Abstract
Human and animal papillomavirus DNA replicates as multicopy nuclear plasmids. Replication requires two viral proteins, the origin-recognition protein E2 and the replicative DNA helicase E1. Using genetic, biochemical, and immunofluorescence assays, we demonstrated that efficient nuclear import of the human papillomavirus (HPV) type 11 E1 protein depends on a codominant bipartite nuclear localization sequence (NLS) and on phosphorylation of the serine residues S89 and S93 by the mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase, and c-Jun N-terminal protein kinase. The NLS and the MAPK substrates are located within a 50-amino-acid-long peptide near the amino terminus, previously designated the localization regulatory region (LRR). The downstream NLS overlaps the cyclin-binding motif RRL, which is necessary for phosphorylation by the cyclin-dependent kinases to inactivate a dominant nuclear export sequence, also in the LRR. Alanine mutations of the MAPK substrates significantly impaired nuclear import, whereas phospho-mimetic mutations partially restored nuclear import. We further identified two MAPK docking motifs near the C terminus of E1 that are conserved among E1 proteins of many HPVs and bovine papillomavirus type 1. Mutations of these MAPK docking motifs or addition of specific MAPK inhibitors significantly reduced nuclear import. Interestingly, a fraction of the NLS-minus E1 protein was cotransported with the E2 protein into the nucleus and supported transient viral DNA replication. In contrast, E1 proteins mutated in the MAPK docking motifs were completely inactive in transient replication, an indication that additional properties were adversely affected by those changes.
Infections by human papillomaviruses (HPVs) can cause benign, hyperproliferative lesions of cutaneous or mucosal epithelium. The virus has a double-stranded circular DNA genome approximately 7,900 bp in length, which replicates as extrachromosomal nuclear plasmids. A low copy number of the viral DNA is maintained in the cycling basal and parabasal keratinocytes of squamous epithelium. Viral DNA amplification to produce progeny virions occurs only in postmitotic, suprabasal cells undergoing terminal differentiation (for a review, see reference 15). Initiation of replication from the origin (ori) of various HPV genotypes and bovine papillomavirus type 1 (BPV-1) depends on the virus-encoded ori binding protein E2 and the replicative DNA helicase E1 (for reviews, see references 16 and 63). The ori consists of several E2 protein binding sites flanking a cluster of E1 protein binding sites. The structures and functions of the E1 and E2 proteins of human and animal papillomaviruses are largely conserved, but significant differences are also noted. In brief, the 42-kDa E2 protein binds as dimers to the palindromic ori sequences, ACCGNNNNCGGT, and recruits the 70-kDa E1 protein via an interaction between the carboxyl terminus of E1 and the amino terminus of E2 (16). E1 then assembles into a dihexameric helicase (28, 44, 45, 60). For HPV type 11 (HPV-11), the heat shock proteins Hsp70 and Hsp40 facilitate the assembly of the E1 protein into the dihexamer (44, 45). In turn, E1 interacts with topoisomerase I (17) and replication protein A (33, 46) and, in the presence of ATP, efficiently unwinds supercoiled DNA (44, 45). Moreover, E1 recruits the DNA polymerase α/primase (4, 20, 50, 54), thereby initiating DNA replication.
The E1 DNA helicase must be actively imported into the nucleus. The classical nuclear localization sequence (NLS) consists of several basic amino acids and was first identified in the simian virus 40 T antigen as the importin α-interacting motif (31). The efficiency of nuclear import is often modulated by phosphorylation (for a review, see reference 38) or sumoylation (66). BPV-1 E1 has a bipartite NLS near the amino terminus (41, 42), and its nuclear translocation is mediated by importin α (8). Cyclin/cylin-dependent kinases (cdk's) also regulate E1 protein subcellular localization and replication activity. The E1 protein of HPV-11 or BPV-1 binds cyclin E with high affinity. BPV-1 E1 is stabilized in vitro by binding to cyclin E (25, 49). In vivo, cyclin E/cdk2 phosphorylation of the BPV-1 E1 protein enhances its nuclear export (35). In contrast, phosphorylation of HPV-11 E1 by cdk2 is critical for nuclear retention and efficient initiation of replication from the ori (27, 43, 48). In HPV-11 E1, the tripeptide R124 R125 L126 constitutes the consensus cyclin binding motif (RxL). There are four potential substrates for cdk's in HPV-11 E1: S89, S93, S107. and T468, each followed by a proline residue. S107 is located within a potent CRM1-dependent nuclear export sequence (NES) (residues 96 to 115), and phosphorylation of this residue by cyclin E/cdk2 or cyclin A/cdk2 in vivo inactivates the NES (27). When cdk2 is inhibited by p21cip1, E1 is shuttled out of the nucleus. In vitro, HPV-11 E1 is phosphorylated by cdk2 and cdk1 in complex with appropriate cyclins but not by cyclin D/cdk4 (48). Thus, the E1 protein remains in the nucleus only during S phase and G2 phase, when cdk2 and cdk1 are active, thereby coupling E1 nuclear retention and HPV DNA replication to the cell cycle.
The T468A mutation does not affect the nucleocytoplasmic localization of the E1 protein, whereas E1 S89A, S93A, or S89,93A mutations, even in combination with an NES mutation, have much-reduced nuclear import relative to that of wild type E1, suggesting the initial need for cytoplasmic kinases for efficient nuclear import (27). The stretch of 50 amino acids (residues 81 to 130) has been designated the localization regulatory region (LRR); it contains the consensus cyclin binding motif, the three serine substrates for kinases specific for S/T-P, and the NES. These motifs are highly conserved among the E1 proteins of many HPV genotypes. By sequence homology with BPV-1 E1 (NLS), the LRR of the HPV-11 E1 protein appears to contain a bipartite NLS as well. But until now, neither the NLS nor the mechanisms that govern HPV E1 nuclear import have been investigated.
In this study, we have characterized in detail the mechanisms that regulate HPV-11 E1 nuclear import. We identified a codominant bipartite NLS of HPV-11 E1 in the LRR, with the downstream NLS overlapping the consensus cyclin-binding motif. We showed that both the NLS and phosphorylation on S89 and S93 by the mitogen-activated protein kinases (MAPKs), extracellular signal-regulated kinase (ERK) and c-Jun N-terminal protein kinase (JNK), are critical for efficient E1 nuclear import. We further identified two consensus MAPK docking motifs near the carboxyl terminus of E1 that are conserved among many HPV E1 proteins and the BPV-1 E1 protein. When the E1 protein had a mutation in either of the two NLS motifs, either of the two MAPK substrate sites, or either of the two MAPK docking motifs, the majority of the HPV-11 E1 protein was localized to the cytoplasm, while exclusively nuclear localization was abolished or significantly reduced. Interestingly, transient-replication assays showed that the E1 proteins with mutations in the NLS were able to support transient replication, because a fraction of the E1 protein was coimported into the nucleus, piggy-backing on the functional partner, the E2 protein, which has a strong NLS of its own (71). In contrast, the E1 proteins with mutations in MAPK docking domains were deleteriously affected in additional properties and no longer supported replication.
MATERIALS AND METHODS
Plasmids.
pUC7730-99, the HPV-11 ori-containing plasmid, and pMT2-H11 E2, which expresses the native HPV-11 E2 protein, were described previously (14, 40). pmRFP-H11 E2 and pmRFP-H11 E2 K239A were made by swapping the enhanced green fluorescent protein (GFP) of the corresponding pGFP-H11 E2 clones (67, 71) with the monomeric red fluorescent protein (mRFP) (9) between the Eco47III and BglII sites. The wild-type HPV-11 E1 construct contains GFP fused to its amino terminus and has mutations that disable the RNA splicing donor site at nucleotide (nt) 847 (GFP-H11 E1dm) without affecting the encoded E1 amino acid sequence (26). All of the E1 mutations were prepared by site-directed mutagenesis using PCR, built on this E1dm background, and cloned into the pGFP-C1 vector (Clontech, Mountain View, CA) between BamHI and KpnI restriction enzyme cleavage sites. All constructs were verified by DNA sequencing.
Cell culture and treatment with inhibitors of MAPKs.
COS7, SiHa, and 293 cell lines were cultivated in Dulbecco's modified Eagle medium containing 10% fetal bovine serum, whereas primary human keratinocytes (PHKs) were cultivated in keratinocyte serum-free medium and 293T cells were cultivated in minimal essential medium containing 10% fetal bovine serum. DNA plasmids were transfected into 5 × 106 COS7, SiHa, or 293 cells by electroporation as described previously (14). Plasmids were transfected into PHKs by using Fugene 6 (Roche, Indianapolis, IN). In some experiments, the MAPK inhibitor, 10 nM U0126 (Cell Signaling, Beverly, MA), 5 nM SB203580 (CalBiochem, La Jolla, CA), or 30 nM SP600125 (CalBiochem) was applied for 20 h starting at 4 h posttransfection. The solvent for these three inhibitors, dimethyl sulfoxide (DMSO) (Sigma, St. Louis, MO), was used as a negative control.
Fluorescence microscopy.
Transfected cells were cultivated on dual-chamber slides for 24 h and fixed with 4% paraformaldehyde, 10 mM MgCl2, 1× phosphate-buffered saline, pH 7.2. Chromosomal DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI). The fluorescence signals were detected with an Olympus AX70 microscope equipped with Speicher filters (Chroma, Rockingham, VT) for fluorescein isothiocyanate (for GFP-H11 E1), Texas Red (for mRFP-H11 E2), and DAPI and recorded with a Zeiss Axiocam digital camera (Carl Zeiss, Oberkochen, Germany). Photoshop (Adobe, San Jose, CA) was used to compose the figures.
In vitro kinase assays.
The HPV-11 E1 wild-type protein was tagged at the amino terminus with glutathione S-transferase (GST). The proteins were expressed in Escherichia coli as described previously (48) and purified by using GST-Sepharose 4B (GE Healthcare/Amersham Biosciences, Piscataway, NJ). ATF-2 (100 ng) (Upstate, Charlottesville, VA) or purified GST-H11 E1 protein (100 or 200 ng) was mixed with 10 μCi [γ-32P]ATP and ERK1 (a gift from Fang-Tsyr Lin, University of Alabama at Birmingham), JNK1 (Upstate), or p38α (Upstate) in 1× kinase buffer (20 mM Tris-Cl [pH 7.4], 50 μM ATP, 10 mM MgCl2, and 10 mM dithiothreitol). The mixtures were incubated at room temperature for 30 min and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The incorporated 32P signals were detected by Storm 860 PhosphorImager (GE Healthcare/Amersham Biosciences/Molecular Dynamics).
Coimmunoprecipitation and Western blot analyses.
Five micrograms (each) of expression vectors of wild-type GFP-E1dm or mutations therein were separately transfected into COS7 cells. After 24 h, cells were harvested in lysis buffer (60 mM Tris-Cl [pH 6.8], 1% SDS), and total protein in each lysate was quantified using the bicinchoninic acid reagent (Pierce, Rockford, IL). The same amount of total protein from each lysate was separated in 10% SDS-PAGE, followed by immunoblotting with mouse monoclonal anti-GFP antibody (Clontech) or horseradish peroxidase (HRP)-conjugated goat antiactin antibody (Santa Cruz Biotech, Santa Cruz, CA). For the MAPK inhibitor experiments, the active or total kinases were detected with rabbit polyclonal anti-phospho-ERK (Promega, Madison, WI), mouse monoclonal anti-ERK (BD Transduction Laboratories, San Jose, CA), rabbit polyclonal anti-phospho-JNK (Cell Signaling), or mouse monoclonal anti-JNK (Santa Cruz Biotech). Phospho-p38 or total p38 protein was detected with rabbit polyclonal anti-phospho-p38 (Promega) or rabbit polyclonal anti-p38 (Santa Cruz Biotech). The p38 substrate HSP27 was detected with rabbit polyclonal anti-phospho-HSP27 (Upstate) or with goat polyclonal anti-HSP27 antibodies (Santa Cruz Biotech). The signals were detected by using X-ray film, using the ECL (enhanced chemiluminescence) system (GE Healthcare/Amersham Biosciences).
For coimmunoprecipitation experiments, an expression plasmid of hemagglutinin (HA)-tagged ERK1 (a gift from Jacques Pouyssegur, University of Nice, Nice, France) or FLAG-tagged JNK1 (a gift from Fang-Tsyr Lin, University of Alabama at Birmingham) was transfected as a calcium phosphate coprecipitate with GFP, GFP-E1dm wild type, DD1m, DD2m, or DD1,2m into the 293T cell line. Cells were harvested in TGH buffer (1% Triton X-100, 10% glycerol, 50 mM HEPES [pH 7.4], 150 mM NaCl, 1 mM EGTA, and 1 mM EDTA) supplemented with a mixture of protease inhibitors (phenylmethylsulfonyl fluoride, leupeptin, antipain, benzamidine, chymostatin, and pepstatin A) and phosphatase inhibitors (sodium pyrophosphate, sodium fluoride, and sodium orthovanadate) as described previously (70) and coimmunoprecipitated with anti-HA beads (Covance, Philadelphia, PA) or anti-FLAG M2 beads (Sigma) overnight. After washing with TGH buffer, the precipitates were separated by 10% SDS-PAGE. The Western blots were then probed with a polyclonal anti-GFP (Santa Cruz Biotech), a polyclonal anti-FLAG (Sigma), or a polyclonal anti-HA (Santa Cruz Biotech) antibody.
Transient replication assay.
Five micrograms of pMT2-H11 E2, 1 μg of pGFP-H11 E1dm (wild type or mutation), and 0.5 μg of HPV-11 ori-containing plasmid were cotransfected into 293 cells by electroporation as described previously (26). The low-molecular-weight DNA was harvested 48 h posttransfection, using methods reported previously (14). The purified DNA was digested with HindIII alone or with HindIII and DpnI together, separated by 0.8% agarose gel electrophoresis, and Southern blotted onto Hybond-N+ membrane filters (GE Healthcare/Amersham Biosciences). [α-32P]dCTP-labeled ori DNA probes were synthesized with the Megaprime DNA labeling system (GE Healthcare/Amersham Biosciences) and used as hybridization probes. The amounts of the radioisotope incorporated into the de novo-synthesized DNA were quantified using the ImageQuant program (GE Healthcare/Amersham Biosciences).
RESULTS
The HPV-11 E1 protein is encoded by an open reading frame spanning genomic nt 832 to 2781. We have shown that a fusion protein of GFP and HPV-11 E1 (GFP-H11 E1) supports transient replication as efficiently as the wild-type E1 protein (26). However, the majority of the transcripts from a native E1 or GFP-E1 fusion expression vector contain an intragenic splice from the preferred splice donor site at nt 847 (within the sixth codon) to nt 2622 near the carboxyl terminus, just upstream of the E2 open reading frame, which overlaps the E1 coding region. This splice leads to a frame shift and translation termination just a few residues downstream of the splice acceptor. Thus, the great majority of the protein generated from the expression vector is a short peptide or a GFP fused to this short peptide (26). Consequently, using the wild-type gene, it is not possible to observe the nucleocytoplasmic localization of the full-length E1 fusion protein by fluorescence microscopy. However, site-directed mutation in the dominant splicing-donor site that does not affect the encoded protein sequence (GFP-H11 E1dm) efficiently abolishes the predominant splice and results in abundant full-length GFP-H11 E1, the location of which can be easily and accurately tracked. Moreover, relative to the E1 or GFP-H11 E1 wild type, only 1/10 to 1/5 of the GFP-H11 E1dm expression plasmid is sufficient to support efficient transient replication (26, 27). Therefore, all the E1 mutations used in this study were built on this GFP-E1dm backbone.
HPV-11 E1 possesses a bipartite NLS, with the downstream component overlapping the consensus cyclin binding motif (RxL).
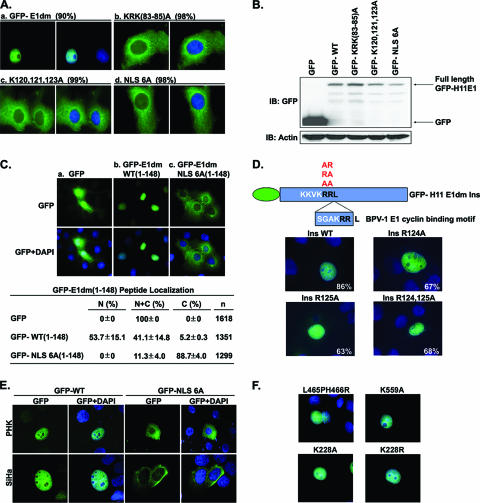
On the basis of sequence homology with BPV-1 E1, the putative bipartite NLS of HPV-11 E1 is predicted to consist of KRK (residues 83 to 85) and KKVK (residues 120 to 123) (see Fig. 5A). We mutated the arginine and lysine residues in one or both (NLS 6A) of these motifs to alanine residues in GFP-E1dm. The expression vectors were then separately transfected into COS7 cells, and the nucleocytoplasmic distribution of the GFP fusion protein was visualized microscopically. Five hundred GFP-positive cells were scored in each experiment. Either or both mutations virtually abolished nuclear localization of GFP-E1dm, with 98% to 99% of the cells exhibiting exclusively cytoplasmic signals (Fig. 1Ab, c, and d). In contrast, about 80 to 90% of cells exhibited exclusively nuclear signals when transfected with the vector expressing GFP-E1dm (27) (Fig. 1Aa; see also Fig. 2A). To ensure that GFP-E1 localization was not compromised by protein degradation, protein products expressed from the plasmids were analyzed by Western blotting using a polyclonal antibody to GFP (Fig. 1B). The majority of the GFP fused to either the wild type or mutated forms of E1 was a full-length protein. There was little difference in the small amounts of shorter fragments in cells transfected with different expression vectors. Therefore, the dramatic distinction in subcellular localization among the wild-type and mutated E1 fusion proteins cannot be attributed to differential protein degradation.
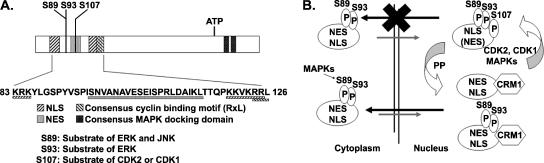
FIG. 5.
Mechanisms governing HPV-11 E1 nucleocytoplasmic localization. (A) Interdigitated regulatory elements in the LRR near the amino terminus of the HPV-11 E1 protein that control its subcellular localization, as identified in the present study and in our previous report (27, 48). (B) Model for mechanisms governing E1 nucleocytoplasmic shuttling. The E1 protein is largely cytoplasmic until signal transductions activate MAPKs, in particular ERK and JNK, which in turn activate the E1 NLS by phosphorylating S89 and S93. Once imported into the nucleus, the protein is efficiently exported back to the cytoplasm by the dominant CRM-1-dependent NES unless its NES is inactivated upon phosphorylation of S107 by cdk2 or cdk1, kinases that are available during S and G2 phase in the presence of the appropriate cyclins. When the cell exits the G2 phase or the cell cycle all together, E1 dephosphorylated on S107 by protein phosphatases is exported by the potent NES out of the nucleus. Thus, E1 nuclear retention is coupled to viral DNA replication and the cell cycle so that it will not unwind the viral DNA when cells cannot support viral DNA replication for lack of host DNA replication machinery or substrates.
FIG. 1.
Nuclear localization of wild-type and mutant forms of HPV-11 E1 fused to GFP. (A) Identification of a codominant bipartite NLS. Expression plasmids of GFP-E1dm wild type or with the KRK(83-85)A, K120,121,123A, or NLS 6A mutation were separately transfected into COS7 cells. Four hundred to five hundred GFP-positive cells were scored, and representative images are presented. Numbers indicate percentages of transfected cells with exclusively nuclear (panel a) or exclusively cytoplasmic (panels b to d) GFP signals. (B) Immunoblot analysis for GFP fusion protein expression. Expression of GFP or GFP-11 E1 protein was detected by anti-GFP antibody. Actin was detected by antiactin antibody as an internal loading control. (C) Representative images of GFP (panel a), GFP-E1dm WT(1-148) (panel b), or GFP-E1dm NLS 6A(1-148) (panel c) fusion peptide in transfected COS7 cells. Numerical data and standard deviations of the subcellular distribution of GFP fusion signals in all positive cells (n) are also presented. N, exclusively nuclear; C, exclusively cytoplasmic; N+C, pan-cellular. The experiments were repeated three times independently. (D) The downstream NLS overlaps the cyclin binding motif. Top panel, a schematic representation of GFP-E1dm with an insertion of six amino acids, SGAKRR, which constitute the putative BPV-1 E1 cyclin binding motif. Bottom panels, representative images of COS7 cells transfected with expression vectors of GFP-E1dm with the SGAKRR insertion or with this insertion as well as the R124A, R125A, or R124,125A mutations. Numbers represent the percentages of GFP-positive cells with exclusively nuclear GFP signals. (E) GFP-H11E1 wild type or NLS 6A localization in PHKs or HPV-16-transformed cervical cancer cell line, SiHa. (F) Nuclear localization of GFP-H11 E1 with mutations in residues that might affect sumoylation based on homology to BPV-1 E1 protein or consensus SUMO site. The GFP-H11E1 L465PH466R, K559A, K228A, or K228R protein was individually expressed in COS7 cells. Nuclei were stained blue with DAPI in Fig. 1A, C, D, E, and F.
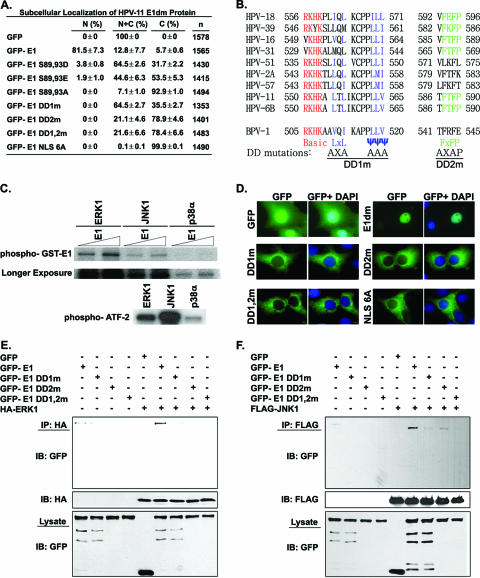
FIG. 2.
HPV-11 E1 is a substrate of ERK and JNK. (A) Numerical data on the nucleocytoplasmic localization of GFP-E1 proteins, with approximately 500 positive cells counted for each of three independent experiments. N, exclusively nuclear; N+C, pan-cellular; C, exclusively cytoplasmic. (B) Amino acid sequence alignment of the carboxyl-terminal portion of E1 helicase from different papillomaviruses. The consensus MAPK docking motif and the mutations DD1m and DD2m are illustrated. (C) HPV-11 E1 is a substrate of ERK and JNK in vitro. One hundred nanograms of ATF-2 and 100 or 200 ng of bacterially expressed GST-11E1 protein were each incubated with individual MAPKs in kinase assays in vitro. (D) Nucleocytoplasmic localization of E1 mutated in the putative MAPK docking domains DD1 and DD2. Expression vectors of GFP-E1dm wild type, DD1m, DD2m, DD1,2m, NLS 6A, or GFP were separately transfected into the COS7 cell line. Representative images are presented. Nuclei were stained blue with DAPI. (E) Coimmunoprecipitation of HA-ERK1 and GFP, GFP-11E1 wild type, DD1m, DD2m, or DD1,2m from transfected cells. (F) Coimmunoprecipitation of FLAG-JNK1 and GFP, GFP-11E1 wild type, DD1m, DD2m, or DD1,2m from transfected cells. The lysates and precipitates were immunoblotted with the indicated antibodies.
To verify the conclusion that the short basic peptides identified above indeed function as an NLS, we fused GFP to the peptide spanning amino acids (aa) 1 to 148 from wild-type GFP-E1dm or the NLS 6A mutated form and examined their localization in COS7 cells. GFP was dispersed throughout the cell as expected (Fig. 1Ca). The GFP-E1dm(1-148) wild-type peptide had about 54% exclusively nuclear GFP signal, indicative of NLS activity (Fig. 1Cb). In contrast, the GFP-E1dm NLS 6A(1-148) peptide was exclusively cytoplasmic in 88.7% of the GFP-positive cells (Fig. 1Cc). These observations support our interpretation that the two basic peptides identified by mutational analysis function as NLS.
Since the two arginine residues (R124 and R125) in the consensus cyclin binding motif RRL (48) immediately follow the downstream NLS (i.e., KKVKRRL), we wanted to know if these two additional residues are a part of the NLS. The RRL sequence in HPV-11 E1 is essential for cyclin binding, and phosphorylation by cdk2 is essential for E1 nuclear retention and for efficient transient or cell-free DNA replication (27, 43, 48). To avoid affecting E1 phosphorylation by cdk2, we inserted six amino acid residues (SGAKRR) between R125 and L126 (E1dm Ins). This insertion generated a second copy of a putative cyclin-binding motif RRL, which is found in BPV-1 E1 (Fig. 1D). Then, into this insertion mutation, Arg124, Arg125, or both were then mutated to alanine residues. For GFP-E1dm, which has the wild-type NLS and the SGAKRR insertion (E1dm Ins), 86% of the GFP-positive cells exhibited a nuclear GFP signal, similar to the parental GFP-E1dm without the insertion. Thus, this putative BPV-1 cyclin E binding motif indeed functions in the sequence context of the HPV-11 E1 protein as well as the original HPV-11 cyclin binding sequence. As shown in Fig. 1D, when either or both of the R124 and R125 residues were mutated to alanine in GFP-E1dm Ins, the percentage of the cells exhibiting exclusively nuclear GFP-E1dm signals was reduced to 63% to 68%, whereas 26% to 22% of the cells showed both nuclear and cytoplasmic signals and about 10% of the cells had exclusively cytoplasmic signals. Thus, we conclude that the downstream NLS overlaps and includes part of the consensus cyclin binding motif, but the contribution of R124 and R125 is not as significant as that of K120, K121, and K123.
To rule out the possibility that the E1 localization observed in the simian virus 40-transformed COS7 cells may not reflect the true properties of E1, the localization of the GFP-E1dm wild type or the NLS 6A form was examined in primary human keratinocytes, the natural host for HPVs, and in SiHa cells, an HPV-16-transformed carcinoma cell line. Although both cell types had a significantly lower transfection efficiency and reduced GFP-H11 E1dm protein expression relative to those of COS7 cells, the conclusions were the same. Only the GFP-E1 wild type but not the GFP-E1 NLS 6A mutant was imported into the nucleus (Fig. 1E). These observations validate the use of COS7 cells in E1 localization studies. Collectively, these results demonstrate that E1 contains a bipartite NLS near its N terminus and that either motif alone is not sufficient to promote nuclear import.
HPV-11 E1 nuclear import is enhanced by negative charges on Ser89 and Ser93.
Because alanine substitutions for Ser89 and Ser93 caused a reduction of 11E1 nuclear import (27), we inferred that the phosphorylation of those two serine residues by kinases could be important for efficient nuclear localization. To examine this possibility, we constructed phosphor-mimetic GFP-E1dm mutants in which S89 and S93 were mutated to Glu or Asp. These mutants were transfected into COS7 cells. Figure 2A summarizes the subcellular localization of each fusion protein. Exclusively cytoplasmic signals were observed in 92.9% of the cells expressing E1 89,93A, with no cell showing an exclusively nuclear signal. In contrast, the S89,93D or S89,93E mutations were seen with increased E1 nuclear import, with 2 to 4% of the cells showing purely nuclear signals and 64.5% and 44.6% of cells showing pan-cellular signals, respectively. Although these substitutions did not restore nuclear import to the extent observed with the wild-type protein, the results are consistent with the interpretation that negative charges on these residues, normally conferred by phosphorylation, enhance HPV-11 E1 nuclear import.
MAPKs phosphorylate HPV-11 E1 in vitro.
Since cyclin E/cdk2 and cyclin A/cdk2 are nuclear kinases, there must be cytoplasmic kinases that phosphorylate S89 and S93 to facilitate E1 nuclear import. Two additional families of kinases are known to phosphorylate serine or threonine followed by a proline residue. One is glycogen synthase kinase 3β, which phosphorylates S/T after a +4 S/T is phosphorylated by other kinases (for a review, see reference 19). Thus, in principle, S89 but not S93 could be a substrate. However, we consider this possibility unlikely, because in HPV lesions or in cells expressing viral E5 or E7, AKT is activated (7, 39, 56, 68), and it would have inactivated glycogen synthase kinase 3β (18, 21, 62). The other functionally logical candidates are the mitogen-activated protein kinases that respond to various signaling pathways. MAPKs comprise three major families, ERK, JNK, and p38, that are themselves activated in a signaling cascade mediated via phosphorylation by MAPK kinase on the TXY motif (for a review, see reference 51). The ERK family is predominantly activated when cells undergo proliferation, whereas the activation of JNK and that of the p38 family are usually associated with the stress and inflammatory responses (for a review, see reference 65).
To examine the possibility that MAPKs can phosphorylate the E1 protein, we first looked for the presence of the MAPK docking motif in the E1 protein. The classical docking sequence is a basic amino acid cluster followed by LxL or a hydrophobic ψψψ motif (for a review, see reference 65). A novel docking sequence, FxFP, is also recognized by ERK and p38α (29, 37). Sequence inspection revealed the potential existence of both types of docking motifs near the carboxyl terminus of the HPV-11 E1 protein. We designated them MAPK DD1 (the upstream docking domain motif) and DD2 (the downstream motif), both of which are highly conserved among the E1 protein sequences of many HPV genotypes and BPV-1 (Fig. 2B).
Next, we determined whether one or more MAPKs can indeed phosphorylate the E1 protein by conducting in vitro kinase assays on a bacterially expressed HPV-11 E1 protein tagged with GST (GST-E1). The purified ATF-2 protein was used as a positive control. The results showed that E1 was phosphorylated by ERK1 and JNK1 in vitro, whereas phosphorylation by p38α was very inefficient. However, p38α also had low activity on the ATF-2 substrate in this assay relative to those of the other MAPKs (Fig. 2C). The positive control, ATF-2, was phosphorylated by each of the MAPKs much more efficiently than E1. This difference could be attributed to two factors: first, the ATPase activity of E1 would reduce the concentration of [α-32P]ATP, the substrate for the kinases, hence their activities. Second, ATF-2 has more substrate sites for each of the kinases than has E1.
HPV-11 E1 mutations in the putative MAPK docking sequences reduce binding to MAPKs and nuclear import.
To investigate whether the DD1 or DD2 motif indeed binds the MAPKs, we constructed GFP-E1dm with a mutation in either or both of these putative docking sequences, designated DD1m, DD2m, and DD1,2m. HA-tagged ERK1 or FLAG-tagged JNK1 was then cotransfected with GFP, GFP-E1dm wild type, or GFP fused to single or double docking motif mutations. Coimmunoprecipitation followed by immunoblotting showed that single and double mutations in the putative docking motifs abolished binding to ERK1, even though the mutated E1 fusion proteins were each expressed to a level similar to that of wild-type GFP-E1 (Fig. 2E). Thus, both docking motifs are necessary for E1 association with ERK1. GFP-E1 DD1m and DD2m each reduced the binding to JNK1. As was observed with ERK1, the double mutation abolished binding to JNK1 (Fig. 2F). Thus, JNK1 can bind to either docking motif, but the complex is more stable upon binding to both sequences.
To examine whether MAPKs regulate E1 nuclear import, expression vectors of the docking motif mutations were separately transfected into COS7 cells and the localization of GFP-E1 was determined by fluorescence microscopy. No cells harboring the single or double MAPK docking motif mutations exhibited a purely nuclear E1 signal. Rather, signals with the single mutations either were completely cytoplasmic or were pan-cellular. The defective DD2 mutation was more severe than that of the DD1 mutation. However, the effect of the double mutation was not more severe than that of DD2m alone. Parallel controls consisted of cells expressing GFP, GFP-E1dm, or GFP-E1dm NLS 6A, each of which had the expected localization (Fig. 2A and D). These results suggest that phosphorylation by MAPKs is critical to promote efficient E1 nuclear import mediated by the NLS.
Inhibitors of ERK and JNK reduce GFP-H11 E1 nuclear import.
To substantiate the above interpretations, we treated COS7 cells with one of several specific MAPK inhibitors after transfection of COS7 cells with the GFP-E1dm expression vector. First, we examined the efficacies of these inhibitors for the respective MAPKs under the conditions used for localization of GFP-E1. Western blots for phosphorylated ERK, JNK, and HSP27, which is a p38-specific substrate (5), confirmed that each kinase was effectively inactivated, but only by the respective inhibitor. Interestingly, compensatory induction of untargeted MAPKs was evident (Fig. 3A).
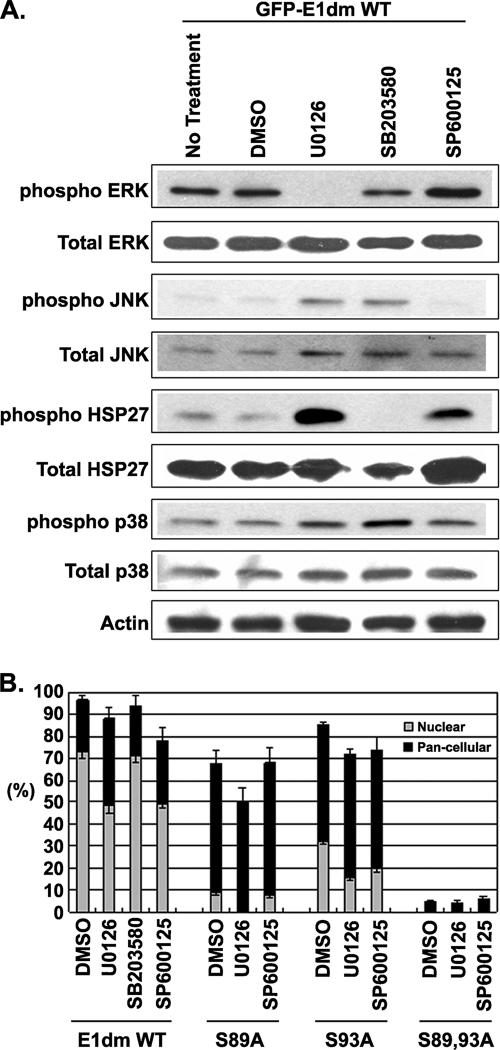
FIG. 3.
Decreased E1 nuclear import in the presence of MAPK inhibitors. (A) Western blot for phosphorylated or total ERK, JNK, HSP27, and p38 in the presence of specific inhibitors, the solvent DMSO, or no treatment. Each agent was specific and effective in the total inhibition of the target MAPKs. Compensatory inductions of untargeted MAPKs were evident. (B) Identification of serine substrates of MAP kinases in GFP-E1dm, E1 89A, E1 93A, and E1 89,93A. E1 expression plasmids were individually transfected into COS7 cells. Four hours posttransfection, the cells were treated for 20 h with the indicated MAPK inhibitor or with control DMSO solvent. Three independent experiments were performed, and approximately 500 positive cells were scored in each transfection, with error bars to indicate the standard deviations. Boxes represent the percentages of cells with exclusively nuclear GFP signals (gray) or pan-cellular signals (black). ERK and JNK inhibitors, but not a p38α inhibitor, reduced nuclear import of all E1 proteins, with the exception of E1 S89,93A, which was not affected.
Next, we examined GFP-E1 localization. Treatment with the solvent DMSO reduced the fraction of cells having an exclusively nuclear signal from 90% to about 73% of all positive cells. In addition, 23.7% of the treated cells exhibited pan-cellular signals. SB203580, an inhibitor of p38α, had no effect on this distribution pattern, indicating that it phosphorylates E1 very inefficiently in vivo, as observed in vitro (Fig. 3B). However, it is possible that its small contribution was masked by the compensatory induction in ERK and JNK (see Fig. 3A). In contrast, U0126 and SP600125, which specifically inhibit ERK and JNK, respectively, each reduced the proportion of cells exhibiting purely nuclear signals to about 50% (Fig. 3B), while cells with pan-cellular signals increased to 39.4% and 28.9%, respectively. Collectively, these results support the conclusion that ERK and JNK can activate E1 nuclear import.
Identification of the substrates of ERK and JNK.
We were curious as to why specific and effective inhibition of MAPKs by individual inhibitors (Fig. 3A) only partially reduced E1 nuclear import compared to the effects of the S89 and S93A mutations in the absence of any inhibitor (Fig. 2A and 3B). One possibility is that at least one of the Ser residues can be phosphorylated by both kinases such that a single inhibitor cannot completely abolish phosphorylation. Significant cytotoxicity prevented us from testing this hypothesis by treating the cells with both ERK and JNK inhibitors simultaneously. Thus, we examined the effects of MAPK inhibitors on GFP-E1dm S89A and GFP-E1dm S93A. COS7 cells were separately transfected with each of the expression vectors. The cells were then treated with the ERK or JNK inhibitor or with DMSO as just described, and the results are presented in Fig. 3B.
In the presence of the control solvent DMSO, the E1dm S89A and E1dm S93A mutations reduced the percentages of cells with entirely nuclear GFP-E1 signals to 9% and 32%, respectively, compared to 73% for the wild-type protein. Conversely, the pan-cellular GFP-E1 signal increased to 59% and 53%, respectively, compared to 24% for the wild-type protein. These observations suggest that phosphorylation on S89 has a greater positive impact on NLS activity than phosphorylation on S93. Upon treatment with the ERK inhibitor U0126, exclusively nuclear localization by the E1dm S89A mutant form was virtually abolished (0.4%) while the percentage of cells with exclusively nuclear E1dm S93A signal was reduced from 32% to 16%. In contrast, the JNK inhibitor SP600125 had no effect on the nucleocytoplasmic localization of the S89A mutation, but it reduced the proportion of cells with exclusively nuclear E1 S93A signals from 32% to 20%. We interpret these data to mean that ERK can phosphorylate both S89 and S93, whereas JNK can phosphorylate only S89. This interpretation is consistent with the partial reduction of E1 nuclear localization by either MAPK inhibitor. It would explain the significant fraction of cells with exclusively nuclear and pan-cellular E1 S93A in the presence of either MAPK inhibitor, because E1 S89 can be phosphorylated by the remaining active MAPK. It would also account for cells with exclusively nuclear and pan-cellular E1 S89A in the presence of the JNK inhibitor (Fig. 3B), because the ERK can still phosphorylate S93. However, we were unable to verify this interpretation by in vitro kinase reactions on E1 S89A and E1 S93A due to extensive degradation of bacterially expressed proteins.
We also examined the GFP-E1dm S89,93A mutant form of E1 in parallel experiments. In the presence of DMSO, more than 95% of this mutant form of E1 was localized exclusively in the cytoplasm, with the balance being pan-cellular. Importantly, this protein distribution pattern was not affected by either ERK or JNK inhibitor (Fig. 3B), ruling out the possibility of additional MAPK substrates (such as S107 and T468) that can significantly influence nuclear import. Collectively, the data verify that phosphorylation of both S89 and S93 is critical for efficient HPV-11 E1 nuclear import.
E1 with NLS mutations can support viral DNA replication in vivo with various efficiencies.
Since nuclear localization is essential to E1 helicase function during DNA replication, once nuclear E1 protein is reduced or abolished by the NLS mutations, viral DNA replication can be expected to be negatively affected. We tested several of the NLS mutation proteins for transient replication in 293 cells. E1dm Ins functioned as well as the wild-type E1 protein. Not surprisingly, GFP-E1dm Ins R124,125A, which was transported into the nucleus with only a slightly reduced efficiency relative to that of the parental protein, supported transient replication efficiently (Fig. 4A, compare lanes 10, 12, and 2). But notably, the E1 KRK(83-85)A, K120,121,123A, and NLS 6A proteins, which were virtually incapable of nuclear import by themselves, also supported transient replication. E1dm KRK(83-85)A had a replication efficiency comparable to that of the wild-type protein, while E1dm K120,121,123A and E1dm NLS 6A exhibited reduced efficiencies (Fig. 4A, compare lanes 2, 4, 6, and 8). Western blots showed that the amount of each E1 protein expressed was not significantly different in cells cotransfected with E1 and E2 expression plasmids (data not shown). The transient-replication assays were repeated two more times, and the results were similar to those shown in Fig. 4B, in which E1 with NLS mutations supported replication with a reduced efficiency relative to that of wild-type E1.
FIG. 4.
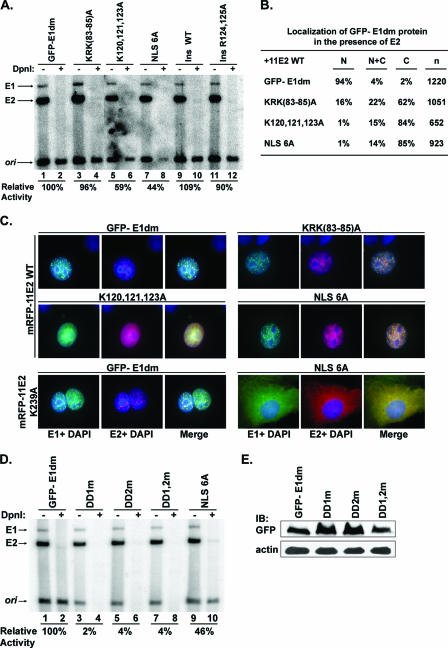
NLS mutations but not MAPK docking domain mutations support transient replication. (A) NLS mutations support transient DNA replication from the HPV-11 origin. GFP-E1dm wild type or with NLS mutations was cotransfected with pMT2-H11 E2 and HPV-11 ori-containing DNA into 293 cells. The low-molecular-weight DNA was digested with Hind III alone or with HindIII plus DpnI and then subjected to Southern blot hybridization. DpnI I-resistant bands represent newly synthesized ori DNA. (B) Nuclear import of GFP-E1dm with NLS mutations in the presence of the wild-type HPV-11 E2 protein. GFP-E1dm or NLS mutants were cotransfected into COS7 cells with pMT2-H11 E2. Six hundred to twelve hundred GFP-positive cells were scored. The numbers represent the percentages of transfected cells with exclusively nuclear (N), pan-cellular (N+C), or exclusively cytoplasmic (C) GFP signals. n, total number of positive cells. (C) Localization of GFP-E1dm or NLS mutant forms in the presence of wild-type or mutant forms of HPV-11 E2, each fused to mRFP. GFP-E1dm expression plasmids were each cotransfected with the expression plasmid of mRFP-H11 E2 wild type (top and middle rows) or with mRFP-H11 E2 K239A, which is mutated in the NLS (67) (bottom row). Nuclei were stained blue with DAPI. (D) Transient HPV ori replication in 293 cells in the presence of the native HPV-11 E2 and GFP-E1dm wild type, MAPK docking motif mutation proteins, or NLS 6A protein. (E) Expression of wild-type E1 and MAPK docking motif mutants in COS7 cells. The GFP-E1dm protein was detected with monoclonal anti-GFP antibody followed by HRP-conjugated antimouse secondary antibody. Actin was detected with HRP-conjugated goat antiactin antibody as a loading control. These experiments were performed twice, with the same results.
The NLS-mutated E1 protein can be coimported with the E2 protein into the nucleus.
The ability of the mutant forms of E1 impaired in nuclear import to support transient HPV ori replication suggests that the known E1-E2 protein interaction may have enabled nuclear coimportation of at least some of the mutated E1 protein. This hypothesis was tested with COS7 cells after cotransfection of expression vectors of HPV-11 E2 and GFP-E1dm wild type or with NLS mutations. Indeed, the fractions of cells exhibiting nuclear signals increased for both the wild-type and the mutant forms of GFP-E1dm (Fig. 4B). For wild-type GFP-E1dm, 94% of the cells had exclusively nuclear signals. Fourteen to twenty-two percent of the cells expressing E1 with mutations in the bipartite NLS exhibited pan-cellular signals. Moreover, 16% of cells expressing E1dm KRK(83-85)A had an exclusively nuclear signal. In contrast, the fraction of cells with exclusively nuclear E1dm K120,121,123A or E1 NLS 6A remained very low. Interestingly, these last two mutant forms of the E1 protein had a reduced replication efficiency relative to that of E1 KRK(83-85)A (Fig. 4A). Thus, replication activities of the E1 protein correlate with the efficiency of nuclear import in the presence of E2.
The hypothesis that cells containing nuclear E1 indeed also expressed the E2 protein was further tested. Expression plasmids of GFP-E1dm and mRFP-H11 E2 were cotransfected into COS7 cells (Fig. 4C, top and middle panels). The HPV-11 E1 and E2 proteins colocalized as nuclear foci (64, 71). Colocalized GFP-E1dm and mRFP-H11 E2 over DAPI would appear as white dots. Indeed, white nuclear dots were observed with E1dm, E1dm KRK(83-85)A, and E1dm NLS 6A. For reasons not yet understood, in repeated experiments with E1dm K120,121,123A, few such punctate signals were observed when the E1 protein was located exclusively in the nucleus; but nuclear dots were observed when the E1 protein was pan-cellular (data not shown). In the control experiments, GFP or mRFP alone was pan-cellular and did not affect the localization of mRFP-H11 E2 or GFP-E1dm, respectively (data not shown). Furthermore, cotransfection of GFP-E1dm NLS 6A and mRFP-H11 E2 K239A, which is cytoplasmic due to an inactivated NLS (67), resulted in only cytoplasmic E1 and E2 (Fig. 4C, bottom panels). These data strongly suggest that a fraction of the E1 protein mutated in the NLS can be coimported with the E2 protein into the nucleus when both are expressed in relatively high abundance from expression vectors.
E1 proteins with mutated MAPK docking motifs do not support transient replication.
A significant fraction (21% or 64%) of the cells expressing GFP-E1 mutated in the MAPK docking motifs exhibited a pan-cellular localization (Fig. 2A). However, these fusion proteins were totally inactive in transient replication in repeated experiments (Fig. 4D; also data not shown). Western blots of transfected COS7 cells revealed that the mutant forms of the E1 protein were expressed to levels comparable to that of the wild type protein (Fig. 4E). These observations suggest that MAPK docking mutations have additional defects.
Mutant forms of HPV-11 E1dm comparable to those critical for BPV-1 E1 sumoylation.
Posttranslational modification by SUMO, the small ubiquitin-like modifier, is similar to the ubiquitination process and needs E1, E2, and E3 ligase. The SUMO protein is activated by E1-activating enzyme, transferred onto the E2-conjugating enzyme ubc9, and then ligated to target proteins by E3-like ligases (i.e., PIAS, Pc2, or RanBP2). Sumoylation has been reported to affect the target proteins in nuclear domain localization, transcriptional activity, and nucleocytoplasmic translocation (for a review, see reference 52). Binding to ubc9 and sumoylation of BPV-1 E1 were reported to be critical for the nuclear import of BPV-1 (57, 58). The HPV-11 E1 protein is sumoylated in vitro by the PIAS family of E3 ligases (except PIASy), but the site of modification was not determined, nor was its impact on E1 nuclear import known (59). We were curious whether sumoylation also affects the nucleocytoplasmic localization of the HPV-11 E1 protein. L465 and H466 of HPV-11 E1 are equivalent to BPV-1 E1 residues critical for ubc9 binding, whereas K559 is equivalent to the BPV-1 E1 K514 sumoylation site. In addition, we identified in HPV-11 E1 a short peptide, FKSD (residues 227 to 230), which fits the consensus sumoylation ΨKXD/E motif (for a review, see reference 61). We constructed and tested four GFP-E1dm mutations, E1dm L465P, H466R, E1dm K559A, E1dm K228A, and E1dm K228R. All four mutant forms of E1 exhibited a normal phenotype in nuclear import (Fig. 1F).
DISCUSSION
In this study, we have determined that multiple mechanisms regulate nuclear import of the HPV-11 E1 protein. We have demonstrated that efficient nuclear import requires both a bipartite NLS and phosphorylation on S89 and S93 by the MAPKs ERK and JNK. We note both similarities and differences to mechanisms that govern the nuclear localization of the BPV-1 E1 protein. We present a model to explain how these mechanisms might operate in the context of a productive HPV infection in the squamous epithelium.
A comparison of mechanisms governing the nuclear localization of HPV-11 E1 and BPV-E1.
We have identified within the HPV-11 E1 protein a codominant bipartite nuclear localization sequence, KRK (aa 83 to 85) and KKVKRR (aa 120 to 125). Both motifs are found within the LRR near the amino terminus. We have shown that the downstream NLS component overlaps the consensus cyclin binding motif, RRL. Interestingly, the NLS functions much more efficiently in the sequence context of the full-length protein than with the amino-terminal peptide (Fig. 1A and C), because the primary MAPK docking domains are located at the carboxyl terminus of the protein (Fig. 2B) (to be discussed). Conversely, the amino-terminal peptide, which lacks the MAPK docking motifs, was imported into the nucleus more efficiently than E1dm with mutations in the MAPK docking motifs (compared Fig. 1C and 2A). The latter result might be attributed in part to the small size of the fusion peptide, allowing a low level of nuclear import by diffusion. It is also possible that a relatively less structured conformation of the short peptide may have allowed it to interact with MAPKs in the absence of the docking motifs or may have facilitated its interaction with importins, leading to increased nuclear import.
The bipartite nature of the HPV E1 NLS is similar to that found in the BPV-1 E1 protein, located at residues 84 to 86 and 105 to 108. But unlike the codominant HPV-11 E1 NLS, the upstream NLS of the BPV-1 E1 dominates over the downstream sequence (42). Additional, more significant differences are noted in the mechanisms that regulate the nucleocytoplasmic localization of the E1 proteins of HPV-11 and BPV-1. First, our experiments show that in the region comparable to the LRR of the HPV-11 E1 protein, the BPV-1 E1 protein has a similar cycling binding motif (Fig. 1D) but it lacks the NES. Furthermore, phosphorylation by cyclin E/cdk2 promotes BPV-1 E1 nuclear export (35), as opposed to the nuclear retention observed for HPV-11 E1 (27). Second, unlike the case with HPV-11 E1, MAPKs have not been implicated in the regulation of the nucleocytoplasmic localization of BPV-1 E1, although a conserved MAPK docking domain is also found in the carboxyl terminus of the BPV-1 E1 protein (Fig. 2B). Third, sumoylation of BPV-1 E1 was reported to be critical for the nuclear import of BPV-1 (57, 58). Our mutational analysis of comparable mutations in HPV-11 E1 did not reveal any altered properties relative to the wild-type protein (Fig. 1F). However, we had no data to suggest whether or not any of these mutations affected E1 sumoylation. The possible significance of sumoylation of HPV-11 E1 remains to be determined.
Activation of HPV-11 E1 NLS by MAPKs.
We presented several lines of evidence strongly suggesting that the HPV-11 E1 ATPase/DNA helicase is a substrate of MAPKs and that phosphorylation of both S89 and S93, in addition to the NLS, is crucial for efficient E1 nuclear import. First, E1 binds ERK1 and JNK1 in vivo (Fig. 2E and F) and is phosphorylated in vitro by ERK and JNK (Fig. 2C). Second, we identified a MAPK docking domain near the carboxyl terminus (aa 550 to 590 in HPV-11 E1) which is conserved among E1 proteins for many HPV genotypes as well as for BPV-1 (Fig. 2B). This domain is comprised of two docking motifs, designated DD1 (RKHRALTLIKCPPLLV) and DD2 (FTFP). Mutation of DD2 affected E1 nuclear importation more severely than that of DD1. Since the effects of the two mutations were not additive, these MAPKs may simultaneously contact the two closely spaced motifs. Indeed, mutation in either or both motifs reduces or abolishes binding to ERK1 and JNK1 in vivo (Fig. 2E and F). Third, the S89,93A mutation significantly reduced nuclear import, whereas the phospho-mimetic mutations S89,93D/E partially restored nuclear import (Fig. 2A). Fourth, inhibitors of ERK and JNK reduced nuclear import (Fig. 3). By using these inhibitors in conjunction with the S89A or S93A mutation, we were able to deduce that ERK can phosphorylate S89 and S93, whereas JNK can phosphorylate only S89. Our data also showed that phosphorylation on S89 more effectively promotes nuclear import than phosphorylation on S93. Consistent with these interpretations, neither inhibitor completely abolishes E1 nuclear import (Fig. 3B).
The results with E1 S89,S93A are particular revealing. Only 5 to 7% of the cells exhibited a pan-cellular E1 signal, whereas the remaining cells had exclusively cytoplasmic signals (Fig. 2A and 3B). Since this low level of nuclear import was not affected by either MAPK inhibitor (Fig. 3B), we attributed it to the intrinsic activity of the HPV-11 E1 NLS. These observations further highlight the critical role of E1 phosphorylation by MAPKs to promote efficient nuclear import. It is interesting to note that the MAPK docking motifs are located at the carboxyl terminus, whereas their substrates are near the amino terminus. Thus, the two ends of the molecule may well be in close juxtaposition, at least transiently. Alternatively, MAPKs may phosphorylate E1 via an intersubunit interaction in a dimeric or multimeric E1 complex.
Although the inhibitor of p38α did not affect E1 nuclear import (Fig. 3B), indirect evidence suggested a minor role of MAPKs other than ERK and JNK in E1 nuclear import. For instance, a significant fraction of the cells exhibited pan-cellular GFP-E1dm S89A in the presence of the ERK inhibitor U0126 (Fig. 3B). The inhibitor should have blocked S93 phosphorylation by ERK, and E1 nuclear import should have been reduced to the basal level conferred by the NLS alone. The presence of the pan-cellular E1 signals would suggest that E1 might be phosphorylated inefficiently by other MAPKs, such as the U0126-insensitive ERK family kinases ERK7 and ERK8 (2, 3), or possibly p38α, which is highly induced by the ERK inhibitor. Indeed, the ERK inhibitor UO126 induced JNK and p38α (and perhaps other kinases) to a greater extent than was the case with the induction of ERK and p38α by the JNK inhibitor SP600125 (Fig. 3A). This difference might have led to a slightly higher level of E1 phosphorylation and a somewhat higher pan-cellular signal in cells treated with the ERK inhibitor than in those treated with the JNK inhibitor.
Differential effects of mutations in the NLS or in the MAPK docking domain on the ability of E1 protein to support transient replication.
The HPV-11 E1 NLS mutations or phosphorylation site mutations that are associated with severe defects in nuclear import can still support transient HPV-11 ori-specific DNA replication (Fig. 4A) (27). This is attributable to E1-E2 cotransport into the nucleus (Fig. 4B and C). This interpretation is supported by the inability of an E2 protein inactivated in its own NLS to promote E1 nuclear import (Fig. 4C). It is also consistent with our previous observation that a fraction of the HPV-11 E2 protein mutated in its NLS is transported into the nucleus when coexpressed with a wild-type HPV-11 E1 protein (71). Although we cannot exclude it, we do not favor the possibility that the nuclear E2 or E1 protein may have modified the nuclear import machinery such that some of the NLS-mutated E1 or E2 protein was coimported into the nucleus. Regardless of the mechanism for E1 and E2 conuclear import, the high levels of both proteins expressed from vectors might have made it possible. In the context of the naturally infected tissues, the low concentrations of these viral proteins, especially in the undifferentiated basal and parabasal epithelial cells, where viral DNA copy number and transcriptional activities are both low, might greatly reduce the probability of the cotransport of the two proteins into the nucleus. The fact that both proteins possess an NLS also indicates their importance during viral persistence or the productive phase.
Unlike the NLS mutations, the MAPK docking domain mutations were completely inactive in transient replication (Fig. 4D) even though a fraction of the proteins was in the nucleus (Fig. 2A). Based on the crystallographic structure of the HPV-18 E1 helicase domain in complex with the E2 transacting domain (1), one of the E2-interacting regions (loop II) and the arginine finger which affects E1 ATPase activity are located very near the conserved MAPK docking motif DD2. Loop I, which contains the basic amino acid residues for E1-DNA interaction, is located close to the classical MAPK docking motif DD1. Therefore, it is conceivable that mutations in the MAPK docking domain might have affected the interactions between E1 and E2 or E1 and DNA or its ATPase function. Deletion mutations have broadly delineated the interaction domain between E1 and DNA polymerase α/primase subunit p68 to the E1 carboxyl terminus, which spans the MAPK docking domain (4, 20, 50). Thus, it is also possible the MAPK docking domain mutations have affected the ability of E1 to recruit DNA polymerase α/primase, which is essential for viral DNA replication. We were not able to purify bacterially expressed E1 proteins mutated in docking domains to test these possibilities due to extensive protein degradation, which itself could be an indication of critical alterations in the tertiary structure of the E1 protein.
A model to explain the regulation of HPV-11 E1 nuclear localization in the context of natural infections.
Why do papillomaviruses use MAPKs as one of their mechanisms to regulate E1 nuclear import? We suggest that because E1 is a potent DNA helicase, its nuclear presence must be carefully controlled so as not to unwind the viral DNA before host DNA replication machinery is available to support viral DNA replication. To do so, these viruses have deftly adapted the normal cellular mechanisms to establish a persistent infection. In various cell types, different MAPKs are activated to promote cell proliferation and migration during the wound healing process. For instance, ERK, JNK, and p38 are all activated in airway epithelial cells and are responsible for cell migration after injury (69). During wound healing in keratinocytes, JNK promotes cell migration and ERK is required for reepithelialization (30, 36). The HPV E5 transmembrane protein, the E6 oncoprotein, or the cellular uptake of virus-like particles can also activate MAPKs (10-12, 22-24, 32, 55). In addition, Oda et al. (53) and Harris et al. (34) suggested that E6 might stabilize an activated Src, which is upstream in the ERK signaling pathway (47).
On the basis of the results presented in this and our previous studies (27, 48), the regulatory elements identified in the HPV-11 E1 location regulatory region can now be depicted, as shown in Fig. 5A. The LRR contains interdigitated NLS, NES, S89, and S93 MAP kinase substrates, a cyclin binding motif which partially overlaps the downstream NLS, and S107, which is the substrate of cyclin/cdk2 and cyclin/cdk1 to inactivate the NES. We propose the following regulatory network and program to describe the exquisite control of the HPV-11 E1 nucleocytoplasmic localization in the context of papillomavirus infection, persistence, and the production program, summarized in Fig. 5B.
After virions gain entry into the basal keratinocytes through a wound in the epithelium and during the ensuing healing period, HPV promoters are temporarily activated to synthesize early messages (15). The E1 protein would be phosphorylated by cytoplasmic MAPKs present during wound healing, resulting in its nuclear entry, and the nuclear export signal would then be inactivated by phosphorylation on S107 by the cdk2 and cdk1 complexes, enabling the DNA helicase to remain in the nucleus and to support viral DNA replication. As long as the host keratinocytes continue to cycle and divide, the nuclear E1 protein collaborates with the E2 origin recognition protein and promotes transient and low levels of viral DNA amplification to establish the initial focus of infected cells and to set the steady-state viral DNA copy number.
Once the healing phase is over, the basal cells return to quiescence, whereas the parabasal cells continue to cycle regularly to maintain a differentiating squamous epithelium, which turns over and renews every 2 to 3 weeks. The viral promoters are down-regulated in the quiescent basal cells but can be reactivated during subsequent wounding and healing or when the host cell reenters the cell cycle on a periodic basis to replace an overlying parabasal cell lost to terminal differentiation. Thus, the E1 and E2 proteins continue to maintain a low viral DNA copy number in the cycling parabasal cells and basal cells.
In the productive phase of the infection, the viral promoters are significantly up-regulated in the differentiated spinous cells, hence the viral mRNA and protein levels dramatically increase. When the cells reenter the cell cycle to reestablished an S-phase milieu in the differentiated cells through the actions of the viral E7 protein (6, 13), the E1 protein is shuttled into and retained in the nucleus via the combined activities of the NLS, MAPKs, and cdk's to support viral DNA amplification. Notably, the ability of the E1 protein to remain in the nucleus and functional as long as cdk2 or cdk1 is active provides the mechanism by which the papillomaviruses can overcome origin-licensing rules and rereplicate many times over to achieve high-copy-number amplification without an intervening cell division.
Our recent data also suggest that once the viral DNA has reached a very high copy number in spinous cells, the E7 activity is extinguished and the keratinocytes exit the cell cycle (H.-K. Wang, A. Duffy, T. R. Broker, and L. T. Chow, unpublished observations). In the absence of active cdk's, S107 in the E1 protein is dephosphorylated by protein phosphatases and the helicase is shuttled out of the nucleus, mediated by the CRM-1 exportin, with cessation of viral DNA amplification (27). Virion morphogenesis occurs in the terminally differentiated cells comprising the superficial strata of squamous epithelia. Progeny virus particles within the cell envelopes are sloughed from the epithelial surface. In summary, by taking advantage of the various cellular regulatory mechanisms and replication machinery, HPVs are able to propagate in the differentiated squamous epithelium while maintaining a persistent infection in the long-surviving basal stem cells or in the transit-amplifying parabasal keratinocytes.
Acknowledgments
This research was supported by USPHS grants CA83679 and CA108733.
We thank Fang-Tsyr Lin for discussions and for sharing the ERK1 kinase and FLAG-JNK1 expression plasmid and Jacques Pouyssegur for sharing the HA-ERK1 expression plasmid.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Abbate, E. A., J. M. Berger, and M. R. Botchan. 2004. The X-ray structure of the papillomavirus helicase in complex with its molecular matchmaker E2. Genes. Dev. 18:1981-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abe, M. K., K. T. Kahle, M. P. Saelzler, K. Orth, J. E. Dixon, and M. R. Rosner. 2001. ERK7 is an autoactivated member of the MAPK family. J. Biol. Chem. 276:21272-21279. [DOI] [PubMed] [Google Scholar]
- 3.Abe, M. K., M. P. Saelzler, R. Espinosa III, K. T. Kahle, M. B. Hershenson, M. M. Le Beau, and M. R. Rosner. 2002. ERK8, a new member of the mitogen-activated protein kinase family. J. Biol. Chem. 277:16733-16743. [DOI] [PubMed] [Google Scholar]
- 4.Amin, A. A., S. Titolo, A. Pelletier, D. Fink, M. G. Cordingley, and J. Archambault. 2000. Identification of domains of the HPV11 E1 protein required for DNA replication in vitro. Virology 272:137-150. [DOI] [PubMed] [Google Scholar]
- 5.Atanasova, G., R. Jans, N. Zhelev, V. Mitev, and Y. Poumay. 2005. Effects of the cyclin-dependent kinase inhibitor CYC202 (R-roscovitine) on the physiology of cultured human keratinocytes. Biochem. Pharmacol. 70:824-836. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee, N. S., L. T. Chow, and T. R. Broker. 2005. Retrovirus-mediated gene transfer to analyze HPV gene regulation and protein functions in organotypic “raft” cultures. Methods Mol. Med. 119:187-202. [DOI] [PubMed] [Google Scholar]
- 7.Bertelsen, B. I., S. J. Steine, R. Sandvei, A. Molven, and O. D. Laerum. 2006. Molecular analysis of the PI3K-AKT pathway in uterine cervical neoplasia: frequent PIK3CA amplification and AKT phosphorylation. Int. J. Cancer 118:1877-1883. [DOI] [PubMed] [Google Scholar]
- 8.Bian, X. L., G. Rosas-Acosta, Y. C. Wu, and V. G. Wilson. 2007. Nuclear import of bovine papillomavirus type 1 E1 protein is mediated by multiple alpha importins and is negatively regulated by phosphorylation near a nuclear localization signal. J. Virol. 81:2899-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell, R. E., O. Tour, A. E. Palmer, P. A. Steinbach, G. S. Baird, D. A. Zacharias, and R. Y. Tsien. 2002. A monomeric red fluorescent protein. Proc. Natl. Acad. Sci. USA 99:7877-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cartin, W., and A. Alonso. 2003. The human papillomavirus HPV2a E5 protein localizes to the Golgi apparatus and modulates signal transduction. Virology 314:572-579. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti, O., K. Veeraraghavalu, V. Tergaonkar, Y. Liu, E. J. Androphy, M. A. Stanley, and S. Krishna. 2004. Human papillomavirus type 16 E6 amino acid 83 variants enhance E6-mediated MAPK signaling and differentially regulate tumorigenesis by notch signaling and oncogenic Ras. J. Virol. 78:5934-5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, S. L., S. T. Lin, T. C. Tsai, W. C. Hsiao, and Y. P. Tsao. 2007. ErbB4 (JM-b/CYT-1)-induced expression and phosphorylation of c-Jun is abrogated by human papillomavirus type 16 E5 protein. Oncogene 26:42-53. [DOI] [PubMed] [Google Scholar]
- 13.Cheng, S., D. C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 9:2335-2349. [DOI] [PubMed] [Google Scholar]
- 14.Chiang, C. M., M. Ustav, A. Stenlund, T. F. Ho, T. R. Broker, and L. T. Chow. 1992. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc. Natl. Acad. Sci. USA 89:5799-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow, L. T., and T. R. Broker. 2007. Human papillomavirus transcription, p. 119-131. In R. L. Garcea and D. OiMaio (ed.), The papillomaviruses. Springer Science and Business Media, New York, N.Y.
- 16.Chow, L. T., and T. R. Broker. 2006. Mechanisms and regulation of papillomavirus DNA replication, p. 53-71. In M. S. Campo (ed.), Papillomavirus research: from natural history to vaccines and beyond. Caister Academic Press, Norwich, United Kingdom.
- 17.Clower, R. V., J. C. Fisk, and T. Melendy. 2006. Papillomavirus E1 protein binds to and stimulates human topoisomerase I. J. Virol. 80:1584-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen, P., D. R. Alessi, and D. A. Cross. 1997. PDK1, one of the missing links in insulin signal transduction? FEBS Lett. 410:3-10. [DOI] [PubMed] [Google Scholar]
- 19.Cohen, P., and S. Frame. 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2:769-776. [DOI] [PubMed] [Google Scholar]
- 20.Conger, K. L., J. S. Liu, S. R. Kuo, L. T. Chow, and T. S. Wang. 1999. Human papillomavirus DNA replication. Interactions between the viral E1 protein and two subunits of human DNA polymerase alpha/primase. J. Biol. Chem. 274:2696-2705. [DOI] [PubMed] [Google Scholar]
- 21.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 22.Crusius, K., E. Auvinen, and A. Alonso. 1997. Enhancement of EGF- and PMA-mediated MAP kinase activation in cells expressing the human papillomavirus type 16 E5 protein. Oncogene 15:1437-1444. [DOI] [PubMed] [Google Scholar]
- 23.Crusius, K., E. Auvinen, B. Steuer, H. Gaissert, and A. Alonso. 1998. The human papillomavirus type 16 E5-protein modulates ligand-dependent activation of the EGF receptor family in the human epithelial cell line HaCaT. Exp. Cell Res. 241:76-83. [DOI] [PubMed] [Google Scholar]
- 24.Crusius, K., I. Rodriguez, and A. Alonso. 2000. The human papillomavirus type 16 E5 protein modulates ERK1/2 and p38 MAP kinase activation by an EGFR-independent process in stressed human keratinocytes. Virus Genes 20:65-69. [DOI] [PubMed] [Google Scholar]
- 25.Cueille, N., R. Nougarede, F. Mechali, M. Philippe, and C. Bonne-Andrea. 1998. Functional interaction between the bovine papillomavirus virus type 1 replicative helicase E1 and cyclin E-Cdk2. J. Virol. 72:7255-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deng, W., G. Jin, B. Y. Lin, B. A. Van Tine, T. R. Broker, and L. T. Chow. 2003. mRNA splicing regulates human papillomavirus type 11 E1 protein production and DNA replication. J. Virol. 77:10213-10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng, W., B. Y. Lin, G. Jin, C. G. Wheeler, T. Ma, J. W. Harper, T. R. Broker, and L. T. Chow. 2004. Cyclin/CDK regulates the nucleocytoplasmic localization of the human papillomavirus E1 DNA helicase. J. Virol. 78:13954-13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fouts, E. T., X. Yu, E. H. Egelman, and M. R. Botchan. 1999. Biochemical and electron microscopic image analysis of the hexameric E1 helicase. J. Biol. Chem. 274:4447-4458. [DOI] [PubMed] [Google Scholar]
- 29.Galanis, A., S. H. Yang, and A. D. Sharrocks. 2001. Selective targeting of MAPKs to the ETS domain transcription factor SAP-1. J. Biol. Chem. 276:965-973. [DOI] [PubMed] [Google Scholar]
- 30.Gangnuss, S., A. J. Cowin, I. S. Daehn, N. Hatzirodos, J. A. Rothnagel, A. Varelias, and T. E. Rayner. 2004. Regulation of MAPK activation, AP-1 transcription factor expression and keratinocyte differentiation in wounded fetal skin. J. Investig. Dermatol. 122:791-804. [DOI] [PubMed] [Google Scholar]
- 31.Goldfarb, D. S., J. Gariepy, G. Schoolnik, and R. D. Kornberg. 1986. Synthetic peptides as nuclear localization signals. Nature 322:641-644. [DOI] [PubMed] [Google Scholar]
- 32.Gu, Z., and G. Matlashewski. 1995. Effect of human papillomavirus type 16 oncogenes on MAP kinase activity. J. Virol. 69:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han, Y., Y. M. Loo, K. T. Militello, and T. Melendy. 1999. Interactions of the papovavirus DNA replication initiator proteins, bovine papillomavirus type 1 E1 and simian virus 40 large T antigen, with human replication protein A. J. Virol. 73:4899-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris, K. F., I. Shoji, E. M. Cooper, S. Kumar, H. Oda, and P. M. Howley. 1999. Ubiquitin-mediated degradation of active Src tyrosine kinase. Proc. Natl. Acad. Sci. USA 96:13738-13743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu, C. Y., F. Mechali, and C. Bonne-Andrea. 2007. Nucleocytoplasmic shuttling of bovine papillomavirus E1 helicase downregulates viral DNA replication in S phase. J. Virol. 81:384-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang, C., Z. Rajfur, C. Borchers, M. D. Schaller, and K. Jacobson. 2003. JNK phosphorylates paxillin and regulates cell migration. Nature 424:219-223. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs, D., D. Glossip, H. Xing, A. J. Muslin, and K. Kornfeld. 1999. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13:163-175. [PMC free article] [PubMed] [Google Scholar]
- 38.Jans, D. A., and S. Hubner. 1996. Regulation of protein transport to the nucleus: Central role of phosphorylation. Physiol. Rev. 76:651-685. [DOI] [PubMed] [Google Scholar]
- 39.Kim, S. H., Y. S. Juhnn, S. Kang, S. W. Park, M. W. Sung, Y. J. Bang, and Y. S. Song. 2006. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1,2 and PI3K/Akt. Cell. Mol. Life Sci. 63:930-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuo, S. R., J. S. Liu, T. R. Broker, and L. T. Chow. 1994. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J. Biol. Chem. 269:24058-24065. [PubMed] [Google Scholar]
- 41.Leng, X., and V. G. Wilson. 1994. Genetically defined nuclear localization signal sequence of bovine papillomavirus E1 protein is necessary and sufficient for the nuclear localization of E1-beta-galactosidase fusion proteins. J. Gen. Virol. 75:2463-2467. [DOI] [PubMed] [Google Scholar]
- 42.Lentz, M. R., D. Pak, I. Mohr, and M. R. Botchan. 1993. The E1 replication protein of bovine papillomavirus type 1 contains an extended nuclear localization signal that includes a p34cdc2 phosphorylation site. J. Virol. 67:1414-1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin, B. Y., T. Ma, J. S. Liu, S. R. Kuo, G. Jin, T. R. Broker, J. W. Harper, and L. T. Chow. 2000. HeLa cells are phenotypically limiting in cyclin E/CDK2 for efficient human papillomavirus DNA replication. J. Biol. Chem. 275:6167-6174. [DOI] [PubMed] [Google Scholar]
- 44.Lin, B. Y., A. M. Makhov, J. D. Griffith, T. R. Broker, and L. T. Chow. 2002. Chaperone proteins abrogate inhibition of the human papillomavirus (HPV) E1 replicative helicase by the HPV E2 protein. Mol. Cell. Biol. 22:6592-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu, J. S., S. R. Kuo, A. M. Makhov, D. M. Cyr, J. D. Griffith, T. R. Broker, and L. T. Chow. 1998. Human Hsp70 and Hsp40 chaperone proteins facilitate human papillomavirus-11 E1 protein binding to the origin and stimulate cell-free DNA replication. J. Biol. Chem. 273:30704-30712. [DOI] [PubMed] [Google Scholar]
- 46.Loo, Y. M., and T. Melendy. 2004. Recruitment of replication protein A by the papillomavirus E1 protein and modulation by single-stranded DNA. J. Virol. 78:1605-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luttrell, L. M., B. E. Hawes, T. van Biesen, D. K. Luttrell, T. J. Lansing, and R. J. Lefkowitz. 1996. Role of c-Src tyrosine kinase in G protein-coupled receptor- and Gβγ subunit-mediated activation of mitogen-activated protein kinases. J. Biol. Chem. 271:19443-19450. [DOI] [PubMed] [Google Scholar]
- 48.Ma, T., N. Zou, B. Y. Lin, L. T. Chow, and J. W. Harper. 1999. Interaction between cyclin-dependent kinases and human papillomavirus replication-initiation protein E1 is required for efficient viral replication. Proc. Natl. Acad. Sci. USA 96:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malcles, M. H., N. Cueille, F. Mechali, O. Coux, and C. Bonne-Andrea. 2002. Regulation of bovine papillomavirus replicative helicase e1 by the ubiquitin-proteasome pathway. J. Virol. 76:11350-11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masterson, P. J., M. A. Stanley, A. P. Lewis, and M. A. Romanos. 1998. A C-terminal helicase domain of the human papillomavirus E1 protein binds E2 and the DNA polymerase alpha-primase p68 subunit. J. Virol. 72:7407-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyata, Y., and E. Nishida. 1999. Distantly related cousins of MAP kinase: biochemical properties and possible physiological functions. Biochem. Biophys. Res. Commun. 266:291-295. [DOI] [PubMed] [Google Scholar]
- 52.Muller, S., A. Ledl, and D. Schmidt. 2004. SUMO: a regulator of gene expression and genome integrity. Oncogene 23:1998-2008. [DOI] [PubMed] [Google Scholar]
- 53.Oda, H., S. Kumar, and P. M. Howley. 1999. Regulation of the Src family tyrosine kinase Blk through E6AP-mediated ubiquitination. Proc. Natl. Acad. Sci. USA 96:9557-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park, P., W. Copeland, L. Yang, T. Wang, M. R. Botchan, and I. J. Mohr. 1994. The cellular DNA polymerase alpha-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc. Natl. Acad. Sci. USA 91:8700-8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Payne, E., M. R. Bowles, A. Don, J. F. Hancock, and N. A. McMillan. 2001. Human papillomavirus type 6b virus-like particles are able to activate the Ras-MAP kinase pathway and induce cell proliferation. J. Virol. 75:4150-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pim, D., P. Massimi, S. M. Dilworth, and L. Banks. 2005. Activation of the protein kinase B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene 24:7830-7838. [DOI] [PubMed] [Google Scholar]
- 57.Rangasamy, D., and V. G. Wilson. 2000. Bovine papillomavirus E1 protein is sumoylated by the host cell Ubc9 protein. J. Biol. Chem. 275:30487-30495. [DOI] [PubMed] [Google Scholar]
- 58.Rangasamy, D., K. Woytek, S. A. Khan, and V. G. Wilson. 2000. SUMO-1 modification of bovine papillomavirus E1 protein is required for intranuclear accumulation. J. Biol. Chem. 275:37999-38004. [DOI] [PubMed] [Google Scholar]
- 59.Rosas-Acosta, G., M. A. Langereis, A. Deyrieux, and V. G. Wilson. 2005. Proteins of the PIAS family enhance the sumoylation of the papillomavirus E1 protein. Virology 331:190-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schuck, S., and A. Stenlund. 2005. Assembly of a double hexameric helicase. Mol. Cell 20:377-389. [DOI] [PubMed] [Google Scholar]
- 61.Seeler, J. S., and A. Dejean. 2003. Nuclear and unclear functions of SUMO. Nat. Rev. Mol. Cell Biol. 4:690-699. [DOI] [PubMed] [Google Scholar]
- 62.Shaw, M., P. Cohen, and D. R. Alessi. 1997. Further evidence that the inhibition of glycogen synthase kinase-3beta by IGF-1 is mediated by PDK1/PKB-induced phosphorylation of Ser-9 and not by dephosphorylation of Tyr-216. FEBS Lett. 416:307-311. [DOI] [PubMed] [Google Scholar]
- 63.Stenlund, A. 2003. Initiation of DNA replication: lessons from viral initiator proteins. Nat. Rev. Mol. Cell Biol. 4:777-785. [DOI] [PubMed] [Google Scholar]
- 64.Swindle, C. S., N. Zou, B. A. Van Tine, G. M. Shaw, J. A. Engler, and L. T. Chow. 1999. Human papillomavirus DNA replication compartments in a transient DNA replication system. J. Virol. 73:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tanoue, T., and E. Nishida. 2003. Molecular recognitions in the MAP kinase cascades. Cell. Signal. 15:455-462. [DOI] [PubMed] [Google Scholar]
- 66.Terui, Y., N. Saad, S. Jia, F. McKeon, and J. Yuan. 2004. Dual role of sumoylation in the nuclear localization and transcriptional activation of NFAT1. J. Biol. Chem. 279:28257-28265. [DOI] [PubMed] [Google Scholar]
- 67.Van Tine, B. A., L. D. Dao, S. Y. Wu, T. M. Sonbuchner, B. Y. Lin, N. Zou, C. M. Chiang, T. R. Broker, and L. T. Chow. 2004. Human papillomavirus (HPV) origin-binding protein associates with mitotic spindles to enable viral DNA partitioning. Proc. Natl. Acad. Sci. USA 101:4030-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Westbrook, T. F., D. X. Nguyen, B. R. Thrash, and D. J. McCance. 2002. E7 abolishes raf-induced arrest via mislocalization of p21(Cip1). Mol. Cell. Biol. 22:7041-7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.White, S. R., R. Tse, and B. A. Marroquin. 2005. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 32:301-310. [DOI] [PubMed] [Google Scholar]
- 70.Xu, J., Y. J. Lai, W. C. Lin, and F. T. Lin. 2004. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J. Biol. Chem. 279:10459-10468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou, N., B. Y. Lin, F. Duan, K. Y. Lee, G. Jin, R. Guan, G. Yao, E. J. Lefkowitz, T. R. Broker, and L. T. Chow. 2000. The hinge of the human papillomavirus type 11 E2 protein contains major determinants for nuclear localization and nuclear matrix association. J. Virol. 74:3761-3770. [DOI] [PMC free article] [PubMed] [Google Scholar]