Abstract
The Epstein-Barr virus (EBV) BGLF4 gene product is a protein kinase (PK). Although this kinase has been characterized and several of its targets have been identified, its biological role remains enigmatic. We have generated and assessed a BGLF4 knockdown phenotype by means of RNA interference and report the following: (i) BGLF4-targeting small interfering RNA effectively inhibited the expression of its product, the viral PK, during lytic reactivation, (ii) BGLF4 knockdown partially inhibited viral DNA replication and expression of selected late viral genes, (iii) the absence of EBV PK resulted in retention of the viral nucleocapsids in the nuclei, and (iv) as a result of the nuclear retention, release of infectious virions is significantly retarded. Our results provide evidence that EBV PK plays an important role in nuclear egress of the virus and ultimately is crucial for lytic virus replication.
Phosphorylation/dephosphorylation is one of the most common ways to regulate the activity of proteins, and viruses often hijack cellular kinases or encode their own, with the result that cellular machinery is subverted into support of viral replication. All human herpesviruses encode at least one protein kinase (PK), and these PKs can be divided into two groups, exemplified by the alphaherpesvirus-encoded PKs. These PKs have been suggested to play roles in viral gene expression (37), inhibition of apoptosis (26), viral DNA synthesis and encapsidation (44), and nuclear egress (24, 27, 39). The group exemplified by the herpes simplex virus (HSV) UL13 PK is encoded by all herpesviruses, and its conservation across the different herpesvirus subfamilies (alpha-, beta-, and gammaherpesviruses) (4, 41) indicates the significant role of this group of PKs in viral replication and pathogenesis.
The Epstein-Barr virus (EBV) BGLF4 gene product, a UL13 homologue, is a serine/threonine PK and is the only PK identified in the EBV genome (4, 41). EBV PK has an early expression kinetics, and its levels remain high throughout the EBV lytic program (14). It is detected mainly in the nuclei of EBV-infected cells (14, 43). Although only a limited number of targets for EBV PK have been identified thus far, their variety implies a multiplicity of processes and steps in viral replication in which this PK is involved. The EBV PK targets identified to date are as follows: the EBV BMRF1 gene product (5, 15), the viral DNA polymerase processivity factor; EBNA2 (46), a key EBV latency transcriptional regulator; the EBNA2 coactivator EBNA-LP (19); BGLF4 itself (5, 13, 18, 19); the EBV BZLF1 gene product (1), a multifunctional protein, best known as initiator of the EBV lytic program (22, 40); and cellular translation elongation factor 1δ (18, 20). Similar to other UL13 homologues, EBV PK is a part of the tegument (1, 43), a virion structural element whose components are thought to play significant roles in establishing favorable conditions for viral replication. EBV PK demonstrates a reasonable functional similarity to other members of the group (20, 21); however, compounds that inhibited the enzymatic activity of human cytomegalovirus (HCMV) UL97 (homologous to EBV PK) (24, 28, 29, 48) failed to inhibit EBV PK in vitro (13). Interestingly, maribavir, an antiviral compound that inhibits replication of both HCMV (3, 33) and EBV (47) and is thought to act through the viral PK, failed to inhibit EBV PK as well (13). The biological significance of EBV PK-mediated phosphorylation is unclear for all of its targets, and even though this phosphorylation has been linked to reduction of transcriptional activity for EBNA2 and EBNA-LP (19, 45, 46), its consequences in the context of viral infection have never been explored.
Thus, one of the major questions that remained unanswered is that of the precise role(s) of EBV PK in the viral life cycle. While HSV-1 UL13 and HCMV UL97 deletion mutants have been created and their phenotypes characterized (6, 34, 35), an EBV BGLF4 deletion mutant has not been characterized yet. Here, we address this question by knocking down EBV BGLF4 expression by using RNA interference (RNAi) techniques during reactivation of the viral lytic cycle. We take advantage of 293 cells that harbor recombinant EBV, which expresses a hygromycin resistance gene and green fluorescent protein (GFP) (7), and in which lytic infection can be easily induced by EBV BZLF1 expression. In this system, we demonstrate that (i) EBV PK protein expression diminished to undetectable levels upon expression of BGLF4-targeting small interfering RNA (siRNA); (ii) EBV PK knockdown partially inhibited viral DNA synthesis and expression of selected late genes; (iii) in contrast, this knockdown greatly reduced the amount of infectious virus released during viral lytic reactivation; and (iv) virion release is blocked at the stage of nuclear egress, likely through its interaction with components of the primary envelopment complex.
Inhibition of EBV BGLF4 expression by RNAi.
In order to generate a BGLF4 knockdown phenotype, we designed a number of siRNAs targeting different regions of BGLF4 mRNA and screened their abilities to inhibit transient EBV PK expression in the 293 HEK cell line (data not shown). Both the negative control (si-NC) and the BGLF4-targeting siRNAs were cloned into pHTPsiRNA (a gift from William Reed, University of North Carolina at Chapel Hill). Transfections were performed using Lipofectamine 2000 (Invitrogen), following the manufacturer's procedure. The best-performing siRNA (designated si-PK) was selected for further experiments. We first tested the ability of si-PK to inhibit EBV PK expression during viral lytic reactivation. Viral lytic reactivation was achieved by transient expression of the EBV BZLF1 gene in 293/EBV+ cells (pSG5/BZLF1 is a gift from Shannon Kenney, University of North Carolina at Chapel Hill). 293/EBV+ (a gift from Henri-Jacques Delecluse, German Cancer Research Center, Heidelberg, Germany) is a 293 human embryonic kidney cell line harboring a recombinant EBV genome that expresses GFP and a hygromycin resistance gene (7). Expression of the EBV PK, EBV EA-D, and EBV BZLF1 proteins upon induction of the lytic program was analyzed by immunoblotting (Fig. 1A). Monoclonal antibodies (MAb) against γ-tubulin were purchased from Sigma (St. Louis, MO); MAb against EA-D were purchased from Bioworld Consulting Laboratories (Mt. Airy, MD); polyclonal EBV PK antibody was generated and described previously (14). MAb against p-ERK and secondary donkey anti-mouse and anti-rabbit horseradish peroxidase-conjugated antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Preparation of the whole-cell lysates and immunoblotting were described previously (15).
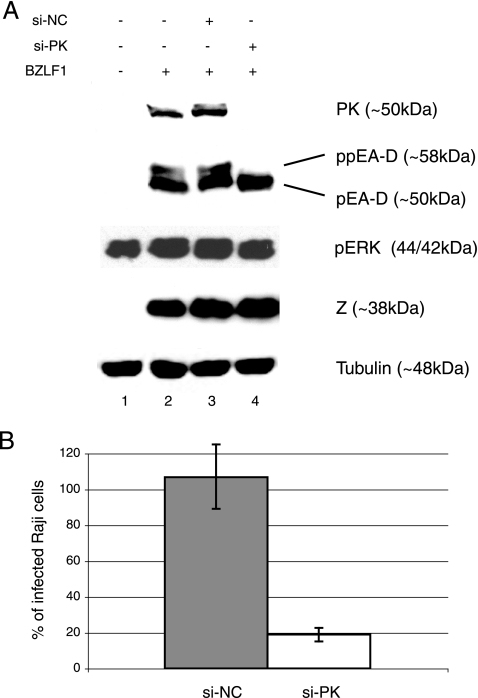
FIG. 1.
EBV BGLF4-targeting siRNA (si-PK) abolishes expression of EBV PK and hyperphosphorylation of EA-D during viral lytic reactivation and reduces EBV infectivity. (A) The viral lytic program was induced in 293/EBV+ cells by the expression of EBV BZLF1 alone or in combination with si-NC (negative control) or si-PK. Whole-cell lysates were prepared at 48 h postinduction, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by immunoblotting. Tubulin served as a loading control. (B) Raji cells were coincubated with the supernatants collected at 48 h posttransfection, and the number of infected (GFP-expressing) cells was determined by fluorescence-activated cell sorting analysis. The graph represents results from three independent experiments and shows percentages of infected cells, normalized to negative (0%) and positive (100%) controls, ± standard deviations.
None of the viral lytic proteins was expressed in mock-transfected cells (Fig. 1A, lane 1), whereas EBV-BZLF1 expression resulted in the appearance of both EBV PK and EA-D proteins (Fig. 1A, lane 2). While si-NC did not significantly affect the expression profiles of the analyzed proteins, si-PK abolished EBV PK expression and also resulted in loss of the hyperphosphorylated form of EA-D, a known EBV PK target (5, 15), as shown by loss of the more slowly migrating upper band (Fig. 1A, lanes 3 and 4). Equal levels of transfected BZLF1 protein in all lanes in Fig. 1A suggest comparable levels of viral reactivation in all samples. Equal levels of phosphorylated ERK (p44/p42) indicate that overall PK activities in the cell remain unaffected. These data confirmed the validity of the RNAi approach in generating a viral knockdown phenotype(s) by targeting respective viral genes.
EBV PK knockdown reduces infectivity of released virus.
Since EBV titers cannot be evaluated by classical plaque assays, there have been no established means for assessing the final outcome of viral lytic infection. The use of recombinant EBV that expresses GFP allows semiquantitation of viral titers through a superinfection of susceptible cell lines (such as Raji, a Burkitt's lymphoma cell line latently infected with EBV [36]) and analysis of GFP expression. In application of this method, 900 μl of virus-containing supernatants collected from 293/EBV+ transfected cells was used to infect 2 × 105 Raji cells (Fig. 1B), and after 48 to 72 h, GFP expression was analyzed by flow cytometry. The assays demonstrated that EBV PK knockdown resulted in significant reduction of viral titers (up to 90%) as reflected by the number of GFP-expressing Raji cells. The results indicate that EBV PK is critical for production of infectious virus.
Effect of EBV PK knockdown on viral DNA synthesis.
EBV PK has previously been shown to hyperphosphorylate viral DNA polymerase processivity factor EA-D (the BMRF1 gene product) (5, 14, 15), although the importance of this modification remains unclear. Assuming that the knockdown of EBV PK could affect DNA viral synthesis, we have assessed it by quantitative PCR using a TaqMan probe targeting the EBV BamHI fragment, which has previously been described in detail (8, 9). Briefly, total genomic DNA was isolated from 293/EBV+ cells 48 h after viral reactivation using a DNeasy tissue kit (QIAGEN). One microliter of this DNA was analyzed by real-time PCR using an ABI 7900HT real-time PCR system under the following cycle conditions: 50°C for 2 min (1 cycle), 95°C for 10 min (1 cycle), and 95°C for 15 s and 60°C for 1 min (40 cycles). Viral DNA copy numbers were calculated from external standards of known concentrations of EBV B95-8 DNA (purchased from Advanced Biotechnologies Inc., MD). The primers and the probe used for PCR are EBVW-1 (5′-GCAGCCGCCCAGTCTCT-3′), EBVW-2 (5′-ACAGACAGTGCACAGGAGCCT-3′), and EBVW-FAM (5′-FAM [6-carboxyfluorescein]-AAAAGCTGGCGCCCTTGCCTG-TAMRA [6-carboxytetramethylrhodamine]-3′).
In the absence of viral lytic reactivation, 6.04 × 103 copies/ng of total DNA were detected. Upon reactivation, the copy numbers increased about 10-fold, reaching 5.85 × 104 copies/ng. Expression of si-NC resulted in a slight increase (6.76 × 104 copies/ng), while expression of si-PK resulted in about a 30% decrease (4.2 × 104 copies/ng) in copy numbers. Thus, although these results indicate that EBV DNA synthesis is affected by the absence of the viral PK, a significant drop in viral infectivity can hardly be explained by this reduction in viral DNA synthesis. The results are summarized in Table 1.
TABLE 1.
Quantitation of intracellular and extracellular viral DNA by real-time PCRa
| Viral DNA type | EBV DNA copy number for indicated treatment
|
|||
|---|---|---|---|---|
| Mock treated | Z | si-NC | si-PK | |
| Extracellularb | 7.98 × 102 | 7.67 × 104 | 7.68 × 104 | 5.48 × 103 |
| Intracellularc | 6.04 × 103 | 5.85 × 104 | 6.76 × 104 | 4.2 × 104 |
| e/i ratiod | 0.132 | 1.311 | 1.136 | 0.131 |
Total DNA was isolated from cells and DNase-treated supernatants as described in the text. The viral DNA was quantified in 1 μl of total cellular or 4 μl of supernatant-derived DNA by real-time PCR.
Extracellular viral DNA levels are expressed as numbers of copies per ml of the supernatant.
Intracellular viral DNA levels are expressed as numbers of copies per ng of the total DNA.
e, extracellular viral DNA; i, intracellular viral DNA.
Viral protein expression during EBV PK knockdown.
Next, we analyzed the consequences of EBV PK knockdown in the context of viral lytic infection. Expression levels for viral proteins from latent, immediate-early, early, and late classes were evaluated by immunofluorescence (IF) and/or immunoblotting. For IF, cells were fixed with 3.7% formaldehyde, followed by permeabilization in 0.1% Triton X-100. Viral proteins were detected by sequential incubation with respective primary antibodies followed by donkey anti-mouse Alexa-594-conjugated antibody (Molecular Probes). MAb against EA-D, gp350/220, and viral capsid antigen (VCA) were purchased from Bioworld Consulting Laboratories (Mt. Airy, MD); MAb against BFRF1 and BFLF2 were gifts from A. Faggioni (Università di Roma La Sapienza, Rome, Italy). Samples were analyzed with a Zeiss Axiovert 200 microscope with a 5× or 10× objective, and digital images were captured with OpenLab imaging software (Improvision) and an Axiocam cooled charge-coupled-device camera.
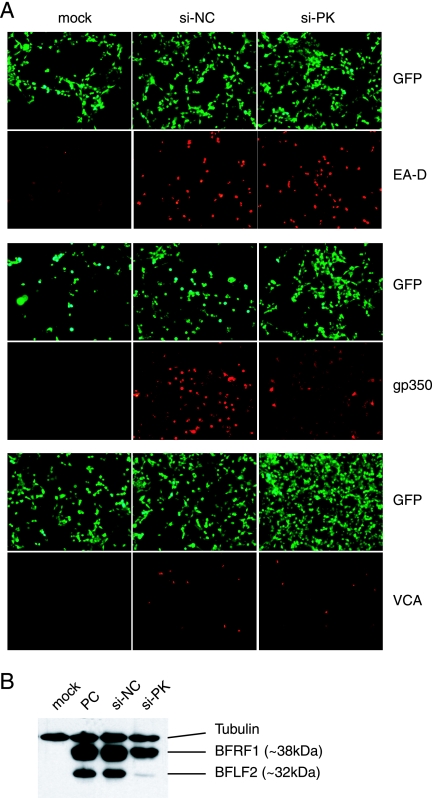
Based on these assays, the tested proteins can be divided into three groups: (i) proteins whose expression did not change, (ii) proteins whose expression changed slightly, and finally, (iii) proteins whose expression changed drastically during EBV PK knockdown. The first group consists of immediate-early protein R as well as several early proteins: EA-D (Fig. 1A and 2A) and the large (BORF2 gene product) and the small (BaRF1 gene product) subunits of the viral ribonucleotide reductase (not shown). The second group is exemplified by the BFRF1 protein, one of the factors involved in nuclear egress (Fig. 2B), and the late proteins gp350/220 and VCA (Fig. 2A). The third group includes BFLF2, another nuclear egress factor, whose expression was almost abolished. None of the proteins could be detected in negative controls (not shown) or in mock-induced cells (Fig. 2B). In contrast, all of them could be readily detected during lytic reactivation (Fig. 1A and 2A and B). These observations demonstrated a potential involvement of EBV PK in the regulation of several pathways during the viral lytic program. In addition, the abolishment of BFLF2 expression suggests a potential role for the kinase in nuclear egress.
FIG. 2.
Effects of EBV PK knockdown on the course of the viral lytic program. 293/EBV+ cells were treated as described for Fig. 1. (A) Expression of early (EA-D) and late (gp350 and VCA) viral proteins was visualized by indirect IF microscopy with the respective antibodies. GFP expression indicates the total number of EBV+ cells. (B) Whole-cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the expression of the EBV BFRF1 and BFLF2 proteins was determined by immunoblotting. PC, positive control.
EBV PK knockdown inhibits production of infectious virus at nuclear egress.
The markedly reduced capacities of supernatants from cells in which EBV PK has been knocked down to infect Raji cells (Fig. 1B) and the loss of BFLF2 expression suggest that the kinase may be involved in regulation of nuclear egress. To examine this hypothesis, we quantitated levels of virion-associated DNA in supernatants by real-time PCR. For the preparation of DNA from intact virions, 100 μl of the filtered supernatants was treated with 2 μl of RQ1 DNase (Promega) for 1 h at 37°C, and reactions were stopped by the addition of EDTA. Total DNAs were prepared from supernatants with a DNeasy tissue kit (QIAGEN) according to the manufacturer's instructions. The viral DNA was quantified by real-time PCR as described above.
Supernatants from the mock-transfected 293/EBV+ cells contained 7.98 × 102 copies/ml of supernatant. Viral reactivation resulted in an approximately 100-fold increase in copy numbers, reaching 7.67 × 104 copies/ml for the positive control (addition of EBV Z only) and 7.68 × 104 copies/ml for the si-NC sample. In contrast, si-PK expression resulted in an almost 15-fold drop in EBV genome copy numbers (5.48 × 103 copies/ml). Moreover, due to the fact that si-PK slightly inhibited viral DNA replication, we calculated the ratio of extracellular, DNase-resistant viral DNA to total intracellular viral DNA and found that this ratio for the si-PK-treated group is very close to that for the mock-treated group (0.131 and 0.132, respectively), while during viral reactivation or si-NC treatment, the ratios were significantly higher (1.311 and 1.136, respectively) (Table 1). These data reiterate the Raji infectivity data (Fig. 1B) and suggest EBV PK involvement in regulation of viral nuclear egress.
Finally, we have assessed subcellular distribution of EBV particles in 293/EBV+ cells by electron microscopy. The cells were fixed with 2% glutaraldehyde and then postfixed in 1% osmium tetroxide in veronal acetate buffer (pH 7.4) for 1 h at 25°C, stained with 0.1% tannic acid in the same buffer for 30 min at 25°C and with uranyl acetate (5 mg/ml) for 1 h at 25°C, dehydrated in acetone, and embedded in Epon 812. Thin sections were examined either unstained or poststained with uranyl acetate and lead hydroxide, using a Morgagni 268D electron microscope (Philips Electron Optics, Eindhoven, The Netherlands). For each transfectant, 25 sections of cells positive for the presence of nucleocapsids in the nucleus were recorded with a charge-coupled-device camera and analyzed with Analysis software (Soft Imaging System GmbH, Münster, Germany). Figure 3 depicts representative samples used for these analyses.
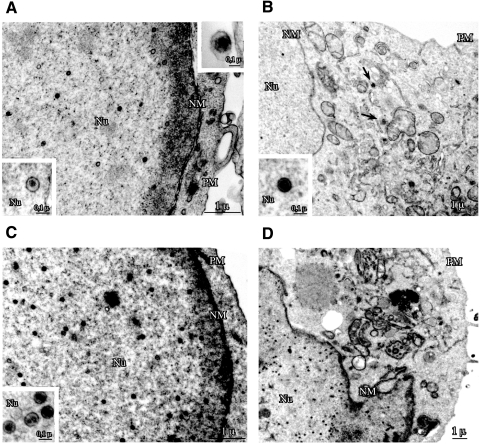
FIG. 3.
EBV PK knockdown causes nuclear retention of viral capsids. The lytic program was reactivated in 293/EBV+ cells as described for Fig. 1, and the cells were fixed in 2% glutaraldehyde at 48 h postreactivation. Ultrathin sections were analyzed by electron microscopy. The cells were transfected with BZLF1 only (A) to reactivate the viral lytic program or cotransfected with si-NC (B) or si-PK (C and D). Arrows point to capsids in the cytoplasm (B). Nu, nucleus; NM, nuclear membrane; PM, plasma membrane.
As shown in Table 2, expression of si-NC practically did not change the number or the distribution of virions, whereas in the presence of si-PK, the majority of viral particles were detected in the nucleus, and virtually no virions were detected in the cytoplasm. Taken together, these observations are consistent with a block of viral nuclear egress with subsequent sequestration of nucleocapsids in the nucleus.
TABLE 2.
Quantitation of nucleocapsid distribution during lytic viral reactivation in 293/EBV+ cellsa
| Cell type | No. of nucleocapsids/ 10-μm2 nuclear areab | Total nuclear area (μm2) | No. of virions/10-μm2 cytoplasmic areac | Total cytoplasmic area (μm2) |
|---|---|---|---|---|
| PC | 5.48 ± 0.97 | 2,601.8 | 0.44 ± 0.20 | 2,916.7 |
| si-PK | 13.1 ± 1.86 | 2,201.4 | 0.02 ± 0.01 | 1,796.9 |
| si-NC | 3.45 ± 0.53 | 2,348.1 | 0.24 ± 0.07 | 2,310.8 |
293/EBV+ cells were transfected for 48 h and then fixed in 2% glutaraldehyde and analyzed by electron microscopy. PC, positive control (cells transfected with EBV BZLF1 only); si-PK, cells cotransfected with BZLF1 and BGLF4-targeting siRNA; si-NC, cells cotransfected with BZLF1 and irrelevant siRNA. For each transfectant, 25 sections of cells positive for the presence of nucleocapsids in the nucleus were recorded with a charge-coupled-device camera. Results are presented as means ± standard errors.
P values (Student's t test) were as follows: for PC versus si-PK, <0.01; for PC versus si-NC, not significant; for si-PK versus si-NC, <0.001.
P values (Student's t test) were as follows: for PC versus si-PK, <0.05; for PC versus si-NC, not significant; for si-PK versus si-NC, <0.01.
Discussion.
In this study, we have generated an EBV BGLF4 knockdown phenotype in order to begin to understand the biological role of the PK encoded by this gene. The results of our analyses provide strong evidence for a role for EBV PK in regulation of primary envelopment, a process in which a maturing viral particle is enveloped while crossing the nuclear membrane. This assertion is based on similarities between the BGLF4 knockdown phenotype and a BFRF1 knockout phenotype reported previously (10). The BFRF1/BFLF2 complex is homologous both positionally and most likely functionally to the UL34/UL31 complex of the alphaherpesviruses (2, 30). Nuclear egress and viral components involved in this process have been best studied with alphaherpesviruses (31, 32). Comparison of these components among the different groups of herpesviruses suggests a high degree of functional conservation (2, 30). Requirement of the US3 kinase for the optimal function of the UL34/UL31 complex has been demonstrated by several groups (17, 23, 38, 39, 42), and both components of the complex are phosphoproteins (10, 11, 16). EBV encodes no homologs to US3, but the EBV BGLF4, a homolog of the HSV UL13 kinase, potentially substitutes for functions of both US3 and UL13. Lake and Hutt-Fletcher have attempted to show the involvement of EBV PK in the distribution of the BFRF1/BFLF2 complex but could not detect differences in the distribution or phosphorylation profile of either protein (25). In contrast, several lines of evidence, including our data, indicate EBV PK involvement. First, HCMV UL97 PK, a homolog of EBV PK, is required for efficient nuclear egress of HCMV (24) although it is unclear whether UL97 is able to directly phosphorylate the p35/p38 complex (homologous to UL34/UL31), or it functions indirectly and is involved in nuclear lamina disruption through interaction with p32 (lamina-associated protein) and phosphorylation of the lamin B receptor (27). Second, UL13 PK may regulate distribution of the UL34/UL31 complex directly or through the phosphorylation of US3 kinase, as suggested by Kato et al. based on comparison of UL13 and US3 deletion mutant phenotypes and the ability of UL13 to phosphorylate US3 in vitro (17). Finally, our preliminary results indicate that EBV PK is able to phosphorylate at least one component of the BFRF1/BFLF2 complex in vitro (data not shown); effects of this phosphorylation on EBV lytic reactivation are currently being evaluated. Thus, although the precise mechanism by which the EBV PK regulates nuclear egress remains uncertain, the data clearly demonstrate for the first time a role for this protein in the context of EBV infection. It is important to emphasize two major limitations in our approach. First, the demonstrated effect of EBV PK knockdown on primary envelopment does not exclude the possibility of EBV PK involvement in other aspects of infection. For instance, with this approach we could not assess the potential contribution of the kinase to tegumentation and primary infection, which may be controlled in part by the kinase as well. Second, the 293/EBV+ cells used in this study represent an attractive model system of epithelial cells susceptible to EBV infection (7, 12); however, the observed phenotype may not be identical in B lymphocytes.
In summary, we have shown that BGLF4 gene expression is crucial for production of infectious EBV progeny, which makes its product, a unique PK, a potential target for antiviral therapy. Our results suggest a role for this kinase in EBV biology as a regulator of viral nuclear egress.
Acknowledgments
We are thankful to K. Hong, C. Meier, and D. Scheswohl for technical assistance and J. Shackelford for critical discussions. We thank A. Faggioni for antibody against EBV BFLF2.
This work is supported by research grant HL064851 from the National Institutes of Health and by grants from MIUR, Ministero della Salute, and Associazione Italiana per la Ricerca sul Cancro (AIRC), Italy.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Asai, R., A. Kato, K. Kato, M. Kanamori-Koyama, K. Sugimoto, T. Sairenji, Y. Nishiyama, and Y. Kawaguchi. 2006. Epstein-Barr virus protein kinase BGLF4 is a virion tegument protein that dissociates from virions in a phosphorylation-dependent process and phosphorylates the viral immediate-early protein BZLF1. J. Virol. 80:5125-5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, et al. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature 310:207-211. [DOI] [PubMed] [Google Scholar]
- 3.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole l-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chee, M. S., G. L. Lawrence, and B. G. Barrell. 1989. Alpha-, beta- and gammaherpesviruses encode a putative phosphotransferase. J. Gen. Virol. 70:1151-1160. [DOI] [PubMed] [Google Scholar]
- 5.Chen, M. R., S. J. Chang, H. Huang, and J. Y. Chen. 2000. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J. Virol. 74:3093-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulter, L. J., H. W. Moss, J. Lang, and D. J. McGeoch. 1993. A mutant of herpes simplex virus type 1 in which the UL13 protein kinase gene is disrupted. J. Gen. Virol. 74:387-395. [DOI] [PubMed] [Google Scholar]
- 7.Delecluse, H. J., T. Hilsendegen, D. Pich, R. Zeidler, and W. Hammerschmidt. 1998. Propagation and recovery of intact, infectious Epstein-Barr virus from prokaryotic to human cells. Proc. Natl. Acad. Sci. USA 95:8245-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan, H., and M. L. Gulley. 2001. Epstein-Barr viral load measurement as a marker of EBV-related disease. Mol. Diagn. 6:279-289. [DOI] [PubMed] [Google Scholar]
- 9.Fan, H., S. C. Kim, C. O. Chima, B. F. Israel, K. M. Lawless, P. A. Eagan, S. Elmore, D. T. Moore, S. A. Schichman, L. J. Swinnen, and M. L. Gulley. 2005. Epstein-Barr viral load as a marker of lymphoma in AIDS patients. J. Med. Virol. 75:59-69. [DOI] [PubMed] [Google Scholar]
- 10.Farina, A., R. Feederle, S. Raffa, R. Gonnella, R. Santarelli, L. Frati, A. Angeloni, M. R. Torrisi, A. Faggioni, and H. J. Delecluse. 2005. BFRF1 of Epstein-Barr virus is essential for efficient primary viral envelopment and egress. J. Virol. 79:3703-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farina, A., R. Santarelli, R. Gonnella, R. Bei, R. Muraro, G. Cardinali, S. Uccini, G. Ragona, L. Frati, A. Faggioni, and A. Angeloni. 2000. The BFRF1 gene of Epstein-Barr virus encodes a novel protein. J. Virol. 74:3235-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fingeroth, J. D., M. E. Diamond, D. R. Sage, J. Hayman, and J. L. Yates. 1999. CD21-dependent infection of an epithelial cell line, 293, by Epstein-Barr virus. J. Virol. 73:2115-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershburg, E., K. Hong, and J. S. Pagano. 2004. Effects of maribavir and selected indolocarbazoles on Epstein-Barr virus protein kinase BGLF4 and on viral lytic replication. Antimicrob. Agents Chemother. 48:1900-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gershburg, E., M. Marschall, K. Hong, and J. S. Pagano. 2004. Expression and localization of the Epstein-Barr virus-encoded protein kinase. J. Virol. 78:12140-12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gershburg, E., and J. S. Pagano. 2002. Phosphorylation of the Epstein-Barr virus (EBV) DNA polymerase processivity factor EA-D by the EBV-encoded protein kinase and effects of the l-riboside benzimidazole 1263W94. J. Virol. 76:998-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonnella, R., A. Farina, R. Santarelli, S. Raffa, R. Feederle, R. Bei, M. Granato, A. Modesti, L. Frati, H. J. Delecluse, M. R. Torrisi, A. Angeloni, and A. Faggioni. 2005. Characterization and intracellular localization of the Epstein-Barr virus protein BFLF2: interactions with BFRF1 and with the nuclear lamina. J. Virol. 79:3713-3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato, A., M. Yamamoto, T. Ohno, M. Tanaka, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2006. Herpes simplex virus 1-encoded protein kinase UL13 phosphorylates viral Us3 protein kinase and regulates nuclear localization of viral envelopment factors UL34 and UL31. J. Virol. 80:1476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kato, K., Y. Kawaguchi, M. Tanaka, M. Igarashi, A. Yokoyama, G. Matsuda, M. Kanamori, K. Nakajima, Y. Nishimura, M. Shimojima, H. T. Phung, E. Takahashi, and K. Hirai. 2001. Epstein-Barr virus-encoded protein kinase BGLF4 mediates hyperphosphorylation of cellular elongation factor 1delta (EF-1delta): EF-1delta is universally modified by conserved protein kinases of herpesviruses in mammalian cells. J. Gen. Virol. 82:1457-1463. [DOI] [PubMed] [Google Scholar]
- 19.Kato, K., A. Yokoyama, Y. Tohya, H. Akashi, Y. Nishiyama, and Y. Kawaguchi. 2003. Identification of protein kinases responsible for phosphorylation of Epstein-Barr virus nuclear antigen leader protein at serine-35, which regulates its coactivator function. J. Gen. Virol. 84:3381-3392. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi, Y., K. Kato, M. Tanaka, M. Kanamori, Y. Nishiyama, and Y. Yamanashi. 2003. Conserved protein kinases encoded by herpesviruses and cellular protein kinase cdc2 target the same phosphorylation site in eukaryotic elongation factor 1δ. J. Virol. 77:2359-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawaguchi, Y., T. Matsumura, B. Roizman, and K. Hirai. 1999. Cellular elongation factor 1δ is modified in cells infected with representative alpha-, beta-, or gammaherpesviruses. J. Virol. 73:4456-4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2574. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 23.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 24.Krosky, P. M., M. C. Baek, and D. M. Coen. 2003. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 77:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lake, C. M., and L. M. Hutt-Fletcher. 2004. The Epstein-Barr virus BFRF1 and BFLF2 proteins interact and coexpression alters their cellular localization. Virology 320:99-106. [DOI] [PubMed] [Google Scholar]
- 26.Leopardi, R., C. Van Sant, and B. Roizman. 1997. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc. Natl. Acad. Sci. USA 94:7891-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marschall, M., A. Marzi, P. Aus dem Siepen, R. Jochmann, M. Kalmer, S. Auerochs, P. Lischka, M. Leis, and T. Stamminger. 2005. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J. Biol. Chem. 280:33357-33367. [DOI] [PubMed] [Google Scholar]
- 28.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van den Bogaard, and T. Stamminger. 2002. Direct targeting of human cytomegalovirus protein kinase pUL97 by kinase inhibitors is a novel principle for antiviral therapy. J. Gen. Virol. 83:1013-1023. [DOI] [PubMed] [Google Scholar]
- 29.Marschall, M., M. Stein-Gerlach, M. Freitag, R. Kupfer, M. van Den Bogaard, and T. Stamminger. 2001. Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97. J. Gen. Virol. 82:1439-1450. [DOI] [PubMed] [Google Scholar]
- 30.McGeoch, D. J., M. A. Dalrymple, A. J. Davison, A. Dolan, M. C. Frame, D. McNab, L. J. Perry, J. E. Scott, and P. Taylor. 1988. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J. Gen. Virol. 69:1531-1574. [DOI] [PubMed] [Google Scholar]
- 31.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 32.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migawa, M. T., J. L. Girardet, J. A. Walker II, G. W. Koszalka, S. D. Chamberlain, J. C. Drach, and L. B. Townsend. 1998. Design, synthesis, and antiviral activity of alpha-nucleosides: D- and L-isomers of lyxofuranosyl- and (5-deoxylyxofuranosyl)benzimidazoles. J. Med. Chem. 41:1242-1251. [DOI] [PubMed] [Google Scholar]
- 34.Overton, H., D. McMillan, L. Hope, and P. Wong-Kai-In. 1994. Production of host shutoff-defective mutants of herpes simplex virus type 1 by inactivation of the UL13 gene. Virology 202:97-106. [DOI] [PubMed] [Google Scholar]
- 35.Prichard, M. N., N. Gao, S. Jairath, G. Mulamba, P. Krosky, D. M. Coen, B. O. Parker, and G. S. Pari. 1999. A recombinant human cytomegalovirus with a large deletion in UL97 has a severe replication deficiency. J. Virol. 73:5663-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulvertaft, R. J. 1965. A study of malignant tumors in Nigeria by short-term culture. J. Clin. Pathol. 18:261-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purves, F. C., W. O. Ogle, and B. Roizman. 1993. Processing of the herpes simplex virus regulatory protein alpha 22 mediated by the UL13 protein kinase determines the accumulation of a subset of alpha and gamma mRNAs and proteins in infected cells. Proc. Natl. Acad. Sci. USA 90:6701-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynolds, A. E., B. J. Ryckman, J. D. Baines, Y. Zhou, L. Liang, and R. J. Roller. 2001. UL31 and UL34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J. Virol. 75:8803-8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reynolds, A. E., E. G. Wills, R. J. Roller, B. J. Ryckman, and J. D. Baines. 2002. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J. Virol. 76:8939-8952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rickinson, A. B., and E. Kieff. 2001. Epstein-Barr virus, p. 2575-2628. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Wiliams & Wilkins, Philadelphia, PA. [Google Scholar]
- 41.Smith, R. F., and T. F. Smith. 1989. Identification of new protein kinase-related genes in three herpesviruses, herpes simplex virus, varicella-zoster virus, and Epstein-Barr virus. J. Virol. 63:450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagenaar, F., J. M. Pol, B. Peeters, A. L. Gielkens, N. de Wind, and T. G. Kimman. 1995. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J. Gen. Virol. 76:1851-1859. [DOI] [PubMed] [Google Scholar]
- 43.Wang, J. T., P. W. Yang, C. P. Lee, C. H. Han, C. H. Tsai, and M. R. Chen. 2005. Detection of Epstein-Barr virus BGLF4 protein kinase in virus replication compartments and virus particles. J. Gen. Virol. 86:3215-3225. [DOI] [PubMed] [Google Scholar]
- 44.Wolf, D. G., C. T. Courcelle, M. N. Prichard, and E. S. Mocarski. 2001. Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation. Proc. Natl. Acad. Sci. USA 98:1895-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoyama, A., M. Tanaka, G. Matsuda, K. Kato, M. Kanamori, H. Kawasaki, H. Hirano, I. Kitabayashi, M. Ohki, K. Hirai, and Y. Kawaguchi. 2001. Identification of major phosphorylation sites of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP): ability of EBNA-LP to induce latent membrane protein 1 cooperatively with EBNA-2 is regulated by phosphorylation. J. Virol. 75:5119-5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue, W., E. Gershburg, and J. S. Pagano. 2005. Hyperphosphorylation of EBNA2 by Epstein-Barr virus protein kinase suppresses transactivation of the LMP1 promoter. J. Virol. 79:5880-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zacny, V. L., E. Gershburg, M. G. Davis, K. K. Biron, and J. S. Pagano. 1999. Inhibition of Epstein-Barr virus replication by a benzimidazole l-riboside: novel antiviral mechanism of 5,6-dichloro-2-(isopropylamino)-1-β-l-ribofuranosyl-1H-benzimidazole. J. Virol. 73:7271-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zimmermann, A., H. Wilts, M. Lenhardt, M. Hahn, and T. Mertens. 2000. Indolocarbazoles exhibit strong antiviral activity against human cytomegalovirus and are potent inhibitors of the pUL97 protein kinase. Antivir. Res. 48:49-60. [DOI] [PubMed] [Google Scholar]