Abstract
Hepatitis B virus splice-generated protein (HBSP), encoded by a spliced hepatitis B virus RNA, was recently identified in liver biopsy specimens from patients with chronic active hepatitis B. We investigated the possible generation of immunogenic peptides by the processing of this protein in vivo. We identified a panel of potential epitopes in HBSP by using predictive computational algorithms for peptide binding to HLA molecules. We used transgenic mice devoid of murine major histocompatibility complex (MHC) class I molecules and positive for human MHC class I molecules to characterize immune responses specific for HBSP. Two HLA-A2-restricted peptides and one immunodominant HLA-B7-restricted epitope were identified following the immunization of mice with DNA vectors encoding HBSP. Most importantly, a set of overlapping peptides covering the HBSP sequence induced significant HBSP-specific T-cell responses in peripheral blood mononuclear cells from patients with chronic hepatitis B. The response was multispecific, as several epitopes were recognized by CD8+ and CD4+ human T cells. This study provides the first evidence that this protein generated in vivo from an alternative reading frame of the hepatitis B virus genome activates T-cell responses in hepatitis B virus-infected patients. Given that hepatitis B is an immune response-mediated disease, the detection of T-cell responses directed against HBSP in patients with chronic hepatitis B suggests a potential role for this protein in liver disease progression.
Hepatitis B virus (HBV) is a small, partially double-stranded DNA virus with four conserved and overlapping open reading frames (ORF) encoding the viral proteins. Persistent infection with HBV is a major health problem worldwide, with more than 350 million patients at risk of developing liver cirrhosis or hepatocellular carcinoma. HBV is not directly hepatotoxic, but some HBV proteins have been directly implicated in liver pathogenesis. The accumulation of the large envelope protein has been shown to have a direct toxic effect on hepatocytes in a transgenic (Tg) mouse model (8). The transactivation of some cellular genes by the expression product of the 3′-truncated preS/S sequence of HBV, which integrates into the genomic DNA of liver cells (12), and the HBV X protein, which regulates proteasome function, thereby controlling the degradation of cellular and viral proteins, have also been implicated as possible causes of HBV-associated oncogenesis (4, 13). However, several lines of evidence suggest that the immune response also plays a central role in pathogenesis and liver disease outcome (7, 26). HBV proteins can trigger immune responses and may participate indirectly in various steps in liver disease. Chronic inflammation and the effects of cytokines secreted by immune system cells are major factors in the development of fibrosis and liver cell proliferation.
Recently, the HBV splice-generated protein (HBSP) was identified. HBSP is encoded by a 2.2-kb singly spliced RNA, and the protein results from the fusion of a sequence encoding the N-terminal part of the polymerase (Pol) and a new ORF created by splicing events. It has been suggested that this protein may also play a role in the natural history and pathogenesis of HBV infection (31). Antibodies against HBSP have been found in sera from chronic HBV carriers, suggesting that this protein is immunogenic at the B-cell level. Moreover, the detection of anti-HBSP antibodies is significantly associated with severe liver fibrosis (32). Various proteins encoded by alternative reading frames (ARF) can activate epitope-specific immune responses. Nontraditional epitopes derived from ARF in influenza virus (5) and hepatitis C virus (1) and the antigens of several cancers, including melanoma (17, 29), have been described previously. We therefore investigated the possible generation of immunogenic epitopes by the processing of the ARF-generated HBSP in vivo, leading to the activation of specific T cells. We show here that HBSP is immunogenic in vivo in humanized mice. More importantly, using peripheral blood mononuclear cells (PBMCs) from chronically HBV-infected individuals, we further demonstrate that HBSP-derived peptides can reactivate a multispecific T-cell response. These T-cell responses may ultimately result in liver damage and contribute to HBV pathogenesis.
MATERIALS AND METHODS
HBSP expression vectors.
The HBSP sequence was cloned from a patient with chronic HBV (genotype A) infection and was inserted into pcDNA3.1/myc-His (Invitrogen, Cergy Pontoise, France). pHBSP expresses HBSP, which corresponds to a fusion between the first 46 amino acids (aa) of the HBV Pol and 65 aa of a novel HBV sequence generated by a frameshift (MPLSYQHFRRLLLLDEEAGPLEEELPRLADEDLNRRVAEDLHLQLPNDPRPPVRDPAKPARLLLKATLCIPHVAVQNLRTEIAPVFPSHHLGLSQNTYGSGPQSVSLGSVY) (31). The pHBSP 39-111 plasmid was derived from the pCMV-S2.S vector (18), which encodes the small (S) and middle (preS2-S) envelope proteins of the HBV envelope under the control of the cytomegalovirus immediate early gene promoter. In pHBSP 39-111, part of the amino-terminal sequence of the middle HBV envelope protein (preS2 domain) was replaced with the carboxy-terminal part of the HBSP sequence (aa 39 to 111), resulting in a fusion protein.
The TNT T7-coupled reticulocyte lysate system (Promega, France) was used for the in vitro translation of plasmid-encoded proteins. Proteins were analyzed by electrophoresis in 18% acrylamide gels.
Plasmids were purified using DNA purification columns (Endofree plasmid kit [QIAGEN, Hilden, Germany]; pHBSP 39-111) or were obtained from Plasmid Factory (Bielefeld, Germany; pHBSP). Both vectors were resuspended at a concentration of 1 mg/ml in endotoxin-free phosphate-buffered saline (PBS; Sigma, St. Quentin Fallavier, France).
Synthetic peptides.
Bioinformatics and Molecular Analysis Section (BIMAS) software (National Institutes of Health, Washington, DC; http://bimas.dcrt.nih.gov/molbio/hla_bind/) and the SYFPEITHI algorithm (http://www.syfpeithi.de/) (25) were used to identify HBSP-derived peptides likely to bind to the HLA-A*0201 or HLA-B*0702 molecule. Four HLA-A*0201 (Pol A2-1, Pol A2-2, HBSP A2-1, and HBSP A2-2) and five HLA-B*0702 (Pol B7-1, HBSP B7-1, HBSP B7-2, HBSP B7-3, and HBSP B7-4) peptides were selected on the basis of binding affinity. HLA-A2-restricted HBV peptides (HBV surface [HBs] protein-derived peptides spanning aa 183 to 191 [HBs 183-191] and HBs 348-357 [7] and an HBV core peptide spanning aa 18 to 27 [core 18-27] [24]) and HLA-B7-restricted HBV peptides (HBs 232-240 and core 19-27 [2]) were used as controls in cytotoxicity assays or to stimulate frozen PBMCs.
Overlapping 15-mer peptides (10-residue overlap) covering aa 39 to 111 of the HBSP (genotype A) were synthesized. Stock solutions of all synthetic peptides were prepared at a concentration of 1 mg/ml in water or, for a few peptides, in acetonitrile-H2O (vol/vol), depending on the supplier. Peptides were used individually or in pools of three to four peptides for the in vitro stimulation of PBMCs and for enzyme-linked immunospot (ELISPOT) assays. Peptides with a minimum purity level of 80% were purchased from NeoMPS (Strasbourg, France).
Mice.
Tg mice positive for the HLA-A*0201 (HHD+/+ β2 m−/− H-2 Db−/−) or the HLA-B*0702 (HLA-B7mα3; H-2 Kb−/− Db−/−) molecule (23, 27) were used for immunization. Groups of 6- to 10-week-old female mice received two injections, 3 weeks apart, of pHBSP or pHBSP 39-111. Intramuscular nucleic acid immunization was carried out under anesthesia by injecting 100 μg of plasmid DNA into regenerating (i.e., cardiotoxin-treated) tibialis anterior muscles as previously described (16). The mice used were reared in the animal facilities of the Institut Pasteur, in accordance with the recommendations of the institutional ethics committee.
HBV-infected study population.
Frozen PBMCs were obtained from nine HLA-A2-positive and seven HLA-B7-positive HBV-infected individuals (patients P1 to P9 and P10 to P16, respectively) from a previous study (9). All patients had biopsy-proven chronic hepatitis, with or without active HBV replication, but none were immunocompromised or infected with human immunodeficiency virus, hepatitis C virus, or hepatitis D virus. PBMCs from three HBV-negative HLA-A2-positive (C1 to C3) and three HBV-negative HLA-B7-positive (C3 to C6) individuals were also included in the study.
In vitro expansion of mouse splenocyte and human PBMC populations.
Mouse spleen cells were collected and depleted of red blood cells 7 to 10 days after the second DNA injection. Splenocytes were cultured (107 cells/well in 24-well plates) in 2 ml of α-minimal essential medium supplemented with 10 mM HEPES, nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM glutamine (Invitrogen, Cergy Pontoise, France), 50 nM β-mercaptoethanol, and 10% fetal calf sera (HyClone, Perbio, Belgium). Murine HLA-A2 splenocytes were stimulated by incubation for 7 days with peptide-pulsed (10 μg/ml), lipopolysaccharide-induced, γ-irradiated (100 Gy) syngeneic lymphoblasts as antigen-presenting cells at an effector/presenting cell ratio of 1:1 (14). HLA-B7 splenocytes were stimulated with peptide only (10 μg/ml) for 1 week.
Ficoll-purified human PBMCs stored in liquid nitrogen were thawed, washed, and incubated overnight at 37°C in complete medium (RPMI 1640 medium supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% human AB serum [Institut Jacques Boy, Reims, France]) in an atmosphere containing 5% CO2. PBMCs were counted (3 × 106 cells/ml) and resuspended in complete medium supplemented with 20 ng/ml interleukin-7 (IL-7; Roche, Meylan, France) in 48-well plates. Cells were then stimulated by incubation for 3 days with 10 μg/ml HLA-A2- or HLA-B7-restricted peptides or with pools of 15-mer peptides derived from the HBSP region from aa 39 to 111 (final concentration of each peptide, 2 μg/ml). Every 3 to 4 days, half the medium was replaced with complete medium supplemented with recombinant IL-2 (50 IU/ml; Roche, Meylan, France). After 10 days of culture, gamma interferon (IFN-γ)-producing cells were specifically quantified by using ELISPOT assays.
Cytolysis assay.
The levels of cytotoxic T-lymphocyte (CTL) activity of HLA-A2 and HLA-B7 mouse splenocytes were measured in a short-term 51Cr release assay against RMA-S HHD (23) and RMA-B7 (27) target cells, respectively. Target cells were pulsed with 10 μg/ml of HBSP- or HBs-derived peptides or with HLA-restricted irrelevant core peptides. After 4 h of incubation at 37°C in an atmosphere containing 5% CO2, 50 μl of the supernatant was collected and evaluated with a beta counter. Levels of spontaneous and maximum release from targets incubated with either medium alone or lysis buffer (5% Triton X-100, 1% sodium dodecyl sulfate) were determined. The percentage of lysis was calculated in triplicate as follows: (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. Results were considered positive if specific lysis (obtained by subtracting the lysis of targets loaded with the irrelevant peptide) was ≥10% for two consecutive effector/target ratios.
IFN-γ ELISPOT assays.
IFN-γ-producing splenocytes were quantified by ELISPOT assays after a 24-h period of stimulation with peptides, as previously described (14). Briefly, sterile 96-well nitrocellulose HA plates (Millipore, Bedford, MA) were coated with 50 μl of mouse monoclonal antibody (mAb) against IFN-γ (R4-6A2; 5 μg/ml; BD Biosciences, Le Pont de Claix, France) in 0.1 M bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Freshly isolated splenocytes were incubated with individual HBSP peptides at a concentration of 2 μg/ml in supplemented α-minimal essential medium. Wells containing cells in culture medium alone were used as negative controls for the evaluation of background values.
A human IFN-γ capture mAb (1-D1K; 15 μg/ml; Mabtech, Stockholm, Sweden) was used with PBMCs. The wells were blocked by incubation with 200 μl of 5% human AB serum in PBS at room temperature for 2 h. The coated wells were filled in triplicate with in vitro-stimulated cells (105/well) in complete medium and the appropriate peptides (1 μg/ml).
A Zeiss ELISPOT automatic counter was used to score the number of spots. Each cell population was titrated in triplicate. The response was considered positive if the median number of spot-forming cells (SFC) in triplicate wells was at least twice that in control wells containing medium and if at least 20 SFC per 106 splenocytes or 50 SFC per 106 PBMCs were detected after the subtraction of background values.
Intracellular staining assays.
For intracellular cytokine staining experiments, freshly isolated splenocytes or in vitro-expanded populations of PBMCs were plated in the presence or absence of peptides (1 μg/ml) and brefeldin A (2 μg/ml; Sigma). Cells were washed and incubated with either anti-mouse or anti-human CD8-peridinin chlorophyll protein- and CD4-phycoerythrin-conjugated antibodies. Surface-stained cells were fixed with 2% formaldehyde in PBS. Fixed cells were resuspended in permeabilization buffer (PBS, 0.5% bovine serum albumin, 0.5% saponin, and 0.05% sodium azide) and incubated with fluorescein isothiocyanate-conjugated anti-IFN-γ or fluorescein isothiocyanate-conjugated anti-IL-2 mAb (BD Biosciences, Le Pont de Claix, France) for flow cytometry analysis of peptide-specific T cells.
RESULTS
Production of HBSP from DNA plasmids.
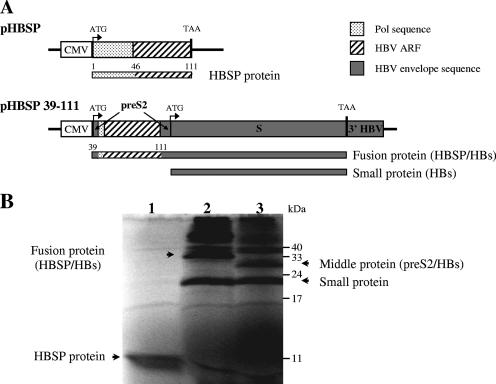
Two plasmids containing all or part of the HBSP sequence were constructed (Fig. 1A). The pHBSP vector expresses a fusion between the first 46 residues of HBV Pol and 65 aa encoded by the HBV ARF generated after the splicing of the 3.5-kb HBV mRNA (31). In the pHBSP 39-111 vector, sequences derived from the HBV ARF are fused in-frame within the preS2 part of the pCMV-S2.S vector, leading to the production of a fusion protein along with the small HBV envelope protein.
FIG. 1.
DNA plasmids used in the study. (A) Schematic diagram of HBSP expression vectors. The expected protein products are indicated by bars below the plasmids. CMV, cytomegalovirus promoter; 3′ HBV, the untranslated region of the HBV genome was used to provide a polyadenylation signal for mRNA. (B) Polyacrylamide gel electrophoresis of the proteins expressed by pHBSP (lane 1), pHBSP 39-111 (lane 2), and pCMV-S2.S (lane 3), translated in vitro. The small and middle HBV envelope proteins, the HBSP-HBs fusion protein, and HBSP are indicated by arrows.
In vitro translation was used to check that the expected proteins were produced from the HBSP constructs (Fig. 1B). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed that the pHBSP vector generated a protein with the expected size of 12 kDa (lane 1). Due to the presence of two in-frame ATG codons, the pCMV-S2.S vector used as a control generated the middle and small HBV envelope proteins (lane 3) (18). As expected, pHBSP 39-111 generated a 35-kDa protein corresponding to the fusion of HBSP and the small HBV envelope protein (lane 2). Bands of higher molecular masses corresponded to dimeric forms of HBV envelope proteins.
Prediction of major histocompatibility complex (MHC) class I-restricted candidate epitopes from HBSP.
Taking advantage of the predominance of the HLA-A*0201 and HLA-B*0702 class I molecules in the human population and the availability of transgenic mice expressing these molecules, we analyzed the amino acid sequence of the HBSP polypeptide (see Materials and Methods) by using the SYFPEITHI (25) and BIMAS (21) algorithms to identify the 9-mer peptides most likely to bind these two molecules. Several different HBSP peptides were selected on the basis of scores of 19 or higher from the SYFPEITHI database plus the prediction by the BIMAS algorithm of stable, high-affinity binding to the HLA-A2 or HLA-B7 molecule (Table 1). Three of these peptides corresponded to the pol-derived sequence and six corresponded to the new ORF. The HBSP B7-2 peptide, with a score below 19 from the SYFPEITHI database and no prediction of binding by the BIMAS algorithm, was used as a negative control.
TABLE 1.
HLA-A2 and HLA-B7 computer-predicted peptides and HBSP B7-1 epitope variants
| Peptide | HLA restriction | Amino acid sequencea | Positionb | Computer-predicted scorec
|
|
|---|---|---|---|---|---|
| SYFPEITHI | BIMAS | ||||
| Pol A2-1 | A2 | RLLLLDEEA | 10-18 | 19 | 9 |
| Pol A2-2 | A2 | LLDEEAGPL | 13-21 | 24 | 7.7 |
| HBSP A2-1 | A2 | LLLKATLCI | 62-70 | 25 | 65.5 |
| HBSP A2-2 | A2 | TLCIPHVAV | 67-75 | 24 | 69.5 |
| Pol B7-1 | B7 | LPRLADEDL | 25-33 | 22 | 800 |
| HBSP B7-1 | B7 | APVFPSHHL | 83-91 | 25 | 360 |
| HBSP B7-2 | B7 | VFPSHHLGL | 85-93 | 12 | NP |
| HBSP B7-3 | B7 | LPNDPRPPV | 45-53 | 22 | 9 |
| HBSP B7-4 | B7 | DPAKPARLL | 55-63 | 25 | 120 |
| HBSP B7-1 V1 | B7 | ALVFPSHHP | 83-91 | 5 | NP |
| HBSP B7-1 V2 | B7 | APVFPSHHP | 83-91 | 15 | NP |
| HBSP B7-1 V3 | B7 | ALVFPSHHH | 83-91 | 5 | NP |
Mutations occurring in the HBSP B7-1 epitope are shown in bold.
Amino acid position in the HBSP sequence derived from HBV genotype A.
NP, not predicted by the BIMAS program.
Induction of HBSP-specific CTLs after immunization with DNA plasmids encoding HBSP.
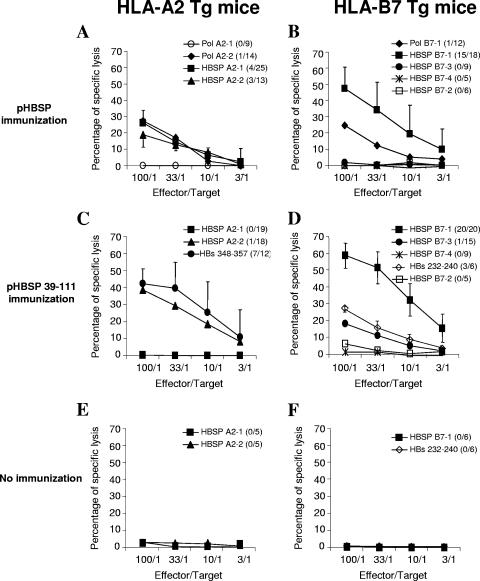
We investigated whether the processing of HBSP could generate the peptides predicted by the algorithms and whether MHC class I binding determinants could specifically stimulate CD8 T cells. Transgenic mice expressing the human HLA-A*0201 or HLA-B*0702 molecule but deficient in mouse MHC class I molecules (23, 27) were immunized twice with plasmids encoding HBSP. Spleen cells obtained from mice 7 to 10 days after injection were stimulated in vitro with identified peptides. Seven days later, activated splenic T lymphocytes were used as effector cells for the assessment of cytotoxic activity against target cells pulsed with the corresponding peptides or with control peptides. Following pHBSP immunization, specific CD8+-T-cell responses were detected in the spleens of immunized HLA-A2-Tg mice after in vitro stimulation with three of the four HLA-A2-restricted peptides (Fig. 2A) but not after stimulation with the HBSP B7-1 peptide used as a control (data not shown). Two of these stimulating peptides corresponded to the new ORF (HBSP A2-1 and HBSP A2-2), whereas the third corresponded to the Pol domain of HBSP (Pol A2-2). However, these responses were sporadic, as only 1 in 14, 4 in 25, and 3 in 13 immunized mice developed cytotoxic responses specific for Pol A2-2, HBSP A2-1, and HBSP A2-2, respectively.
FIG. 2.
Cytotoxic activity of splenocytes from immunized Tg mice. Cytotoxic assays were performed after 7 days of culture of splenocytes from HLA-A2 (A, C, and E)- and HLA-B7 (B, D, and F)-Tg mice immunized with either pHBSP (A and B) or pHBSP 39-111 (C and D) and splenocytes from nonimmunized mice (E and F). Percentages of specific lysis were obtained after the subtraction of background lysis tested with target cells loaded with irrelevant peptides (HLA-A2 core 18-27 and HLA-B7 core 19-27). The numbers of mice with positive responses and the numbers of tested mice are indicated in parentheses. Results are expressed as the mean percentages of specific lysis of T cells from responding mice.
CTL activity against the Pol-derived B7 epitope (Pol B7-1) in HLA-B7-Tg mice was detected, with only 1 of the 12 immunized mice responding (Fig. 2B). No cytotoxic activity against target cells loaded with the HBSP B7-3 or HBSP B7-4 epitope predicted by computer analysis was found. Targets loaded with HBSP B7-2, a peptide with a low predicted binding affinity, were not lysed by primed splenocytes either. In contrast, 15 of the 18 immunized mice generated an efficient specific CTL response against HBSP B7-1-pulsed target cells.
We also assessed cytotoxic activity in mice immunized with the pHBSP 39-111 vector, which encodes a fusion between the C-terminal part of HBSP and the small HBV envelope protein. As a positive control of CTL induction, we checked the response against the well-described HBs-derived HLA-A2-restricted epitope HBs 348-357 in HLA-A2-Tg mice. Efficient specific lysis in 7 of 12 mice was observed (Fig. 2C). Cytotoxic activity against HBSP epitopes was observed only with the HBSP A2-2 peptide, in a single mouse.
Among HLA-B7-Tg immunized mice, splenocytes from only one animal responded to HBSP B7-3 and none responded to HBSP B7-4 or HBSP B7-2. As a control for HBs-specific responses, three of six mice had cytotoxic T cells specific for the HLA-B7-restricted HBs 232-240 epitope (2). By contrast, efficient specific lysis was found with effector cells from all HLA-B7-immunized mice and target cells loaded with the HBSP B7-1 peptide (Fig. 2D). To confirm the specificity of the CTL responses, cross-stimulation experiments were performed. Following the in vitro stimulation of splenocytes from HLA-A2 or HLA-B7 immunized Tg mice with HLA-B7 or HLA-A2 peptides, respectively, less than 5% lysis of target cells was observed. The stimulation of spleen cells from nonimmunized mice with predicted HLA-A2 or HLA-B7 peptides showed no in vitro activation of HBSP-specific T cells (Fig. 2E and F).
Thus, HBSP is immunogenic in vivo and plasmids encoding HBSP prime cytotoxic CD8+ T cells after DNA injection. However, the T-cell response was highly dependent on the MHC class I molecule, with an immunodominant response observed in HLA-B7-Tg mice.
Induction of IFN-γ-secreting T cells after immunization with DNA plasmids encoding HBSP.
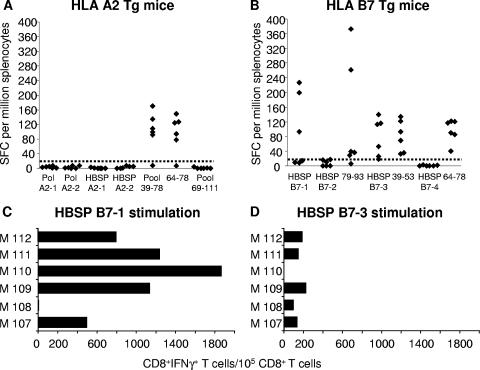
Spleen cells from immunized mice were used in an ex vivo ELISPOT assay for the complete mapping of HBSP peptides recognized by T cells. This assay was used to detect IFN-γ-secreting T cells after overnight stimulation with 9-mer peptides predicted to bind MHC class I molecules or with four pools of 15-mer overlapping peptides covering the HBSP ARF sequence.
In pHBSP-immunized HLA-A2-Tg mice, IFN-γ-secreting T cells were observed only after stimulation with peptide pool 39-78 (including peptides covering the sequence from aa 39 to 78). Fine characterization of the T-cell response showed it to have been induced by the individual peptide spanning aa 64 to 78 (peptide 64-78) from this pool (Fig. 3A). In samples from the 25 mice tested, no IFN-γ-secreting T cells were found after ex vivo stimulation with peptides identified by computer analysis. When the pHBSP 39-111 vector was used, the T-cell response was directed against the HLA-A2-restricted epitope HBs 348-357 only (data not shown). This indicates that the frequency of CD8+ T cells recognizing HBSP A2-1 and HBSP A2-2 was probably low, with these cells being detectable only after in vitro stimulation (see above).
FIG. 3.
Detection of IFN-γ-producing cells among splenocytes from immunized Tg mice. ELISPOT assays were performed ex vivo with splenocytes from HLA-A2-Tg mice immunized with pHBSP (A) and HLA-B7-Tg mice immunized with pHBSP 39-111 (B), and the splenocytes were stimulated with either individual or pooled peptides. Each diamond represents the result for an individual mouse from a representative experiment. Responses are expressed as numbers of SFC per 106 splenocytes. The dotted lines indicate the cutoff point for the ELISPOT assay. Ex vivo frequencies of HBSP B7-1 (C)- and HBSP B7-3 (D)-specific IFN-γ-producing CD8+ T cells were determined using intracellular staining for IFN-γ and surface labeling of splenocytes from six pHBSP 39-111-immunized HLA-B7-Tg mice (M107 to M112). Results are expressed as numbers of IFN-γ-producing cells per 105 CD8+ T cells.
When HLA-B7-Tg mice were immunized with pHBSP 39-111, IFN-γ-secreting T cells were detected after ex vivo activation with the 9-mer peptides HBSP B7-1 (17 positive mice among 47 tested) and HBSP B7-3 (16 positive mice among 41 tested) but not after activation with the HBSP B7-2 (0 positive mice among 31 tested) or HBSP B7-4 (0 positive mice among 25 tested) peptide (data from a representative experiment are presented in Fig. 3B). The quantification of HBSP B7-1-specific T cells by using intracellular staining for IFN-γ-producing cells showed that only CD8+ T cells produced IFN-γ. Frequencies of T cells specific for the immunodominant HBSP B7-1 peptide ranged from 500 to 1,870 IFN-γ-positive cells per 105 CD8+ T cells, whereas frequencies of HBSP B7-3-specific T cells were much lower (Fig. 3C and D).
T-cell reactivity was also observed after the ex vivo stimulation of splenocytes with the 15-mer peptides 79-93 and 39-53, which include the predicted HBSP B7-1 and HBSP B7-3 sequences, respectively. Again, IFN-γ secretion in response to peptide 64-78 was observed. This response occurred in both Tg lineages (Fig. 3A and B) and was due to the activation of CD4+ T cells (data not shown). It was therefore restricted to murine IAb molecules. None of the other 15-mer HBSP peptides activated T cells such that IFN-γ was produced. The immunization of HLA-B7-Tg mice with pHBSP gave similar results, except that no response was observed after HBSP B7-3 peptide stimulation (data not shown). Thus, the processing of the HBSP polypeptide produced in vivo from plasmid DNA efficiently generates peptides that activate T cells, leading to the secretion of IFN-γ. This response focuses primarily on an immunodominant epitope, HBSP B7-1, presented by HLA-B*0702.
Detection of HBSP-specific T-cell responses in HBV-infected patients.
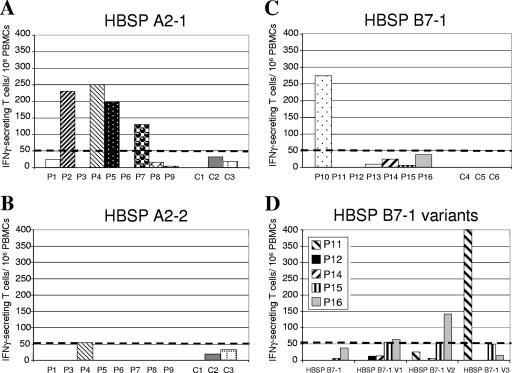
It was then important to determine whether HBSP is processed and presented as peptides during HBV infection in humans. We addressed this question by using frozen PBMCs from patients chronically infected with HBV (9). T-cell responses were first analyzed in an IFN-γ ELISPOT assay by using the two computer-predicted HBSP peptides found to be immunogenic in HLA-A2-Tg mice. PBMCs from four of the nine HLA-A2-positive patients (P2, P4, P5, and P7) displayed significant T-cell activation with the HBSP A2-1 peptide (Fig. 4A). In contrast, neither the HBSP A2-2 peptide nor the peptides derived from Pol induced significant IFN-γ release from the PBMCs of the eight individuals tested (Fig. 4B and data not shown). As expected, no IFN-γ production could be observed after in vitro stimulation of PBMCs from HLA-A2 patients with peptide HBSP B7-1 (Table 2). To appreciate the relevance of the HBSP-specific response during chronic HBV infection, we used three well-described HLA-A2 peptides derived from HBV structural proteins to stimulate PBMCs in parallel assays. Among PBMCs from HLA-A2-positive patients with HBSP-specific T cells, T-cell responses to envelope- and core-derived peptides are not systematically detectable at the same time point (Table 2). As described previously, the number of detectable IFN-γ-secreting T cells is highly variable depending on patient characteristics, the stage of the disease, and the serum viral load (3, 33).
FIG. 4.
Detection of IFN-γ-producing cells among PBMCs from HBV-infected patients in response to peptides identified as potential epitopes by computer analysis. PBMCs from nine HLA-A2-positive patients with chronic HBV infection (P1 to P9) (A and B), seven HBV-infected HLA-B7-positive patients with chronic HBV infection (P10 to P16) (C), and as controls, six HLA-matched healthy HBV-, hepatitis C virus-, and human immunodeficiency virus-seronegative individuals, C1 to C3 (A and B) and C4 to C6 (C), were stimulated with the indicated peptides and analyzed by ELISPOT assays. Results are presented as numbers of IFN-γ-secreting T cells per 106 PBMCs. Responses were considered positive if numbers of IFN-γ-secreting T cells were >50/106 PBMCs and were at least twice the background levels (measured with medium only). (D) PBMCs from five HLA-B7-positive patients with HBV infection were stimulated in vitro with each of the natural variants of the HBSP B7-1 peptide (Table 1), and IFN-γ-secreting T cells against the indicated peptide were quantified. The dotted lines indicate the cutoff point for the ELISPOT assay.
TABLE 2.
Comparison of HBSP-specific T-cell responses to known structural HBV proteins in HLA-A2 patientsa
| Patient | No. of IFN-γ-secreting T cells/106 PBMCs in response to:
|
||||
|---|---|---|---|---|---|
| HBSP A2-1 | Core 18-27 | HBs 183-191 | HBs 348-357 | HBSP B7-1 | |
| P2 | 325 | Neg | Neg | Neg | ND |
| P4 | 92 | Neg | 345 | 183 | Neg |
| P5 | 199 | 2,480 | 1,490 | Neg | Neg |
| P7 | 131 | Neg | Neg | Neg | ND |
Neg, negative response in IFN-γ ELISPOT assays; ND, not determined.
We then performed experiments with peptides predicted to bind the HLA-B7 MHC class I molecule. No response was observed after the in vitro stimulation of PBMCs with HBSP B7-2, HBSP B7-3, and HBSP B7-4 (data not shown). With the peptide corresponding to the HLA-B7-restricted epitope identified as being immunodominant in Tg mice (HBSP B7-1), significant levels of IFN-γ release from PBMCs of only one of seven HLA-B7-positive individuals were detected (Fig. 4C). We checked that the detected responses were HBV specific by analyzing PBMCs from three HBV-negative, HLA-A2-positive and three HBV-negative, HLA-B7-positive individuals. None of these patients had T cells that responded to the HBSP A2-1, HBSP A2-2, or HBSP B7-1 peptide after stimulation (Fig. 4A, B, and C, C1 to C3 and C4 to C6).
We sought an explanation for the low frequency of HBSP B7-1 epitope recognition in humans by checking HBV databases for variations in the sequence of this epitope among natural HBV isolates. PBMCs from five HLA-B7-positive patients displaying no recognition after stimulation with the HBSP B7-1 peptide were stimulated in vitro with each of the naturally occurring variants (V1 to V3) of the HBSP B7-1 epitope (Table 1). ELISPOT assays were then carried out with each of the individual peptides. Among PBMCs from patients P16 and P11, T cells reacting with variant peptides V2 and V3, respectively, were observed (Fig. 4D). Therefore, the HBSP B7-1 epitope was generated during the natural course of chronic HBV infection but could not be considered a dominant epitope in this setting. In contrast, PBMCs from approximately half of the HBV-infected HLA-A2-positive patients tested displayed a response against the HBSP A2-1 epitope.
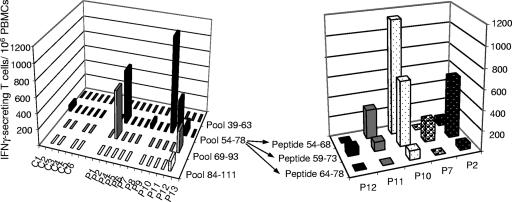
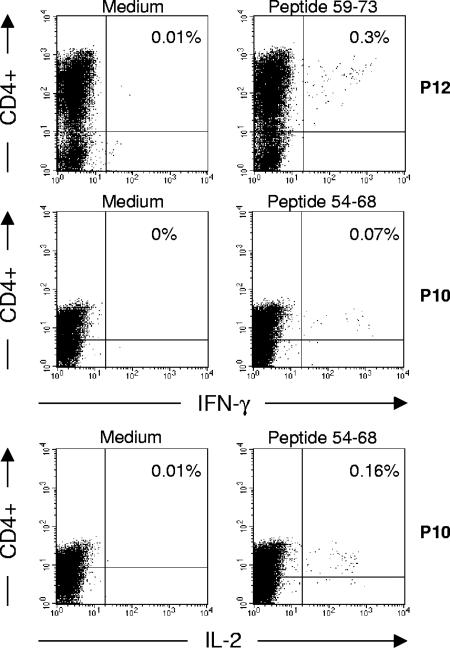
We further documented HBSP-specific responses by using pools of HBSP 15-mers covering the HBSP sequence from aa 39 to 111 translated from the HBV ARF. The PBMCs of the six HBV-negative patients (C1 to C6) did not produce significant amounts of IFN-γ upon exposure to the peptide pools (Fig. 5, left panel). Among PBMCs from five patients with chronic HBV infection, HBSP-specific T cells were found after in vitro stimulation with pools of 15-mer peptides. Incubation with three pools of peptides not corresponding to the N-terminal part of the HBSP region from aa 39 to 111 (aa 39 to 63) induced significant IFN-γ release from T cells. PBMCs could be stimulated by two or three different peptide pools (Fig. 5, left panel, P2 and P12), but the 54-78 pool was the most frequently recognized. When the three peptides of this pool were used individually to activate T cells in ELISPOT assays, all three peptides activated T cells, leading to IFN-γ production (Fig. 5, right panel). However, the highest levels of IFN-γ production were observed following stimulation with peptides 54-68 and 59-73. As the 15-mer peptides used in ELISPOT assays can react with both MHC class I and class II molecules, we analyzed the phenotypes of the IFN-γ-secreting T cells from two patients. Fluorescence-activated cell sorter analysis showed that the HBSP-specific T cells activated by peptides 54-68 and 59-73 were CD4+ T cells (Fig. 6). Intracellular staining for cytokines showed that cells from patient P10 produced both IL-2 and IFN-γ in response to stimulation with peptide 54-68.
FIG. 5.
Detection of IFN-γ-producing cells among PBMCs from HBV-infected patients in response to stimulation with 15-mer peptides. PBMCs from 12 individuals with chronic HBV infection (P1, P2, and P4 to P13) and 6 healthy controls (C1 to C6) were stimulated in vitro with four pools of 15-mer peptides covering the region from amino acids 39 to 111, and IFN-γ-secreting T cells were quantified by ELISPOT assays. Positive responses observed after stimulation with pool 54-78 were further characterized by using the three individual peptides included in the pool. Results are expressed as described in the legend to Fig. 4.
FIG. 6.
Intracellular staining of HBSP-specific T cells. PBMCs from two patients with chronic HBV infection (P10 and P12) were stimulated with medium alone (left panels) or with HBSP-reactive 15-mer peptides (right panels). Phenotypic characterization of cytokine-producing T cells was carried out, together with intracellular staining for IFN-γ and IL-2. The percentages of IFN-γ- or IL-2-producing CD4+ T cells are indicated in the upper right quadrants.
Thus, these results demonstrate the existence of a cellular immune response specific for epitopes derived from HBSP in chronically HBV-infected individuals. This response is multispecific, as several epitopes were recognized, and is mediated by CD8+ and CD4+ T cells.
DISCUSSION
In this study, we used a combination of three approaches to identify T-cell epitopes within a recently identified HBV protein encoded by a 2.2-kb singly spliced HBV RNA. Bioinformatic predictions of peptide-HLA binding and in vivo assays with HLA-transgenic mice were used to define HLA-A2- and HLA-B7-restricted epitopes. Overlapping 15-mer peptides spanning the HBSP sequence were used to characterize the T-cell responses further. HBSP induced potent CD8+-T-cell responses in transgenic mice. Moreover, we found that the epitopes defined by experiments with transgenic mice were able to recall, in vitro, specific IFN-γ-secreting T-cell responses among PBMCs from patients with chronic HBV infection. Using the 15-mer peptide library, we also demonstrated the activation by HBSP of CD4+-T-cell responses in HBV-infected individuals.
HLA-Tg mice with knockouts of murine MHC molecules have proven to be excellent preclinical models for the characterization of epitopes relevant to T-cell recognition in humans. Furthermore, the antigen-processing machinery is highly conserved in mouse and human cells (22). In the first part of this study, we used a strategy for searching for MHC class I-presented stimulating peptides in a previously uninvestigated protein from HBV. The MHC-peptide binding (BIMAS) and epitope prediction (SYFPEITHI) programs identified eight peptides as potential targets of the CD8+-T-cell response in vivo. However, the correlation between predictive scores and the results of T-cell stimulation assays with HLA-Tg mice was not perfect. In our hands, the correlation between epitope prediction and in vivo T-cell responses was better for HLA-B7- than for HLA-A2-restricted epitopes. It should be noted that, although a peptide must bind to MHC molecules to form epitopes, it must first be obtained from the original protein by proteasome cleavage or another processing pathway. There must also be T-cell receptors that bind to these complexes with a sufficiently high affinity for T-cell activation.
We then produced HBSP either alone or as a fusion with the small HBV envelope protein, which carries the HBs antigen. This approach made it possible to compare the relative immunogenicities of these two HBV proteins when coexpressed from the same vector in vivo in HLA-Tg mice. In HLA-B7-transgenic mice immunized with DNA vectors encoding HBSP, the response appeared to focus on an immunodominant epitope (HBSP B7-1), whether HBSP was produced alone or as a fusion with the HBV small envelope protein. However, using the vector encoding the fusion with the HBs antigen, we identified an epitope (HBSP B7-3) not detected after immunization with the vector encoding HBSP only. This finding may be due to the help provided by HBs-specific CD4+ T cells in the development of CD8+-T-cell responses (19). In contrast, in HLA-A2-Tg mice, the response to the full-length HBSP was multiepitopic—targeting at least three epitopes within HBSP—but was sporadic in transgenic animals. When HLA-A2-Tg mice were immunized with the vector encoding the fusion protein, T-cell responses clearly focused on an HLA-A2-restricted HBs-derived epitope. Immunodominance occurs when only a small fraction of all the possible determinants from a given antigen elicit an immune response in vivo (34). HBV envelope proteins have been reported to be strongly immunogenic in both humans and HLA-A2-Tg mice, with well-characterized HLA-A2-restricted epitopes (14, 15, 20). In contrast, few HLA-B7-restricted epitopes derived from envelope proteins have been described as immunogenic in patients (2). This situation is consistent with our findings showing that the HBs 232-240 epitope was less immunogenic than the HBSP B7-1 epitope in immunized HLA-B7-Tg mice (Fig. 2D).
We provide the first evidence of T-cell-mediated immune responses to HBSP in subjects chronically infected with HBV. HBSP has been detected in liver samples from chronic HBV carriers with high levels of viral replication (32). Although we did not directly search for HBSP in the livers of infected individuals in the present study, we were able to identify the signature of the previous or remaining presence of the antigen by demonstrating the presence of both CD4+ and CD8+ T cells specific for HBSP. Several peptides were recognized by memory T cells among PBMCs from chronic HBV-infected individuals but not among PBMCs from control subjects. These findings may reflect the clinical statuses of the patients analyzed. In subjects with chronic HBV infection, other HBV proteins, such as envelope and core proteins, are produced in large amounts and have been implicated in tolerance induction in specific T-cell responses (6, 33). In contrast, HBSP-specific T-cell responses are less likely to be subject to deletion or tolerance induction, probably due to the low levels of HBSP produced.
The relative prevalences of CD8+-T-cell responses specific for the HLA-B7- and HLA-A2-restricted epitopes differed in HLA-Tg mice and humans. Only one of the seven randomly selected HLA-B7-positive patients responded to the HBSP B7-1 epitope found to be immunodominant in immunized HLA-B7-Tg mice. In contrast, T-cell responses to the HBSP A2-1 epitope were more readily detected, as four of the nine patients had T cells specific for this epitope. Variations in the sequence of the HBSP B7-1 epitope of the infecting virus may account for the low prevalence of HLA-B7-positive patients with T cells recognizing this epitope. A comprehensive analysis of naturally occurring HBV viral sequences in the NCBI database (http://www.ncbi.nlm.nih.gov) revealed the existence of several different viral HBSP sequences. The resulting amino acid changes affect the HBSP B7-1 epitope at positions 2 and 9 (Table 1), reducing the efficiency of binding of the corresponding peptides to the HLA-B7 molecule (11, 30). Nevertheless, this region seems to be immunogenic, as peptide variants derived from other HBV strains were able to reactivate T cells in vitro despite the presence of nonconsensus anchor residues in an otherwise conserved sequence. Additional experiments with HLA-B7-Tg mice immunized with the plasmid encoding HBSP and ex vivo stimulation with each of the variant peptides (V1, V2, and V3) revealed no cross-recognition at the T-cell level (data not shown). This finding suggests that the T-cell responses observed in humans probably reflect changes in the DNA sequences of viruses infecting patients rather than the cross-recognition of variant epitopes by T cells.
In addition to HBSP-specific CD8+ T cells, CD4+-T-cell responses were observed after the stimulation of PBMCs from HBV-infected individuals. This observation is consistent with the detection of anti-HBSP antibodies in the sera of patients with chronic hepatitis. HBSP induces apoptosis in vitro (31). If this is also the case in vivo, then we might expect apoptotic bodies generated from HBSP-expressing hepatocytes to activate dendritic cells and to stimulate CD4+ T cells through cross-priming.
HBSP is generated from a spliced HBV RNA found in defective HBV particles (10, 28). These defective forms are maintained in the viral population through trans-complementation with wild-type helper viruses. They have been implicated in viral multiplication during the chronic phase of the disease. In addition, anti-HBSP antibodies are independently associated with viral replication markers, fibrosis severity, and increases in tumor necrosis factor alpha secretion (32). These data suggest that HBSP plays a role in the natural history of HBV infection and may be directly involved in the pathogenicity of HBV infection.
We demonstrate here that HBSP can activate T-cell responses in humans. These T cells, by secreting inflammatory cytokines after the specific recognition of infected liver cells or by recruiting nonspecific inflammatory cells, may ultimately cause liver damage. Therefore, in addition to having a direct effect on HBV pathogenesis, HBSP may be involved in the immunopathogenesis of hepatitis B virus infection.
Acknowledgments
This work was supported by grants from the Agence Nationale de Recherche contre le SIDA et les Hépatites Virales (ANRS) and Institut Pasteur. F. Bayard held an ANRS fellowship. Q. Deng holds a fellowship from the French Ministry of Foreign Affairs and was supported by the French consulate in Shanghai, China.
We thank A. Pajot for assistance with the use of computer algorithms and for helpful discussions.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Bain, C., P. Parroche, J. P. Lavergne, B. Duverger, C. Vieux, V. Dubois, F. Komurian-Pradel, C. Trepo, L. Gebuhrer, G. Paranhos-Baccala, F. Penin, and G. Inchauspe. 2004. Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection. J. Virol. 78:10460-10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertoni, R., J. Sidney, P. Fowler, R. W. Chesnut, F. V. Chisari, and A. Sette. 1997. Human histocompatibility leukocyte antigen-binding supermotifs predict broadly cross-reactive cytotoxic T lymphocyte responses in patients with acute hepatitis. J. Clin. Investig. 100:503-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boni, C., P. Fisicaro, C. Valdatta, B. Amadei, P. Di Vincenzo, T. Giuberti, D. Laccabue, A. Zerbini, A. Cavalli, G. Missale, A. Bertoletti, and C. Ferrari. 2007. Characterization of Hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J. Virol. 81:4215-4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brechot, C. 2004. Pathogenesis of hepatitis B virus-related hepatocellular carcinoma: old and new paradigms. Gastroenterology 127:S56-S61. [DOI] [PubMed] [Google Scholar]
- 5.Bullock, T. N., and L. C. Eisenlohr. 1996. Ribosomal scanning past the primary initiation codon as a mechanism for expression of CTL epitopes encoded in alternative reading frames. J. Exp. Med. 184:1319-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, M., M. Sallberg, J. Hughes, J. Jones, L. G. Guidotti, F. V. Chisari, J. N. Billaud, and D. R. Milich. 2005. Immune tolerance split between hepatitis B virus precore and core proteins. J. Virol. 79:3016-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chisari, F. V. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 8.Chisari, F. V., P. Filippi, J. Buras, A. McLachlan, H. Popper, C. A. Pinkert, R. D. Palmiter, and R. L. Brinster. 1987. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. USA 84:6909-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couillin, I., S. Pol, M. Mancini, F. Driss, C. Brechot, P. Tiollais, and M. L. Michel. 1999. Specific vaccine therapy in chronic hepatitis B: induction of T cell proliferative responses specific for envelope antigens. J. Infect. Dis. 180:15-26. [DOI] [PubMed] [Google Scholar]
- 10.Gunther, S., G. Sommer, A. Iwanska, and H. Will. 1997. Heterogeneity and common features of defective hepatitis B virus genomes derived from spliced pregenomic RNA. Virology 238:363-371. [DOI] [PubMed] [Google Scholar]
- 11.Huczko, E. L., W. M. Bodnar, D. Benjamin, K. Sakaguchi, N. Z. Zhu, J. Shabanowitz, R. A. Henderson, E. Appella, D. F. Hunt, and V. H. Engelhard. 1993. Characteristics of endogenous peptides eluted from the class I MHC molecule HLA-B7 determined by mass spectrometry and computer modeling. J. Immunol. 151:2572-2587. [PubMed] [Google Scholar]
- 12.Koike, K., M. Kobayashi, H. Mizusawa, E. Yoshida, K. Yaginuma, and M. Taira. 1983. Rearrangement of the surface antigen gene of hepatitis B virus integrated in the human hepatoma cell lines. Nucleic Acids Res. 11:5391-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kremsdorf, D., P. Soussan, P. Paterlini-Brechot, and C. Brechot. 2006. Hepatitis B virus-related hepatocellular carcinoma: paradigms for viral-related human carcinogenesis. Oncogene 25:3823-3833. [DOI] [PubMed] [Google Scholar]
- 14.Loirat, D., F. A. Lemonnier, and M. L. Michel. 2000. Multiepitopic HLA-A*0201-restricted immune response against hepatitis B surface antigen after DNA-based immunization. J. Immunol. 165:4748-4755. [DOI] [PubMed] [Google Scholar]
- 15.Maini, M. K., C. Boni, G. S. Ogg, A. S. King, S. Reignat, C. K. Lee, J. R. Larrubia, G. J. Webster, A. J. McMichael, C. Ferrari, R. Williams, D. Vergani, and A. Bertoletti. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8(+) T cells associated with the control of infection. Gastroenterology 117:1386-1396. [DOI] [PubMed] [Google Scholar]
- 16.Mancini, M., H. Davis, P. Tiollais, and M. L. Michel. 1996. DNA-based immunization against the envelope proteins of the hepatitis B virus. J. Biotechnol. 44:47-57. [DOI] [PubMed] [Google Scholar]
- 17.Mayrand, S. M., and W. R. Green. 1998. Non-traditionally derived CTL epitopes: exceptions that prove the rules? Immunol. Today 19:551-556. [DOI] [PubMed] [Google Scholar]
- 18.Michel, M.-L., H. L. Davis, M. Schleef, M. Mancini, P. Tiollais, and R. G. Whalen. 1995. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc. Natl. Acad. Sci. USA 92:5307-5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Milich, D. R., and G. G. Leroux-Roels. 2003. Immunogenetics of the response to HBsAg vaccination. Autoimmun. Rev. 2:248-257. [DOI] [PubMed] [Google Scholar]
- 20.Nayersina, R., P. Fowler, S. Guilhot, G. Missale, A. Cerny, H. J. Schlicht, A. Vitiello, R. Chesnut, J. L. Person, A. G. Redeker, et al. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 150:4659-4671. [PubMed] [Google Scholar]
- 21.Parker, K. C., M. A. Bednarek, and J. E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163-175. [PubMed] [Google Scholar]
- 22.Pascolo, S. 2005. HLA class I transgenic mice: development, utilisation and improvement. Expert Opin. Biol. Ther. 5:919-938. [DOI] [PubMed] [Google Scholar]
- 23.Pascolo, S., N. Bervas, J. M. Ure, A. G. Smith, F. A. Lemonnier, and B. Perarnau. 1997. HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J. Exp. Med. 185:2043-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penna, A., F. V. Chisari, A. Bertoletti, G. Missale, P. Fowler, T. Giuberti, F. Fiaccadori, and C. Ferrari. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 174:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 26.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215-229. [DOI] [PubMed] [Google Scholar]
- 27.Rohrlich, P. S., S. Cardinaud, H. Firat, M. Lamari, P. Briand, N. Escriou, and F. A. Lemonnier. 2003. HLA-B*0702 transgenic, H-2KbDb double-knockout mice: phenotypical and functional characterization in response to influenza virus. Int. Immunol. 15:765-772. [DOI] [PubMed] [Google Scholar]
- 28.Rosmorduc, O., M. A. Petit, S. Pol, F. Capel, F. Bortolotti, P. Berthelot, C. Brechot, and D. Kremsdorf. 1995. In vivo and in vitro expression of defective hepatitis B virus particles generated by spliced hepatitis B virus RNA. Hepatology 22:10-19. [PubMed] [Google Scholar]
- 29.Shastri, N., S. Schwab, and T. Serwold. 2002. Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 20:463-493. [DOI] [PubMed] [Google Scholar]
- 30.Sidney, J., M. F. del Guercio, S. Southwood, V. H. Engelhard, E. Appella, H. G. Rammensee, K. Falk, O. Rotzschke, M. Takiguchi, R. T. Kubo, et al. 1995. Several HLA alleles share overlapping peptide specificities. J. Immunol. 154:247-259. [PubMed] [Google Scholar]
- 31.Soussan, P., F. Garreau, H. Zylberberg, C. Ferray, C. Brechot, and D. Kremsdorf. 2000. In vivo expression of a new hepatitis B virus protein encoded by a spliced RNA. J. Clin. Investig. 105:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soussan, P., R. Tuveri, B. Nalpas, F. Garreau, F. Zavala, A. Masson, S. Pol, C. Brechot, and D. Kremsdorf. 2003. The expression of hepatitis B spliced protein (HBSP) encoded by a spliced hepatitis B virus RNA is associated with viral replication and liver fibrosis. J. Hepatol. 38:343-348. [DOI] [PubMed] [Google Scholar]
- 33.Webster, G. J., S. Reignat, D. Brown, G. S. Ogg, L. Jones, S. L. Seneviratne, R. Williams, G. Dusheiko, and A. Bertoletti. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78:5707-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yewdell, J. W., and J. R. Bennink. 1999. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu. Rev. Immunol. 17:51-88. [DOI] [PubMed] [Google Scholar]