-independent viral control was accompanied with preservation of central memory CD4+ T cells in the chronic phase. Our results suggest that prophylactic CTL vaccine-based nonsterile protection can result in long-term viral containment by adapted CTL responses for AIDS prevention.
Human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections induce acute, massive depletion of CCR5+ CD4+ effector memory T cells from mucosal effector sites. This is followed by chronic immune activation with gradual immune disruption leading to AIDS (7, 15, 20, 25, 26, 33, 34). Acute depletion has an impact on disease course but does not dictate everything that happens in the chronic phase (7, 26). It has also been suggested that persistent viral replication-associated chronic immune activation may be critical for AIDS progression.
Virus-specific CD8+ cytotoxic T-lymphocyte (CTL) responses are crucial for control of HIV and SIV replication (3, 8, 12, 18, 24, 29). Several vaccine regimens eliciting virus-specific CTL responses have been developed and evaluated in macaque AIDS models (6, 21). Some of them have shown protective efficacies leading to viremia control in a model of X4-tropic simian-human immunodeficiency virus (SHIV) infections (1, 16, 22, 23, 28, 31). However, assessment of the ability of vaccines to ameliorate disease progression requires analysis in macaque models of R5-tropic SIV infection (5). Although most CTL-based vaccine trials using rigorous SIV challenges in Indian rhesus macaques have failed, some of them have shown amelioration of acute memory CD4+ T-cell depletion in the vaccinated animals with reduction in viral loads out to a year postinfection (4, 13, 19, 35). These findings have suggested that there may be a clinical benefit conferred by CTL-based AIDS vaccines. Unfortunately, it is still unclear as to how nonsterile protection conferred by prophylactic CTL-based vaccines can result in long-term viral containment and disease control.
We have previously developed a CTL-eliciting AIDS vaccine regimen using a DNA-prime/Gag-expressing Sendai virus (SeV-Gag) vector-boost (16, 32). Our regimen does not utilize Env immunogen that may induce neutralizing antibodies, although this antigen has been used in most of the vaccines except for a few cases (16, 31, 35). We have evaluated efficacy of this Env-independent vaccine against SIVmac239 challenge in Burmese rhesus macaques and found neutralizing antibody-independent, CTL-based control of primary SIV replication in five of eight vaccinees (17). In the present study, we have followed these macaques to examine if long-term viral containment without disease progression is possible by prophylactic CTL-based AIDS vaccines.
MATERIALS AND METHODS
Animal experiments.
Twelve Burmese rhesus macaques (Macaca mulatta) used in our previous SIVmac239 challenge experiment (17) were followed in the present study. These macaques were maintained in accordance with the Guideline for Laboratory Animals of the National Institute of Infectious Diseases and the National Institute of Biomedical Innovation. Four of them were naive, whereas the other eight macaques received a DNA vaccine followed by a single boost with SeV-Gag before an intravenous SIVmac239 challenge. The DNA, CMV-SHIVdEN, used for the vaccination was constructed from an env- and nef-deleted SHIVMD14YE molecular clone DNA (30) and has the genes encoding SIVmac239 Gag, Pol, Vif, and Vpx, SIVmac239-HIV-1DH12 chimeric Vpr, and HIV-1DH12 Tat and Rev as described previously (17). At the DNA vaccination, animals received 5 mg of CMV-SHIVdEN DNA intramuscularly. Six weeks after the DNA prime, animals intranasally received a single boost with 1 × 108 cell infectious units of replication-competent SeV-Gag (V1, V2, V3, and V4) or 6 × 109 cell infectious units of F-deleted replication-defective F(-)SeV-Gag (9, 14, 32). Approximately 3 months after the boost, animals were challenged intravenously with 1,000 50% tissue culture infective doses (TCID50) of SIVmac239 (11).
For CD8+ cell depletion, animals received a single intramuscular inoculation of 10 mg/kg of body weight of monoclonal anti-CD8 antibody (cM-T807) provided by Centocor (Malvern, PA) followed by three intravenous inoculations of 5 mg/kg cM-T807 on days 3, 7, and 10 after the first inoculation. The anti-CD8 antibody administration started at week 118 in macaque V5 and at week 156 in macaques V6 and V8. CD8+ T-cell depletion in peripheral blood was confirmed by immunostaining using fluorescein isothiocyanate-conjugated anti-human CD8 antibody (DK25; Dako, Kyoto, Japan).
All the noncontrollers were euthanized when they showed typical signs of AIDS, such as reduction in peripheral CD4+ T-cell counts, loss of body weight, diarrhea, and general weakness. Autopsy revealed lymphoatrophy or post-persistent generalized lymphadenopathy conditions consistent with AIDS.
Quantitation of plasma viral loads.
Plasma RNA was extracted using the High Pure viral RNA kit (Roche Diagnostics, Tokyo, Japan). Serial fivefold dilutions of RNA samples were amplified in quadruplicate by reverse transcription and nested PCR using SIV gag-specific primers to determine the endpoint. Plasma SIV RNA levels were calculated according to the Reed-Muench method as described previously (17). The lower limit of detection is approximately 4 × 102 copies/ml.
Measurement of virus-specific neutralizing titers.
Serial twofold dilutions of heat-inactivated plasma were prepared in duplicate and mixed with 10 TCID50 of SIVmac239. In each mixture, 5 μl of diluted plasma was incubated with 5 μl of virus. After a 45-min incubation at room temperature, each 10-μl mixture was added to 5 × 104 MT4 cells in a well of a 96-well plate. After 12 days of culture, supernatants were harvested. Progeny virus production in the supernatants was examined by enzyme-linked immunosorbent assay for detection of SIV p27 core antigen (Beckman-Coulter, Tokyo, Japan) to determine the 100% neutralizing endpoint. The lower limit of detection is a titer of 1:2.
Measurement of virus-specific CTL responses.
We measured virus-specific CD8+ T-cell levels by flow cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation as described previously (17). In brief, peripheral blood mononuclear cells (PBMCs) were cocultured with autologous herpesvirus papio-immortalized B-lymphoblastoid cell lines infected with a vaccinia virus vector expressing SIVmac239 Gag for Gag-specific stimulation or a vesicular stomatitis virus G protein (VSV-G)-pseudotyped SIVGP1 for SIV-specific stimulation. The pseudotyped virus was obtained by cotransfection of COS-1 cells with a VSV-G expression plasmid and the SIVGP1 DNA, an env- and nef-deleted SHIV molecular clone DNA. Intracellular IFN-γ staining was performed using a CytofixCytoperm kit (Becton Dickinson, Tokyo, Japan). Peridinin chlorophyll protein-conjugated anti-human CD8, allophycocyanin-conjugated anti-human CD3, and phycoerythrin-conjugated anti-human IFN-γ antibodies (Becton Dickinson) were used. Specific T-cell levels were calculated by subtracting nonspecific IFN-γ+ T-cell frequencies from those after Gag-specific or SIV-specific stimulation. Specific T-cell levels less than 100 cells per million PBMCs are considered negative.
Immunostaining of CD4+ T-cell memory subsets.
Frozen stocks of PBMCs were thawed and subjected to immunofluorescent staining by using fluorescein isothiocyanate-conjugated anti-human CD28, phycoerythrin-conjugated anti-human CD95, peridinin chlorophyll-conjugated anti-human CD4, and allophycocyanin-conjugated anti-human CD3 monoclonal antibodies (Becton Dickinson). Memory and central memory subsets of CD4+ T cells were delineated by CD95+ and CD28+ CD95+ phenotypes, respectively, as described previously (27).
Statistical analysis.
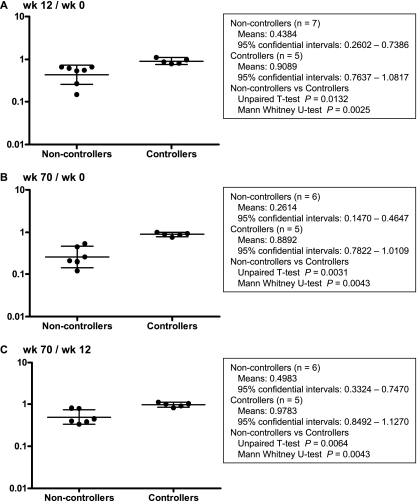
Central memory CD4+ T-cell counts just before SIV challenge (at week zero) were not significantly different between the noncontrollers (n = 7) and the controllers (n = 5) by unpaired t test. We calculated ratios of the counts at week 12 to week 0, week 70 to week 0, and week 70 to week 12 in each animal and performed an unpaired t test and nonparametric Mann-Whitney U-test between the noncontrollers and the controllers by using Prism software version 4.03 (GraphPad Software, Inc., San Diego, CA).
RESULTS
Long-term viral containment without disease progression in the sustained controllers.
We followed up on our vaccinated Burmese rhesus macaques used in the previous trial (17). These macaques were vaccinated using a DNA prime-SeV-Gag boost, and they were challenged with SIVmac239. Five of eight vaccinees controlled viral replication and had undetectable plasma viremia at week 8 postchallenge. The remaining three vaccinees (V1, V2, and V7) and all four unvaccinated macaques (N1, N2, N3, and N4) failed to control viral replication. Of the five controllers, two macaques V3 and V5 (referred to as transient controllers) exhibited viremia reappearance around week 60, but the other three, V4, V6, and V8 (referred to as sustained controllers), maintained viral control (10).
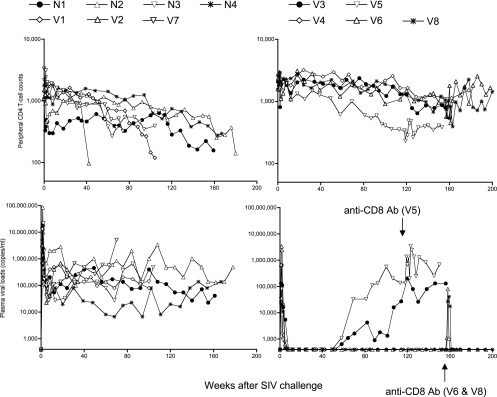
In the present follow-up study, all seven noncontrollers, including three vaccinees and four unvaccinated controls, exhibited persistent viremia and a gradual decline in peripheral CD4+ T-cell counts (Fig. 1). All of them finally developed AIDS and were euthanized at week 42 to 180 postchallenge (Table 1), confirming that failure in control of SIVmac239 replication results in AIDS progression even in Burmese rhesus macaques. In contrast, all three sustained controllers maintained viral control and preserved peripheral CD4+ T cells without disease progression for more than 3 years (Fig. 1).
FIG. 1.
Follow-up of the macaques after SIVmac239 challenge. Upper panels, peripheral CD4+ T-cell counts (cells/μl); lower panels, plasma viral loads (viral RNA copies/ml plasma); left panels, the seven noncontrollers; right panels, the five controllers. All seven noncontrollers developed AIDS and were euthanized during the observation period (Table 1). Macaques V5, V6, and V8 received anti-CD8 antibody treatment starting from week 118, week 156, and week 156, respectively.
TABLE 1.
Summary of responses in macaques challenged with SIVmac239
| Macaque group and no. | MHC-I haplotypea | VL
|
Statusc | CD4 countd at euthanasia | Opportunistic infection at autopsye | |
|---|---|---|---|---|---|---|
| Set pointb | After wk 60 | |||||
| Unvaccinated noncontrollers | ||||||
| N1 | 90-088-Ij | >104 | >104 | Euthanized at wk 161 | 158 | |
| N2 | 90-120-Ia | >104 | >104 | Euthanized at wk 180 | 141 | PCP |
| N3 | 90-122-Ie | >104 | >104 | Euthanized at wk 104 | 393 | |
| N4 | 90-010-Id | >104 | >104 | Euthanized at wk 167 | 296 | CMV |
| Vaccinated noncontrollers | ||||||
| V1 | 90-088-Ij | >104 | >104 | Euthanized at wk 105 | 119 | |
| V2 | 90-120-Ib | >104 | Euthanized at wk 42 | 97 | PCP | |
| V7 | 90-122-Ie | >104 | >104 | Euthanized at wk 77 | 323 | |
| Vaccinated transient controllers | ||||||
| V3 | 90-120-Ia | <400 | >103 | Alive >3 yr | ||
| V5 | 90-120-Ia | <400 | >104 | Euthanized at wk 154* | 384 | |
| Vaccinated sustained controllers | ||||||
| V4 | 90-120-Ia | <400 | <400 | Alive >3 yr | ||
| V6 | 90-122-Ie | <400 | <400 | Alive >3 yr* | ||
| V8 | 90-010-Id | <400 | <400 | Alive >3 yr* | ||
MHC-I haplotype was determined by reference strand-mediated conformation analysis as described previously (2, 17). MHC class I haplotypes 90-120-Ia and 90-120-Ib are derived from breeder R-90-120, 90-122-Ie is from R-90-122, 90-010-Id is from R-90-010, and 90-088-Ij is from R-90-088.
Plasma viral load (VL, in RNA copies/ml plasma) around week 12.
All seven noncontrollers exhibited reduction in peripheral CD4 T-cell count, loss of body weight, and general weakness and were euthanized and subjected to autopsy to be confirmed as AIDS. Macaques V5, V6, and V8 (indicated by asterisks) were administered an anti-CD8 antibody for CD8 cell depletion at weeks 118, 156, and 156, respectively.
Peripheral CD4 T-cell counts.
PCP, pneumocystis pneumonia; CMV, cytomegalovirus infection.
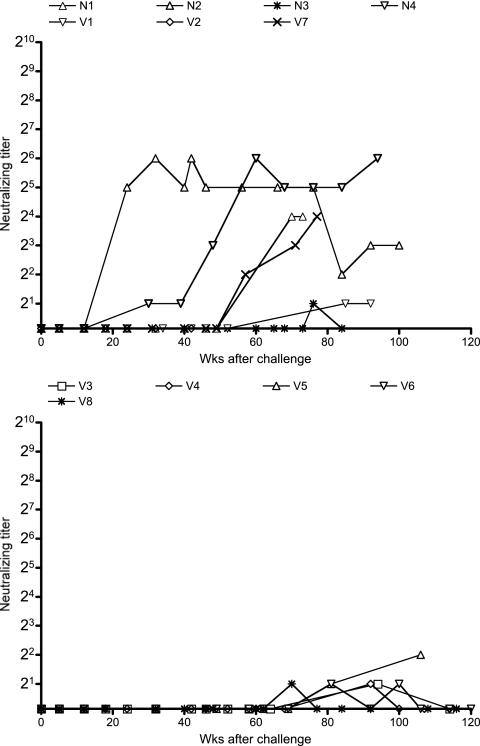
We then examined SIVmac239-specific neutralizing antibody responses by determining the end point plasma titers for killing 10-TCID50 virus replication on MT4 cells (Fig. 2). Our vaccine regimens did not utilize Env as an immunogen, and no neutralizing antibody responses were induced before challenge in any of the vaccinees. Even after challenge, none of the SIVmac239-challenged macaques showed detectable neutralizing antibody responses until 6 months. After that, neutralizing antibody responses became detectable in some of the noncontrollers. In contrast, no or little neutralizing antibody responses were induced in the controllers, even in the chronic phase.
FIG. 2.
SIVmac239-specific neutralizing antibody levels in plasma. Plasma titers for killing 10-TCID50 SIVmac239 replication in the noncontrollers (top panel), including unvaccinated control animals, and in the controllers (bottom panel) are shown.
Shift of antigens targeted by CTLs during the period of viral control.
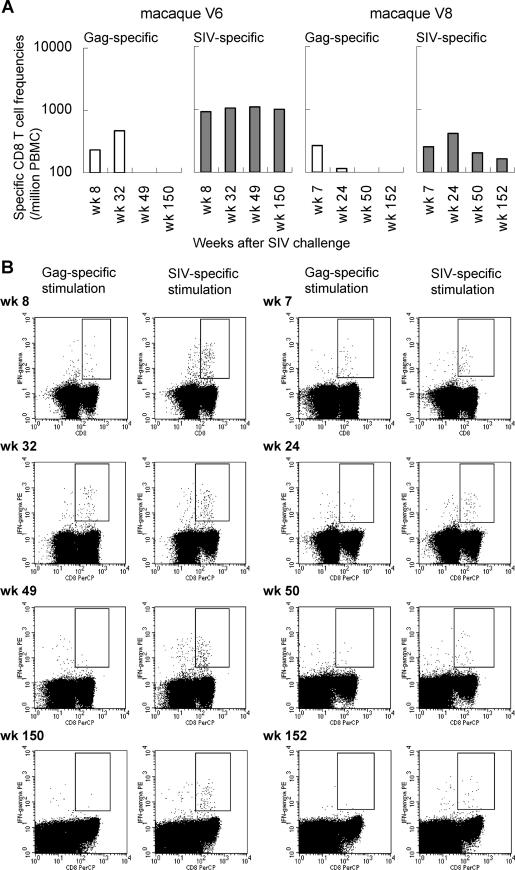
CTLs from all five controllers selected Gag CTL escape mutations soon after infection, indicating that vaccine-induced Gag-specific CTL responses were crucial for viral control in the early phase of SIV infection (17). In one sustained controller, macaque V4, possessing major histocompatibility complex class I haplotype 90-120-Ia, Gag206-216 (IINEEAADWDL) epitope-specific CTLs and Gag241-249 (SSVDEQIQW) epitope-specific CTL responses likely played a central role in control of viral replication in the chronic phase (10). We also analyzed virus-specific CTL responses in the remaining two sustained controllers, V6 and V8, to determine if vaccine-induced Gag-specific CTL responses played a role in control of viral replication in the chronic phase.
We measured Gag-specific and SIV-specific CTL frequencies in macaques V6 and V8 (Fig. 3). In both macaques, Gag-specific CTL frequencies were high around 2 months postchallenge but then decreased to below detection levels around 1 year postchallenge. In contrast, SIV-specific CTL responses against epitopes in other SIV proteins were still detectable 3 years postchallenge. These results suggest that the vaccine-induced Gag-specific CTL responses were diminished soon after challenge and that there was then a predominance of CTLs specific for SIV-derived antigens other than Gag in the chronic phase in both of the sustained controllers, V6 and V8.
FIG. 3.
Virus-specific CD8+ T-cell responses in sustained controllers V6 (left panels) and V8 (right panels). (A) Gag-specific and SIV-specific CD8+ T-cell frequencies in PBMCs. (B) Dot plots gated on CD3+ lymphocytes after Gag-specific or SIV-specific stimulation.
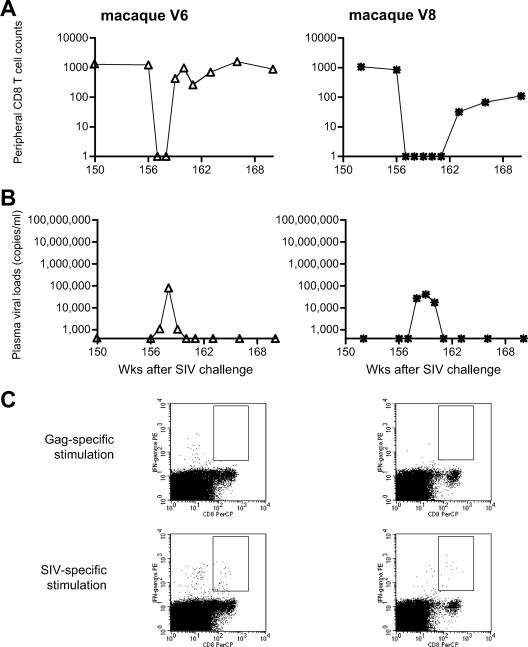
Viremia reappearance by CD8+ cell depletion in the sustained controllers.
In the sustained controllers, V6 and V8, vaccine-induced Gag-specific CTLs involved in viremia control in the early phase became undetectable after approximately 6 months. CTLs specific for SIV-derived antigens other than Gag (referred to as SIV non-Gag-specific CTLs) were elicited or expanded after challenge, and these became predominant in the chronic phase. We then performed CD8+ cell depletion experiments to examine if these SIV non-Gag-specific CTL responses played a role in the maintenance of viremia control in the chronic phase. Administration of the monoclonal anti-CD8 antibody, cM-T807, to macaques V6 and V8 at week 156 postchallenge resulted in transient depletion of peripheral CD8+ T lymphocytes (Fig. 4A). In both macaques, plasma viremia reemerged in 1 or 2 weeks after the initial anti-CD8 antibody treatment and disappeared simultaneously with recovery of peripheral CD8+ T lymphocytes in both of them (Fig. 4B). These results support the notion that, in the sustained controllers V6 and V8, these SIV non-Gag-specific CTL responses, rather than vaccine-induced Gag-specific CTL, played a crucial role in the control of SIV replication in the chronic phase. Analysis of the returning wave of virus-specific CTL responses revealed a predominance of SIV non-Gag-specific CTLs (Fig. 4C).
FIG. 4.
CD8+ cell depletion experiments starting at week 156 in sustained controllers V6 (left panels) and V8 (right panels). (A) Peripheral CD8+ T-cell counts (per μl). (B) Plasma viral loads (viral RNA copies/ml plasma). (C) Virus-specific CTL responses at week 160 in V6 and at week 166 in V8. Dot plots gated on CD3+ lymphocytes after Gag-specific or SIV-specific stimulation are shown.
We also administered the anti-CD8 antibody to macaque V5, a transient controller, at week 118. In this macaque, accumulation of multiple Gag CTL escape mutations resulted in reappearance of plasma viremia around week 60. Transient CD8+ cell depletion by the anti-CD8 antibody treatment resulted in a 1-log increase in plasma viral loads (Fig. 1), suggesting that CTLs still exerted pressure on the replication of the escaped viruses at week 118 in this animal.
Long-term central memory CD4+ T-cell preservation in the sustained controllers.
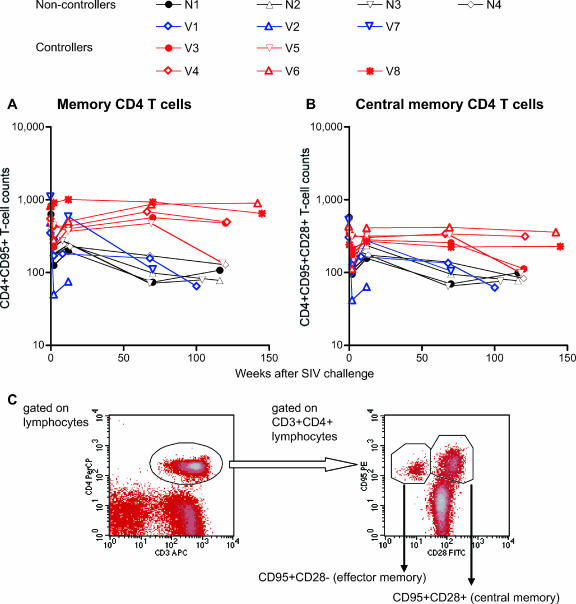
It has recently been suggested that vaccine-based transient control of viral replication can ameliorate central memory CD4+ T-cell loss in the early phase of SIV infections. However, it is unclear if CTL-based sustained control of viral replication can contribute to memory CD4+ T-cell preservation in the chronic phase. We, therefore, compared peripheral memory CD4+ T-cell counts at several time points, prechallenge and around weeks 2, 12, 70, and 120 postchallenge, in the noncontrollers and the controllers (Fig. 5). All the noncontrollers showed significant but partial recovery of peripheral memory CD4+ T-cell counts around week 12 after transient loss during the acute phase. However, memory CD4+ T-cell counts, especially central memory CD4+ T-cell counts at week 12, were lower than prechallenge levels in the noncontrollers. By contrast, such a reduction was not observed in the controllers, suggesting protection from acute memory CD4+ T-cell depletion.
FIG. 5.
Changes in peripheral memory CD4+ T-cell counts. Noncontrollers are indicated in black or blue, and controllers are indicated in red. (A) Peripheral memory CD4+ (CD4+ CD95+) T-cell counts (per μl). (B) Peripheral central memory CD4+ (CD4+ CD95+ CD28+) T-cell counts (per μl). (C) Representative density plots (macaque V4 prechallenge) for determining peripheral memory CD4+ T-cell percentages. The left panel is a density plot gated on lymphocytes, and in this plot, CD3+ CD4+ lymphocytes are gated for the right panel of the density plot. In the right panel, we determined the percentages of central memory (CD95+ CD28+) CD4+ T cells and memory (CD95+ CD28+ plus CD95+ CD28−) CD4+ T cells.
A continuous reduction in memory CD4+ T-cell counts was observed in the noncontrollers. The controllers, however, showed no such reduction in memory CD4+ T-cell counts out to week 70. At approximately week 120, all the sustained controllers still showed preservation of memory and central memory CD4+ T cells. In contrast, both of the transient controllers, V3 and V5, experienced a reduction in central memory CD4+ T-cell counts, although reduction in memory CD4+ T-cell counts was observed in only one of them. These results suggest that CTL-based vaccines that control viral replication can also preserve central memory CD4+ T cells even in the chronic phase. Finally, statistical analysis revealed that there was no significant reduction in central memory CD4+ T cells during the period between weeks 12 and 70 in the controllers (Fig. 6). Thus, CTL vaccine-based, sustained viral control can result in preservation of central memory CD4+ T cells in both the chronic phase as well as the acute phase.
FIG. 6.
Statistical analysis indicating preservation of central memory CD4+ T-cell counts in the controllers. The ratios of central memory CD4+ T-cell counts at week 12 to week 0 (A), week 70 to week 0 (B), and week 70 to week 12 (C) in the noncontrollers (except for rapid progressor V2 in panels B and C) and the controllers are plotted. The longer bars indicate geometric mean values, and the regions between the shorter bars indicate the 95% confidential intervals. Statistical analysis was performed with the t test and nonparametric Mann-Whitney U-test using the Prizm software.
DISCUSSION
Here we followed three Burmese rhesus macaques that maintained CTL vaccine-based control of SIVmac239 replication without disease progression for more than 3 years. The set-point plasma viral loads in SIVmac239-infected Burmese rhesus macaques may be lower than those usually observed in SIVmac239-infected Indian rhesus but are higher than those typically observed in untreated humans infected with HIV-1. All four of the naive control animals along with three vaccinees failed to control viremia after SIVmac239 challenge. They also experienced peripheral CD4+ T-cell loss and developed AIDS in 3 years, indicating that this model of SIVmac239 infection in Burmese rhesus macaques is adequate for evaluation of vaccine efficacies. Our finding of long-term control of viral replication and CD4+ T-cell preservation in three vaccinees in this AIDS model underlines the potential of a prophylactic CTL-based vaccine for AIDS prevention.
Our previous study revealed rapid selection of Gag CTL escape mutations in all the controllers, indicating that vaccine-induced Gag-specific CTL responses played an important role in viral control in the early phase of SIV infection (17). In the chronic phase, neutralizing antibody induction was still inefficient, and our results suggest long-term CTL-based viral containment. Indeed, the vaccine-induced Gag-specific CTL responses have been shown to play a crucial role in viral control even in the chronic phase in one (V4) of three sustained controllers (10). In contrast, Gag-specific CTL responses became undetectable and SIV non-Gag-specific CTL responses, instead, became predominant in macaques V6 and V8. The results obtained from a CD8+ cell depletion experiment are consistent with involvement of these SIV non-Gag-specific CTL responses in the long-term viral control in both sustained controllers, although there might be involvement of other components, such as NK and CD4+ memory T cells. Thus, it can be speculated that vaccine-based control of primary SIV replication can preserve the ability of the immune system to elicit functional CTL responses, leading to reinforcement or adaptation of protective immunity by postchallenge induction or expansion of effective CTL responses. This may contribute to stable viral containment in the chronic phase.
In the natural courses of HIV and SIV infections, the infected hosts exhibit acute, massive depletion of CCR5+ CD4+ effector memory T cells from mucosal effector sites, and the chronic immune activation with gradual immune disruption that follows leads to AIDS (7, 15, 20, 25). The former acute memory loss may influence the latter chronic disease progression (25, 26). The acute depletion results in compromised immune responses at the effector sites and systemic proliferative responses that partially compensate for the loss of mucosal memory CD4+ T-cell populations. Recent reports indicating amelioration of acute mucosal memory CD4+ T-cell depletion and associated central memory CD4+ T-cell loss in the early phase by CTL-based vaccines have suggested that vaccine-based amelioration of acute memory CD4+ T-cell depletion in mucosal effector sites can delay AIDS progression (13, 19, 35). However, this acute memory CD4+ T-cell depletion is not the only cause of chronic disease progression and persistent viral replication-associated immune activation may be responsible for chronic immune disruption leading to AIDS (7). Indeed, in both of the transient controllers, V3 and V5, central memory CD4+ T cells were preserved during the initial, transient period of viremia control but decreased after the reappearance of plasma viremia. This suggests that there may be an association between persistent viral containment and central memory CD4+ T-cell preservation, even in the chronic phase.
Theoretically, protection by CTL-based AIDS vaccines is likely to be nonsterile, and it will be difficult to contain viral replication completely. Additionally, CTL-based viremia control would require CTL activation. Indeed, our CD8+ cell depletion experiment indicated that persistent viral replication was inefficient but not completely contained in the absence of plasma viremia in sustained controllers V6 and V8. Transition of recognition of CTL epitopes from Gag to other non-Gag proteins in the chronic phase suggests that these “new” CTLs were either elicited or expanded by viral replication in the acute phase or by this inefficient persistent viral replication. Nevertheless, these macaques showed long-term viral control with central memory CD4+ T-cell preservation, indicating that nonsterile protection by CTL-based vaccines can result in prevention of chronic central memory CD4+ T-cell loss.
In summary, the present study shows that primary viral control by a CTL-based AIDS vaccine can result in long-term control of SIV replication by adapted CTL responses and preservation of central memory CD4+ T cells without AIDS progression. Our results suggest that CTL-based vaccines can result in long-term viral containment and disease control.
Acknowledgments
This work was supported by a grant from the Ministry of Education, Culture, Sports, Science, and Technology, grants from the Japan Health Sciences Foundation, and grants from the Ministry of Health, Labor, and Welfare in Japan.
The animal experiments were conducted through the Cooperative Research Program in Tsukuba Primate Research Center, National Institute of Biomedical Innovation with the help of the Corporation for Production and Research of Laboratory Primates. We thank Centocor Inc. and K. A. Reimann for providing cM-T807 and DNAVEC Corp. and J. Lifson, Y. Ami, F. Ono, K. Komatsuzaki, A. Hiyaoka, A. Oyama, K. Oto, H. Oto, H. Akari, K. Terao, M. Miyazawa, M. Yasunami, A. Kimura, M. Takiguchi, A. Kato, K. Mori, N. Yamamoto, T. Takemori, T. Sata, T. Kurata, K. Koike, Y. Nagai, and A. Nomoto for their help.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS in rhesus macaques by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Arguello, J. R., A. M. Little, A. L. Pay, D. Gallardo, I. Rojas, S. G. Marsh, J. M. Goldman, and J. A. Madrigal. 1998. Mutation detection and typing of polymorphic loci through double-strand conformation analysis. Nat. Genet. 18:192-194. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Che, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinberg, M. B., and J. P. Moore. 2002. AIDS vaccine models: challenging challenge viruses. Nat. Med. 8:207-210. [DOI] [PubMed] [Google Scholar]
- 6.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4:630-640. [DOI] [PubMed] [Google Scholar]
- 7.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 8.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kato, A., Y. Sakai, T. Shioda, T. Kondo, M. Nakanishi, and Y. Nagai. 1996. Initiation of Sendai virus multiplication from transfected cDNA or RNA with negative or positive sense. Genes Cells 1:569-579. [DOI] [PubMed] [Google Scholar]
- 10.Kawada, M., H. Igarashi, A. Takeda, T. Tsukamoto, H. Yamamoto, S. Dohki, M. Takiguchi, and T. Matano. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 80:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 12.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, H. O., Y. F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y. S. Lee, M. Fukumura, A. Iida, A. Kato, Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 74:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 16.Matano, T., M. Kano, H. Nakamura, A. Takeda, and Y. Nagai. 2001. Rapid appearance of secondary immune responses and protection from acute CD4 depletion after a highly pathogenic immunodeficiency virus challenge in macaques vaccinated with a DNA-prime/Sendai viral vector-boost regimen. J. Virol. 75:11891-11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 199:1709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattapallil, J. J., D. C. Douek, A. Buckler-White, D. C. Montefiori, N. L. Letvin, G. J. Nabel, and M. Roederer. 2006. Vaccination preserves CD4 memory T cells during acute simian immunodeficiency virus challenge. J. Exp. Med. 203:1533-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. A. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 21.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura, Y., C. R. Brown, J. J. Mattapallil, T. Igarashi, A. Buckler-White, B. A. Lafont, V. M. Hirsch, M. Roederer, and M. A. Martin. 2005. Resting naive CD4+ T cells are massively infected and eliminated by X4-tropic simian-human immunodeficiency viruses in macaques. Proc. Natl. Acad. Sci. USA 102:8000-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishimura, Y., T. Igarashi, O. K. Donau, A. Buckler-White, C. Buckler, B. A. Lafont, R. M. Goeken, S. Goldstein, V. M. Hirsch, and M. A. Martin. 2004. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. USA 101:12324-12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 25.Picker, L. J., and D. I. Watkins. 2005. HIV pathogenesis: the first cut is the deepest. Nat. Immunol. 6:430-432. [DOI] [PubMed] [Google Scholar]
- 26.Picker, L. J., S. I. Hagen, R. Lum, E. F. Reed-Inderbitzin, L. M. Daly, A. W. Sylwester, J. M. Walker, D. C. Siess, M. Piatak, Jr., C. Wang, D. B. Allison, V. C. Maino, J. D. Lifson, T. Kodama, and M. K. Axthelm. 2004. Insufficient production and tissue delivery of CD4+ memory T cells in rapidly progressive simian immunodeficiency virus infection. J. Exp. Med. 200:1299-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2004. Development and homeostasis of T cell memory in rhesus macaques. J. Immunol. 168:29-43. [DOI] [PubMed] [Google Scholar]
- 28.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 30.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176:362-373. [DOI] [PubMed] [Google Scholar]
- 31.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 32.Takeda, A., H. Igarashi, H. Nakamura, M. Kano, A. Iida, T. Hirata, M. Hasegawa, Y. Nagai, and T. Matano. 2003. Protective efficacy of an AIDS vaccine, a single DNA-prime followed by a single booster with a recombinant replication-defective Sendai virus vector, in a macaque AIDS model. J. Virol. 77:9710-9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veazey, R. S., K. G. Mansfield, I. C. Tham, A. C. Carville, D. E. Shvetz, A. E. Forand, and A. A. Lackner. 2000. Dynamics of CCR5 expression by CD4+ T cells in lymphoid tissues during simian immunodeficiency virus infection. J. Virol. 74:11001-11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 35.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]