Abstract
A human immunodeficiency virus (HIV)-preventive vaccine will likely need to induce broad immunity that can recognize antigens expressed within circulating strains. To understand the potentially relevant responses that T-cell based vaccines should elicit, we examined the ability of T cells from early infected persons to recognize a broad spectrum of potential T-cell epitopes (PTE) expressed by the products encoded by the HIV type 1 (HIV-1) nef gene, which is commonly included in candidate vaccines. T cells were evaluated for gamma interferon (IFN-γ) secretion using two peptide panels: subtype B consensus (CON) peptides and a novel peptide panel providing 70% coverage of PTE in subtype B HIV-1 Nef. Eighteen of 23 subjects’ T cells recognized HIV-1 Nef. In one subject, Nef-specific T cells were detected with the PTE but not with the CON peptides. The greatest frequency of responses spanned Nef amino acids 65 to 103 and 113 to 147, with multiple epitope variants being recognized. Detection of both the epitope domain number and the response magnitude was enhanced using the PTE peptides. On average, we detected 2.7 epitope domains with the PTE peptides versus 1.7 domains with the CON peptides (P = 0.0034). The average response magnitude was 2,169 spot-forming cells (SFC)/106 peripheral blood mononuclear cells (PBMC) with the PTE peptides versus 1,010 SFC/106 PBMC with CON peptides (P = 0.0046). During early HIV-1 infection, Nef-specific T cells capable of recognizing multiple variants are commonly induced, and these responses are readily detected with the PTE peptide panel. Our findings suggest that Nef responses induced by a given vaccine strain before HIV-1 exposure may be sufficiently broad to recognize most variants within subtype B HIV-1.
Developing a vaccine that protects against the wide range of human immunodeficiency virus type 1 (HIV-1) circulating strains is one of the greatest public health challenges ever confronted. The enormous genetic diversity of HIV-1 creates a major barrier to both the design and the evaluation of HIV-1 vaccines, and it is widely recognized that a globally efficacious vaccine will most likely need to elicit broadly reactive immune responses (31, 34, 35). Group M HIV-1, which dominates the worldwide pandemic, consists of several distinct subtypes (24). Proteins within subtypes vary by approximately 5 to 25% and vary between subtypes by approximately 10 to 40%, with the lower bound representing conserved proteins and the upper bound representing variable proteins (26). A successful vaccine is likely to require an immunogenic sequence or a combination of sequences that can induce T cells recognizing epitopes that are similar to or cross-react with those in circulating viruses. To better understand the potentially relevant responses that T-cell-based vaccines should elicit, we examined the ability of T cells from early infected persons to recognize multiple epitope variants within subtype B HIV-1. We chose subjects with acute and early HIV-1 infection since the immune responses that are measured in these subjects are more likely to be relevant for immune protection and immune control, compared to later stages of infection when the viruses may have already escaped key cytotoxic T-lymphocyte (CTL) responses. Since HIV-1 Nef contains highly immunogenic regions of HIV-1 (1) and is commonly included in candidate HIV-1 vaccines, we focused our investigations on the detection of T-cell responses to a broad spectrum of potential T-cell epitopes (PTE) expressed by the products encoded by the HIV-1 nef gene.
Today, numerous candidate T-cell-based vaccines representative of multiple HIV strains and several subtypes are under evaluation in clinical trials. Vaccine-induced T cells are typically identified by their ability to produce cytokines when stimulated with overlapping 15-mer peptides whose sequences are based on vaccine strains. This approach provides the greatest sensitivity for defining the immunogenicity of the vaccine preparation but does not adequately assess specificities of the immune responses that may be potentially relevant to protection against circulating strains worldwide. The use of peptides based on centrally derived computational sequences has the potential to increase the sequence similarity between the antigen expressed in a vaccine strain and that of circulating viruses. For example, the consensus (CON) method has at each site the most common nucleotide or amino acid residue across a sequence alignment, and this approach has been frequently utilized for the design of peptide reagents in the assessment of T-cell responses (15, 36). Although this method offers improvements over the use of peptide panels based on a single reference strain or patient isolate, there are two disadvantages: first, the CON sequences are artificially derived and may not occur naturally in HIV-1 strains; and second, most T-cell epitopes identified by central-sequence peptides cluster in domains with low sequence variability, with few being detected in regions with moderate and high sequence variability (47).
An alternative strategy is the use of autologous sequence-based peptides. These peptide sets can improve the detection of T-cell determinants in variable regions of HIV-1 proteins and are useful in detailed studies of viral escape mutations resulting from immune pressure in HIV-1-infected subjects (2, 37). However, this approach is not relevant in the examination of immunogenicity in HIV-1-uninfected vaccine recipients and certainly is not feasible in large population studies. Thus, a standard HIV-1 peptide panel is needed that is more representative of the circulating virus strains vaccine recipients are likely to encounter and that permits affordable and objective comparisons of T-cell responses to a variety of candidate vaccines.
To address these issues, we developed a standardized peptide panel, designated PTE-B, that represented PTE contained in diverse circulating subtype B HIV-1 strains. Responses were assessed by gamma interferon enzyme-linked immunospot (IFN-γ ELISPOT) assays of two Nef peptide sets: a PTE-B panel designed to cumulatively cover 70% of PTE among subtype B isolates and the CON-B panel. We provide evidence that T cells in most newly infected subjects are better at recognizing multiple epitope variants than heretofore appreciated when using CON peptide panels. The data have wider implications in relation to vaccine immunogenicity, suggesting that vaccine-induced T cells may be able to recognize epitopes both in and outside the subtype represented in the vaccine. In addition, our findings indicate that the magnitude and breadth of responses detected with PTE-optimized peptide panels are significantly higher than those detected with CON peptides. While initially we used the PTE peptides to evaluate vaccine immunogenicity, this approach laid the framework for the design of a vaccine immunogen with expanded T-cell epitope coverage to provide protection against highly diverse viruses and rapidly changing virus populations.
MATERIALS AND METHODS
Study population.
Subjects with primary HIV-1 infection were recruited and enrolled at the University of Washington Primary Infection Clinic. The duration of infection was typically defined as the time from the onset of clinical signs and/or symptoms suggestive of acute retroviral syndrome (4, 29, 43). All participants were men who have sex with men and presumed to be infected with clade B viruses (42). Some patients at various time points following diagnosis elected to receive combination antiretroviral therapy with nucleoside reverse transcriptase inhibitors and either a protease inhibitor or a nonnucleoside reverse transcriptase inhibitor. Uninfected volunteers at low risk for HIV-1 infection were recruited through the Fred Hutchinson Cancer Research Center HIV Vaccine Trials Unit to serve as donor controls in the evaluation. The Human Subjects Review Board at the institutions approved the studies, and the volunteers provided written consent prior to participation.
Plasma HIV-1 RNA levels, T-cell subset analyses, and HLA typing.
Plasma HIV-1 RNA was determined by quantitative branched-chain DNA (bDNA; Chiron, Emeryville, CA) and ultrasensitive reverse transcriptase-PCR (RT-PCR; Roche Molecular Systems, Branchburg, NJ) assays. Levels were expressed as copies/ml, and the lower levels of sensitivity were 500 copies/ml (bDNA assay) and 50 copies/ml (RT-PCR assay) (12). Absolute blood CD4+ T-cell counts were measured by consensus flow cytometry methodology. HLA typing was performed at the Puget Sound Blood Center by sequence-specific primer PCR as previously described (7).
Analysis of PTE from circulating strains and PTE coverage.
Given that major histocompatibility complex (MHC) class I molecules typically present antigenic peptides that are 8 to 10 amino acids (aa) in length (33, 38, 46), we considered 9 aa as the minimal epitope length and defined PTE as all possible unique 9-mers contained in the virus sequences. The PTE peptide set was designed to capture the most prevalent CTL epitopes among circulating HIV-1 strains (27), with each stretch of nine amino acids within a protein sequence having the potential to be a T-cell epitope. Therefore, for a given HIV-1 protein whose sequence was x amino acids in length, the number of PTEs that could be extracted was x − 8. All possible PTEs from a collection of virus sequences (e.g., all clade B viruses) were combined, and any repeat PTEs were removed. Within this mixture of unique PTEs, each was then classified by its frequency of occurrence relative to the other PTEs reported for HIV-1 strains in the Los Alamos database. We then arbitrarily decided on the extent of coverage desired across all these sequences. For instance, 70% coverage would represent a peptide set that contains 70% of all the potential epitopes within the group of virus sequences under consideration (e.g., clade B).
We then derived the synthetic peptides from the PTE sequences. Either peptides corresponding to the extracted 9-mer PTEs or longer peptides can be made. We chose to synthesize 15-mer PTEs that contained one or more overlapping PTEs and yet retained the natural virus sequence, for the following reasons: (i) peptides of 15 amino acids in length conform to the size of common peptide reagents in use (e.g., consensus peptides) and are less expensive to synthesize than 9-mers; (ii) 15-mer PTEs are preferable to use in screening assays over smaller peptides (e.g., 9-mers) because the latter more often stimulate non-HIV-1-specific T-cell reactivities; and (iii) 15-mers should be recognized by both CD4+ and CD8+ T cells. To design longer peptides such as 15-mers, each unique 15-amino-acid sequence embedded in the group of virus sequences was extracted. These naturally occurring 15-mers were then ordered by their PTE coverage level, which is the summation of the PTE frequencies associated with each of the individual embedded 9-mers. Thus, the higher the PTE coverage, the greater the likelihood that the peptide represented the virus sequences. A peptide reagent set was then built in a stepwise fashion. The first 15-mer was selected as the one with the highest coverage. The PTEs covered by this 15-mer were removed from the set, the coverage of each of the candidate 15-mers was recomputed, and the second 15-mer was selected as the one with the highest (updated) coverage.
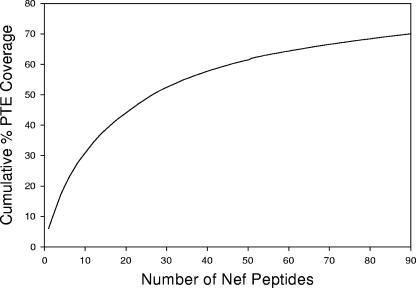
In this study, a total of 46,000 unique 9-mers were extracted from subtype B Nef proteins (n = 110) in the Los Alamos HIV-1 sequence database, and a 15-mer peptide set was then constructed by the forward stepwise algorithm described above. The stepwise selection process was repeated until a mean cumulative PTE coverage of 70% was reached (Fig. 1; also see Table S1 in the supplemental material).
FIG. 1.
Schematic representation of the PTE frequency coverage plot for the Nef PTE peptide set (n = 90 peptides). The PTE frequency coverage plot shows the number of 15-mer peptides relative to the cumulative PTE coverage for 110 Nef sequences. Ninety peptides were required to achieve a cumulative coverage of 70%. The PTE peptides with lower numbers are those that are present at the highest frequency among the virus sequences in the database.
Synthetic HIV-1 peptides.
Synthetic overlapping peptides corresponding to gene products from the HIV-1 Nef CON subtype B were obtained through the NIH AIDS Research and Reference Reagent Program (NARRRP, Bethesda, MD), and the HIV-1 Nef PTE peptides were synthesized at Mimotopes (Raleigh, NC). It should be noted that in validation studies, expected positive and negative responses to both peptide sets were observed for HIV-infected and uninfected donors, respectively. In addition, four frequently recognized Nef CON peptides (aa 69 to 83, PVRPQVPLRPMTYKA; aa 73 to 87, QVPLRPMTYKAAVDL; aa 81 to 95, YKAAVDLSHFLKEKG; and aa 129 to 143, PGPGIRYPLTFGWCF) were obtained from both sources (NARRRP and Mimotopes) and compared for T-cell recognition. T-cell responses to these peptide pairs in both infected and uninfected donors were comparable in breadth and magnitude (data not shown).
For the PTE peptide set (n = 90 peptides) there were 18 pools, and each pool consisted of 9 to 10 peptides (9-by-10 matrix). For the CON peptide set (n = 49 peptides), there were 14 pools, and each pool consisted of 7 peptides (7-by-7 matrix). The peptides were pooled in a matrix format so that each peptide was contained in two pools (5, 21). Additional experiments were performed comparing responses to individual PTE peptides, with variant peptides contained in single pools to ensure that there were no significant antagonist effects on T-cell responses (data not shown). All peptides were used at a final concentration of 2 μg/ml, unless stated otherwise. The final concentration of dimethyl sulfoxide never exceeded 1% in the assays (34).
IFN-γ ELISPOT assay.
ELISPOT assays were performed to detect HIV-1-specific IFN-γ-secreting cells as previously described (9), with modifications. Peripheral blood mononuclear cells (PBMC) were plated (1 × 105 to 2 × 105 cells/well) in 96-well hydrophobic polyvinylidene difluoride-backed plates (Millipore, Bedford, MA) precoated with 50 μl of 10 μg/ml anti-IFN-γ monoclonal antibody (1-D1K, mouse immunoglobulin G1; Mabtech, Nacka, Sweden). Phytohemagglutinin (Murex Biotech, Dartford, United Kingdom) stimulation at 2 μg/ml was used as a positive control, and no peptide stimulation was used as a negative control.
Plates were incubated overnight at 37°C in 5% CO2 and then developed. Spot-forming cells (SFC) were counted using an Immunospot (Cellular Technology Ltd., Cleveland, OH) optical reader. The number of antigen-specific IFN-γ-secreting cells was calculated by subtracting the SFC in the negative control wells from those in the antigen-stimulated wells. The peptides were first tested in matrix pools, and all responses were confirmed at the single-peptide level (5, 21). Responses >3-fold above background and >50 SFC/106 cells above background were scored positive. We detected no false-positive responses in 12 HIV-1-seronegative control subjects with either the CON or the PTE peptide set.
The breadth of the Nef-specific response for an individual subject was defined as the number of distinct epitopic domains recognized (1), and the total magnitude of response was determined by the sum of the response (IFN-γ SFC/106 PBMC) to all epitopic domains. The recognition of two adjacent CON peptides was imputed as one epitopic domain, and the greater of the two responses was enumerated in the total magnitude. Similarly, the recognition of multiple variant PTE peptides with an amino acid overlap of ≥10 aa was imputed as one epitopic domain, and the highest IFN-γ SFC frequencies among all variant epitope responses were used to enumerate the total magnitude of response.
To determine the functional avidity of the T-cell response, the standard IFN-γ ELISPOT assay was performed with the optimal epitopic peptides at serial concentrations between 10,000 and 0.1 ng/ml. The SFC frequencies were plotted against the log10 peptide concentration with ORIGIN 6.0 professional software (Microcal Software, Inc., Northampton, MA). The effective peptide concentration that elicited 50% of the maximum T-cell response, defined as the EC50, was determined with a Sigmoidal Fit tool in the software.
Sequencing of autologous viruses.
DNA was purified from 2 × 106 cryopreserved PBMC using a QIAamp DNA blood mini-kit (QIAGEN, Valencia, CA), according to the manufacturer's recommendations. Alternately, RNA was purified from the plasma virions using a QIAamp viral RNA mini-kit. The HIV-1 Nef sequences were amplified by standard nested PCR as previously described (28). Primers DS7 (F) 8169-8198 and TMMNEF6 (R) 9607-9631 were used in the first-round PCR, resulting in a 1.5-kb product. Primers DS9 (F) 8678-8697 and DS8 (R) 9527-9550 were used in the second-round PCR, resulting in an 872-bp product. The PCR products were cloned into the TA vector using a TOPO TA cloning kit according to the manufacturer's protocol (Invitrogen, San Diego, CA). Plasmids from individual clones were isolated and sequenced using the M13 355-370 and T7 328-347 forward and M13 205-221 and T3 243-262 reverse primers with ABI Prism BigDye Terminator cycle sequence reagents (Applied Biosystems, Foster City, CA). Computational analysis of the sequences was performed using ExPASy Translate Tool (http://us.expasy.org/tools/dna.html), ClustalW (http://www.ebi.ac.uk/clustalw/), and BioEdit.
Scoring of changes in amino acid sequences.
Amino acid substitutions between sequences were scored for similarity using a BLOSUM matrix (20). The matrix is derived from blocks of aligned sequences from homologous proteins with a certain level of identity. Conservative changes have positive scores, and nonconservative changes have negative scores, with the scale extending from −4 to +3. Conservative amino acid substitutions are likely to conserve the physical and chemical properties necessary to maintain the structure and function of the protein, whereas nonconservative substitutions are likely to disrupt essential structural and functional features of the protein.
Statistical analysis.
T-cell responses from CON and PTE peptide sets were compared by the use of two-tailed paired nonparametric t tests with linear (breadth) and log-transformed (magnitude) data. PTE peptide order number versus T-cell responder frequency was examined using the Spearman correlation coefficient.
RESULTS
Study population.
The study population consisted of 23 HIV-1-infected subjects enrolled during primary infection (Table 1). All subjects were men, and the median age was 36 years (range, 23 to 45 years). At enrollment, the subjects had been infected for a median duration of 29 days (range, 8 to 191 days). The median plasma HIV-1 RNA level was 260,605 copies/ml, and CD4+ T-cell counts were 559 cells/mm3. Subjects in this analysis were chosen based on PBMC specimen availability from time points within 1 year of infection. At the time of analysis of the immune response, the subjects had been infected for a median duration of 46 days; and two subjects had received antiretroviral therapy for >1 week (duration, 42 and 57 days, respectively) (Table 1).
TABLE 1.
Demographic, clinical, and virological characteristics of the study population
| Subjecta | Age (yr) | Ethnicityb | Enrollment time point
|
Sampling time point
|
HLA class I type
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days postinfection | HIV-1 RNA copies/ml | CD4+ T cells/mm3 | Days postinfection | Treatment duration (days) | HIV-1 RNA copies/ml | CD4+ T cells/mm3 | A | B | Cw | |||
| 1188 | 23 | H | 79 | 73,074 | 409 | 93 | 5 | <500 | 375 | 23 | 35, 15 | 02, 07 |
| 1212 | 37 | C | 11 | 5,060,000 | 757 | 16 | Noc | 102,045 | 925 | 02, 23 | 44, 62 | 03, 04 |
| 1216 | 33 | C | 129 | 26,000 | 300 | 134 | 2 | 38,400 | 255 | 03, 24 | 07, 39 | 07 |
| 1238 | 42 | C | 45 | 99,600 | 367 | 45 | No | 99,600 | 367 | 01, 03 | 07, 14 | 07, 08 |
| 1242 | 30 | C | 42 | 96,900 | 874 | 105 | 57 | 343 | 787 | 03, 33 | 14, 35 | 04, 08 |
| 1362 | 24 | C | 8 | 8,240,000 | 878 | 8-680 | No | 117,077 | 871 | 02, 25 | 18, 51 | 01, 12 |
| 1484 | 27 | C | 12 | 4,340,700 | 300 | 19 | No | 4,340,700 | 830 | 02, 03 | 07, 35 | 04, 07 |
| 1490 | 41 | C | 55 | 286,272 | 568 | 62 | No | 286,272 | 568 | 02, 11 | 15, 35 | 03, 04 |
| 1509 | 37 | C | 191 | 271 | 486 | 343 | No | 65 | 426 | 01, 02 | 15, 57 | 03, 06 |
| 1576 | 32 | C | 22 | 3,717,000 | 332 | 63 | 42 | 4,129 | 552 | 11, 33 | 49, 51 | 07, 15 |
| 1577 | 32 | C | 89 | 701 | 746 | 177 | No | 24 | 603 | 2 | 27, 49 | 02, 07 |
| 1585 | 39 | C | 123 | 58 | 659 | 150 | No | 56 | 554 | 10, 68 | 03 | 03, 06 |
| 1596 | 36 | C | 19 | 260,605 | 700 | 20 | No | 260,605 | 265 | 03, 26 | 38, 35 | 04,12 |
| 1599 | 30 | C | 46 | 404,463 | 265 | 46 | No | 404,463 | 265 | 01, 02 | 08, 18 | 7 |
| 1686 | 38 | H | 55 | 155,545 | 186 | 70 | No | 219,312 | 241 | 23, 25 | 35, 40 | 03, 04 |
| 1689 | 28 | H | 11 | 2,240,958 | 1206 | 11 | No | 94,708 | 745 | 02, 03 | 35, 40 | 03, 04 |
| 1690 | 42 | C | 21 | 1,031,477 | 396 | 21 | No | 400,163 | 528 | 01 | 08 | 07 |
| 1691 | 23 | B | 29 | 26,713 | 694 | 29 | No | 7,382 | 758 | 30 | 42, 53 | 06, 17 |
| 1692 | 42 | B | 50 | 2,461 | 612 | 50 | No | 2,461 | 612 | 23, 30 | 08, 42 | 07, 17 |
| 1693 | 41 | H | 7 | 377,063 | 559 | 7 | No | 1,912,195 | 472 | 01, 03 | 08, 35 | 04, 07 |
| 1698 | 45 | H | 11 | 734,413 | 458 | 11 | No | 262,437 | 531 | NTd | NT | NT |
| 1699 | 43 | C | 28 | 215 | 1,94 | 28 | No | 296 | 945 | 01, 02 | 08, 57 | 06, 07 |
| 1706 | 28 | C | 25 | 354,860 | 420 | 25 | No | 6,457 | 521 | 02, 32 | 08, 55 | 03, 07 |
All subjects were male.
Ethnicity: C, Caucasian; H, Hispanic; B, black.
No, no treatment.
NT, not tested.
Distribution of reactive PTE peptides versus frequency distribution in virus isolates.
T-cell reactivity among the 23 subjects was quantified using an IFN-γ ELISPOT assay of the PTE and CON peptide sets. IFN-γ-secreting Nef-specific T-cell responses were detected in 18 of 23 HIV-1-infected subjects by using the PTE peptide set and in 17 of 23 subjects by using the CON peptide set (see Table S2 in the supplemental material). The five patients whose T cells failed to recognize the PTE Nef peptides were the same five (of 6) patients who failed to recognize the Con B Nef peptides.
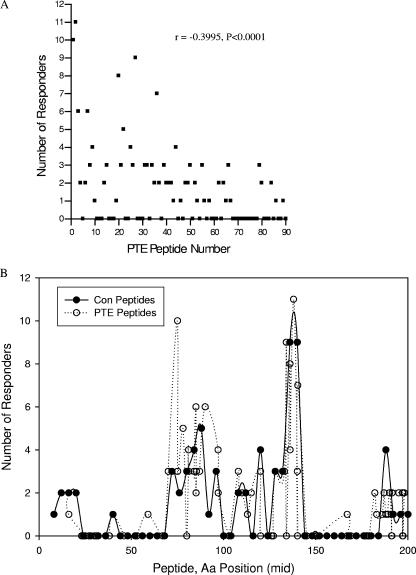
Here, we examined the relationship between the PTE peptide rank and the detection of T-cell responses to the peptide (Fig. 2A). As predicted, the number of responses detected correlated with the frequency ranking of the PTE peptides (r = −0.3995; P < 0.0001). Thus, the greatest frequency of T-cell responses detected was following stimulation with the most frequent PTE among subtype B virus isolates. We also investigated whether T-cell reactivity to certain Nef domains was more likely to be observed than others. We observed clustering of reactive peptides, with major clusters spanning amino acids 65 to 103 and 113 to 147 and minor clusters between amino acids 1 to 27 and 177 to 206. Reactive domains detected with both the CON and PTE peptide sets were similar, with the greatest frequency of responses spanning amino acids 65 to 103 and 113 to 147, respectively (Fig. 2B).
FIG. 2.
Relationship between responder frequency with PTE peptide order (A) and peptide distribution within the Nef protein (B). PBMC were stimulated with HIV-1 PTE and CON peptide sets in a matrix format and tested for IFN-γ production using an ELISPOT assay. Responses >3-fold above background and >50 SFC/106 cells above background were scored positive. All responses were confirmed at the single-peptide level. Shown are the number of subjects responding to each peptide. In panel A, the highest ranking (lower numbers) PTE peptides are those that are present at the highest frequency among the clade B virus sequences in the database. In panel B the position of each CON and PTE peptide is imputed upon the HXB2 position for the middle amino acid in each of the peptides.
Recognition of multiple epitope variants: magnitude and avidity of responses.
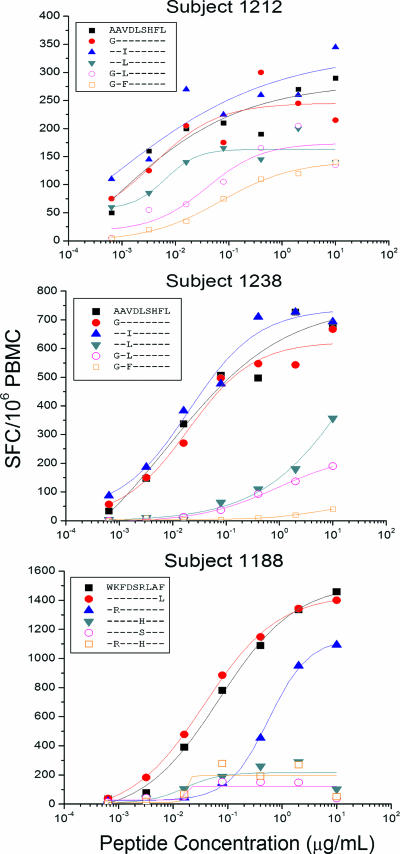
For several of the Nef epitopes, recognition of multiple variants was detected with the PTE peptide set (see Table S2 in the supplemental material). We further characterized T-cell recognition of epitope variants, comparing recognition of the 15-mer PTE peptide with that of optimal 9-mer epitope truncations: the AL9/B62-restricted response in subject 1212, the AL9/Cw08-restricted response in subject 1238, and the WF9/B1503-restricted response in subject 1188 (9). The proportion of variants recognized varied for different epitopes and between subjects (Table 2). For example, four of six variants of the AL9 epitope (AAVDLSHFL, GAVDLSHFL, AALDLSHFL, and GAFDLSHFL) contained within five 15-mer PTE peptides were recognized by PBMC from subject 1212 (Table 2). Notably, responses to all six optimal-length 9-mer variants were detected (Fig. 3, top panel), although responses to the variants containing two amino acid substitutions (GALDLSHFL and GAFDLSHFL) each were 92- to 166-fold lower in avidity than responses to the AAVDLSHFL epitope.
TABLE 2.
IFN-γ ELISPOT responses to epitope variants with PBMC from HIV-1 infected subjects
| PTE peptidea | HXB2 aa position | Epitope sequenceb | BLOSUM score(s)c | Subject/epitope/HLA restrictiond
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1212/AL9/B62
|
1238/AL9/Cw08
|
1188/WF9/B1503
|
||||||||||
| SFC/106 PBMC 15-mer | SFC/106 PBMC 9-mer | EC50 ng/ml 9-mer | SFC/106 PBMC 15-mer | SFC/106 PBMC 9-mer | EC50 ng/ml 9-mer | SFC/106 15-mer | SFC/106 9-mer | EC50 ng/ml 9-mer | ||||
| Epitope AL9 | ||||||||||||
| YKAAVDLSHFLKEKG | 081-095 | AAVDLSHFL | 191 | 270 | 0.42 | 287 | 726 | 7.99 | ||||
| PMTYKAAVDLSHFLK | 078-092 | ......... | 222 | 270 | 0.42 | 690 | 726 | 7.99 | ||||
| RPMTYKGAVDLSHFL | 077-091 | G........ | 0 | 402 | 245 | 3.87 | 700 | 542 | 19.40 | |||
| RPMTYKAALDLSHFL | 077-091 | ..L...... | 1 | 276 | 200 | 0.55 | Not reactive | 179 | 202.00 | |||
| TRRAAIDLSHFLREK | 080-094 | ..I...... | 3 | Not reactive | 260 | 6.46 | 95 | 726 | 20.30 | |||
| PMTFKGALDLSHFLR | 078-092 | G.L...... | 0, 1 | Not reactive | 205 | 39.23 | Not reactive | 136 | 909.00 | |||
| GALDLSHFLKEKGGL | 083-097 | G.L...... | 0, 1 | Not reactive | 205 | 39.23 | Not reactive | 136 | 909.00 | |||
| PMTYKGAFDLSHFLK | 078-092 | G.F...... | 0, −1 | 61 | 120 | 70.80 | Not reactive | Not reactive | ||||
| Epitope WF9 | ||||||||||||
| EREVLEWKFDSRLAF | 177-191 | WKFDSRLAF | 2,826 | 1,335 | 69.60 | |||||||
| VLVWKFDSRLAFRHM | 180-194 | ......... | 1,436 | 1,335 | 69.60 | |||||||
| VLMWKFDSRLAFHHI | 180-194 | ......... | 1,013 | 1,335 | 69.60 | |||||||
| WRFDSRLAFHHMARE | 183-197 | .R....... | 2 | 949 | 950 | 563.43 | ||||||
| EVLQWKFDSRLALRH | 179-193 | ........L | 0 | 753 | 1,343 | 37.55 | ||||||
| LVWKFDSSLAFHHRA | 181-195 | .....S... | −1 | 350 | 148 | 17.23 | ||||||
| LVWKFDSHLAFHHMA | 181-195 | .....H... | 0 | 123 | 290 | 16.44 | ||||||
| VWRFDSHLAFRHMAR | 182-196 | .R...H... | 2, 0 | 106 | 270 | 17.16 | ||||||
Amino acid differences from the CON sequence are underscored. The optimal epitope sequence is in bold type.
The CON B sequence for each epitope is indicated on the top. Identity to the amino acid in the CON sequence is indicated by a dot. All epitopes shown were previously mapped and HLA restriction was performed (9).
Conservative amino acid substitutions are denoted by positive scores and nonconservative substitutions by negative scores. The scoring is on a scale of −4 to +3.
All SFC data shown were obtained from IFN-γ ELISPOT assays performed at 2 μg/ml. Response levels of >50 SFC/106 PBMC were designated reactive. EC50, effective peptide concentration that elicited 50% of the maximum T-cell response.
FIG. 3.
Characterization of the T-cell responses to epitope variants AL9/B62 in subject 1212 (top panel), AL9/Cw08 in subject 1238 (middle panel), and WF9/B1503 in subject 1188 (bottom panel). A standard IFN-γ ELISPOT assay was performed by stimulation with the optimal epitopic peptides and variants at serial concentrations between 10,000 and 0.1 ng/ml. Shown here are PBMC responses from subjects 1238 and 1212 (AL9 epitope variants) and subject 1188 (WF9 epitope variants). The SFC frequencies were plotted against the log10 peptide concentration, and the effective peptide concentration that elicited 50% of the maximum T-cell response was determined using ORIGIN 6.0 professional software. Epitope mapping and HLA restriction data were published previously (9).
PBMC from subject 1238 recognized three of six variants (AAVDLSHFL, GAVDLSHFL, and AAIDLSHFL) contained within four 15-mer PTE peptides (Table 2). When tested with optimal-length 9-mer variants, low-frequency responses with 25- to 114-fold lower avidity were detected for an additional two variants (GALDLSHFL, and AALDLSHFL) (Fig. 3, middle panel). Finally, all five variants of the WF9/B1503 epitope contained within eight different 15-mer PTE peptides were recognized by T cells from subject 1188 (Fig. 3, bottom panel), although amino acid substitutions in presumed T-cell receptor (TCR) contact sites, e.g., WKFDSSLAF (R→S, position 6, BLOSUM score, −1) and WKFDSHLAF (R→H, position 6, BLOSUM score, 0), were associated with a 5- to 10-fold reduction in magnitude compared to responses to the WKFDSRLAF epitope. Smaller differences in the magnitude of responses observed for peptides containing identical optimal epitope sequences, for example, WKFDSRLAF, may be related either to variations in the epitope position within the 15-mer peptides or to the flanking amino acids.
In summary, T cells from early infected persons can recognize multiple epitope variants, although the magnitude and avidity of the responses can vary greatly across peptide variants. Multiple factors may influence the recognition of variants, including the number, the type (i.e., conservative versus nonconservative amino acid substitutions), and the position of the amino acid substitutions. Finally, while we detected IFN-γ-secreting cells in response to stimulation with peptide variants, further investigations are needed to define the quality of the T-cell response to the different variants.
Recognition of epitope variants and autologous virus sequence analyses.
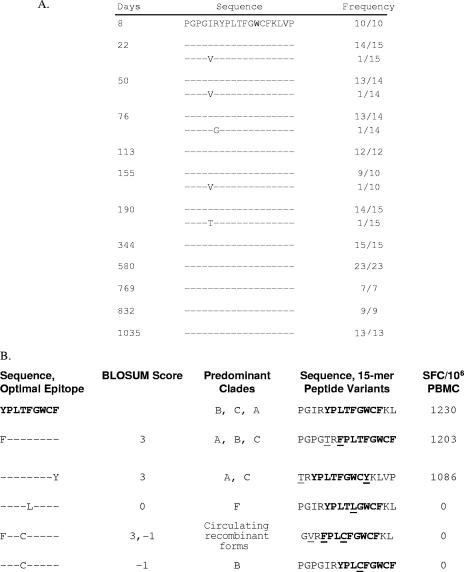
To determine the relationship of the autologous Nef sequences to the epitope variants recognized by the PBMC from an individual, we analyzed an average of 13 Nef sequences from subject 1362 at 12 time points spanning days 8 through 1,035 following infection (Fig. 4). The Nef-specific T-cell response restricted by HLA B18 was previously fine mapped to YPLTFGWCF (9). Although no YPLTFGWCF epitope variants were detected at any of the time points (Fig. 4A), the T cells reacted with three of six 15-mer peptide variants (Fig. 4B). These data indicate that the T-cell clonotypes generated in response to the infecting virus sequence YPLTFGWCF were able to recognize a range of epitope variants.
FIG. 4.
YPLTFGWCF epitope evolution (A) and recognition of epitope variants (B) in subject 1362. Evolutionary changes at the Nef epitope in autologous viral sequences from 12 time points between days 8 and 1,035 after infection are shown. Identity to amino acid in the consensus sequence is indicated by a dot. Numbers at the end of the sequences indicate the proportion of clones from the plasma encoding the given variant. The recognition of YPLTFGWCF variants by PBMC from subject 1362 was assessed by IFN-γ ELISPOT assay. Reactivity to six 15-mer peptides containing the variants was examined, and responses >3-fold background and >50 SFC/106 cells above background were scored positive. The optimal epitope sequence is in bold type. Amino acid differences from the CON sequence are underlined.
Autologous viruses from subject 1188 were sequenced from day 93 following infection and analyzed for heterogeneity in three epitopes: YF9/B35, WF9/B15, and KY11/Cw07 (Table 3). While all 13 sequences contained the FPLTFGWCF sequence (a Y1F substitution, compared to that of CON), PTE peptide variants containing three different epitope sequences were recognized. In addition, while 11 of 12 WF9 autologous sequences matched the CON B sequence, six different variants of the epitope were recognized. Similarly, while the KY11 autologous sequences contained a mix of only two sequences (KRQDILDLWVY and KRQDILDLWIY), all five epitope variants tested showed reactivity (Table 3). In summary, multiple epitope variants were recognized by the PBMC even when these were not contained among the autologous virus sequences.
TABLE 3.
T-cell recognition of PTE peptide variants and the sequence of autologous viruses in subject 1188
| Epitope restricting HLA allelea | ELISPOT responses to 15-mer PTE peptides
|
Autologous sequences
|
|||||
|---|---|---|---|---|---|---|---|
| PTE peptide sequenceb | HXB2 aa position | Epitope sequencec | BLOSUM score(s)d | SFC/106 PBMC | Sequencee | Clonal frequency | |
| YPLTFGWCF | GPGIRYPLTFGWCF KLVPV | ||||||
| YF9/B35 | PGPGTRFPLTFGWCF | 129-143 | F........ | 3 | 1,046 | ...T. F........ ..... | 13/13 |
| PGPGIRYPLTFGWCF | 129-143 | ......... | NA | 523 | |||
| PGPGIRYPLCFGWCF | 129-143 | ....C.... | −1 | 87 | |||
| PGIRYPLTFGWCFKL | 131-145 | ......... | NA | 1,096 | |||
| GVRYPLTLGWCFKLV | 132-146 | ...L..... | 0 | 0 | |||
| GVRFPLCFGWCFKLV | 132-146 | F..C.... | 3, −1 | 9 | |||
| TRYPLTFGWCYKLVP | 133-147 | ........Y | 3 | 409 | |||
| WKFDSRLAF | REVLE WKFDSRLAF HHMAR | ||||||
| WF9/B15 | VLMWKFDSRLAFHHI | 180-194 | ......... | NA | 1,013 | ....M ......... ..... | 6/12 |
| EREVLEWKFDSRLAF | 177-191 | ......... | NA | 2,826 | ....I ......... ..L.. | 5/12 | |
| EVLQWKFDSRLALRH | 179-193 | ........L | 0 | 753 | ....M ...A..... ..... | 1/12 | |
| VLVWKFDSRLAFRHM | 180-194 | ......... | NA | 1,436 | |||
| LVWKFDSHLAFHHMA | 181-195 | .....H... | 0 | 123 | |||
| LVWKFDSSLAFHHRA | 181-195 | .....S... | −1 | 350 | |||
| VWRFDSHLAFRHMAR | 182-196 | .R...H... | 2, 0 | 106 | |||
| WRFDSRLAFHHMARE | 183-197 | .R....... | 2, 0 | 949 | |||
| KRQDILDLWVY | IYSQ KRQDILDLWVY HTQG | ||||||
| KY11/Cw07 | IYSQKRQDILDLWIY | 101-115 | .........I. | 3 | 1,256 | .... .........I. .... | 9/13 |
| KRQDILDLWVYHTQG | 105-119 | ........... | NA | 1,453 | .... ........... .... | 4/13 | |
| IYSRKRQEILDLWIY | 101-115 | R..E.....I. | 2, 2, 1 | 773 | |||
| SQRRQDILDLWVYHT | 103-117 | R.......... | 2 | 1,516 | |||
| RQEILDLWVYHTQGY | 106-120 | ..E....... | 2 | 647 | |||
Epitope WF9 was previously mapped, and HLA restriction was performed (9). Epitopes YF9 and KY11 are presumed based on the subject having the appropriate HLA allele and publication in the Los Alamos database (23).
Amino acid differences from the CON sequence are underlined. The optimal epitope sequence is in bold type.
The CON B sequence for each epitope is indicated on the top. Identity to amino acid in the CON sequence is indicated by a dot.
Conservative amino acid substitutions are denoted by positive scores and nonconservative substitutions by negative scores. The scoring is on a scale of −4 to +3. NA, not applicable.
The CON B sequence for the domain is indicated on the top. Identity to amino acid in the CON sequence is indicated by a dot. Numbers at the end of the sequences indicate the percentage of clones encoding the given variant. The compartment sampled was PBMC.
Breadth and magnitude of response to Nef CON and PTE peptide sets.
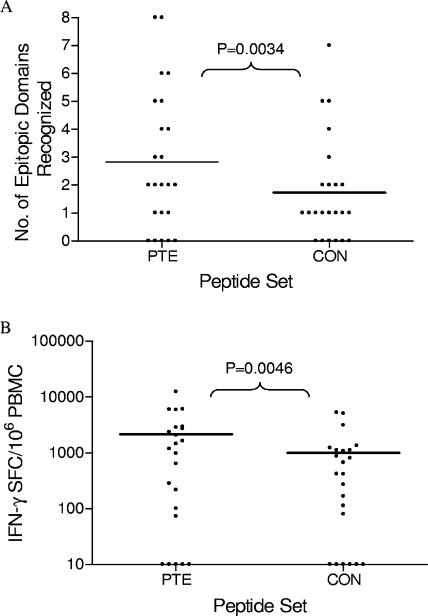
Among the 23 subjects, a mean of 2.7 (median, 2; range, 0 to 8) epitope domains were detected with the PTE peptide set compared to 1.7 (median, 1; range, 0 to 7) epitope domains with the CON peptide set (P = 0.0034) (Fig. 5A). The average (median) magnitude of response was 2,169 SFC/106 PBMC (1,177) with the PTE peptide set compared to 1,010 SFC/106 PBMC (419) with the CON peptide set (P = 0.0046) (Fig. 5B). The recognition of multiple variant PTE peptides with an amino acid overlap of ≥10 aa was imputed as one epitopic domain.
FIG. 5.
Breadth (A) and magnitude (B) of the Nef-specific T-cell responses in 23 HIV-1 infected subjects, using PTE and CON peptide sets in IFN-γ ELISPOT assays. The breadth of response for an individual subject was defined as the sum of the recognized peptides, and the total magnitude of response was determined by the sum of the response to all epitopic regions. The recognition of two adjacent CON peptides was imputed as one epitopic region, and the greater of the two responses was enumerated in the total magnitude. Similarly the recognition of multiple variant PTE peptides with an amino acid overlap of ≥10 amino acids was imputed to be one epitopic region, and the greatest response was enumerated in the total magnitude. T-cell responses between CON and PTE peptide sets were compared by the use of two-tailed paired t tests on linear (breadth) and log-transformed (magnitude) data.
To determine why an increased breadth of response was detected with the PTE peptide set versus the CON peptide set, we compared the sequence of the reactive PTE peptides with the CON peptides. Overall, an additional 29 epitopic domains not detected with the CON peptide set were detected with the PTE peptides (Table 4; also see Table S2 in the supplemental material). Among these, 18 epitopic domains contained one or more amino acid substitutions. In the remaining 11, the position of the domain within the 15-mer differed between the PTE and CON peptides. An additional five epitopic domains not detected with the PTE peptide set were detected with the CON peptides. A compilation of all PTE and CON peptides recognized is presented in Table S2 in the supplemental material.
TABLE 4.
Summary of epitopic domains identified with PTE or CON peptides, in subjects with HIV-1 subtype B infection
| Subject | Presumed epitope/HLA restriction (aa)a | PTE
|
CON
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Peptide | HXB2 aa position | Sequenceb | SFC/106 PBMC | Peptide no. | HXB2 aa position | Sequence | SFC/106 PBMC | ||
| 1188 | RL9/B7-super (77-85) | 9 | 074-088 | IPLRPMTYKGALDLS | 176 | ||||
| 79 | 074-088 | VPLRPMTYRAARDLS | 313 | ||||||
| TL10/B35 (128-137) | 31 | 126-140 | NYTPGPGTRYPLTFG | 82 | |||||
| 27 | 127-141 | YTPGPGVRYPLTFGW | 492 | ||||||
| UNK | 42 | 175-189 | DPEREVLEWRFDSRL | 53 | |||||
| 1212 | UNK | 64 | 009-023 | SVVGWPAVRERMRRA | 54 | ||||
| UNK | 31 | 126-140 | NYTPGPGTRYPLTFG | 66 | |||||
| UNK | 19 | 160-174 | ENNSLLHPMSLHGMD | 56 | |||||
| 1216 | UNK | 46 | 032-046 | AVSRDLERHGAITSS | 82 | ||||
| FL9/B7 (68-76) | 39 | 063-077 | EEEVGFPVKPQVPLR | 117 | |||||
| UNK | 80 | 188-202 | SLAFHHRARELHPEY | 57 | |||||
| 6 | 190-204 | AFHHMARELHPEYYK | 262 | ||||||
| 40 | 190-204 | AFRHVARELHPEYFK | 167 | ||||||
| 1238 | UNK | 1 | 001-015 | MGGKWSKRSVVGWPT | 53 | ||||
| UNK | 9 | 033-047 | VSRDLEKHGAITSSN | 73 | |||||
| FR10/B7 (68-77) | 17 | 065-079 | EVGFPVRPQVPLRPM | 87 | |||||
| 1362 | UNK | 37 | 108-122 | DILDLWIYHTQGYFP | 1,430 | ||||
| 1484 | FL9/B35/B7 (68-76) | 39 | 063-077 | EEEVGFPVKPQVPLR | 618 | ||||
| 1490 | AK10/A11 (83-92) | 7 | 078-092 | PMTYKAAVDLSHFLK | 60 | ||||
| 3 | 083-097 | GALDLSHFLKEKGGL | 67 | ||||||
| UNK | 2 | 131-145 | PGIRYPLTFGWCFKL | 57 | |||||
| 1576 | UNK | 34 | 101-115 | IYSQKRQDILDLWIY | 67 | ||||
| 1585 | UNK | 80 | 188-202 | SLAFHHRARELHPEY | 57 | ||||
| 52 | 191-205 | FHHKARELHPEYYKD | 73 | ||||||
| 1596 | UNK | 2 | 131-145 | PGIRYPLTFGWCFKL | 462 | ||||
| 1599 | UNK | 1 | 068-082 | FPVRPQVPLRPMTYK | 775 | ||||
| AL8/A2 (84-91) | 3 | 083-097 | GALDLSHFLKEKGGL | 72 | |||||
| UNK | 25 | 090-104 | FLKEKGGLEGLIYSQ | 867 | |||||
| 41 | 090-104 | FLREKGGLEGLVYSQ | 157 | ||||||
| UNK | 27 | 127-141 | YTPGPGVRYPLTFGW | 467 | |||||
| UNK | 46 | 181-195 | LEWKFDSRLAFHHMA | 105 | |||||
| 47 | 185-199 | FDSRLAFHHMARELH | 58 | ||||||
| 1686 | VY8/B35 (74-81) | 9 | 074-088 | IPLRPMTYKGALDLS | 829 | ||||
| 79 | 074-088 | VPLRPMTYRAARDLS | 1,976 | ||||||
| 1690 | UNK | 2 | 131-145 | PGIRYPLTFGWCFKL | 690 | ||||
| EL8/B8 (182-189) | 42 | 175-189 | DPEREVLEWRFDSRL | 227 | |||||
| 35 | 180-194 | VLVWKFDSRLAFRHM | 77 | ||||||
| AY8/A01 (195-202) | 52 | 191-205 | FHHKARELHPEYYKD | 170 | |||||
| 1692 | UNK | 1 | 068-082 | FPVRPQVPLRPMTYK | 101 | ||||
| AL9 (190-198) | 10 | 184-198 | KFDSRLAFHHVAREL | 51 | |||||
| 62 | 184-198 | KF*SRLAFHHIAREKH | 88 | ||||||
| 1693 | WM8/B0801 (13-20) | 4 | 013-027 | WPTVRERMRRAEPAA | 110 | ||||
| UNK | 43 | 052-066 | NAACAWLEAQEEEEV | 193 | |||||
| UNK | 39 | 063-077 | EEEVGFPVKPQVPLR | 1,810 | |||||
| 1 | 068-082 | FPVRPQVPLRPMTYK | 180 | ||||||
HLA restriction was inferred based on the subject's known HLA type and published MHC class I restricted CD8+ T-cell epitopes (23), except for epitopes indicated with an asterisk. Optimal epitopes and HLA restrictions for the latter were confirmed using peptide truncations and partially matched and mismatched antigen-presenting cells. UNK, epitope unknown.
Amino acid differences from the CON sequence are underlined.
In summary, both the breadth and magnitude of responses detected by stimulation with the PTE peptide sets were significantly greater than the breadth and magnitude detected with the CON peptide set. The increase in the number of epitopic domains detected with the PTE peptides was a combination of the recognition of peptides whose amino acid sequence differed from the CON sequence and the differences in the position of the epitopic domain within the 15-mer peptides.
DISCUSSION
Our findings indicate that Nef is frequently targeted during early HIV-1 infection and that T cells in these newly infected subjects are capable of recognizing multiple epitope variants. Several variants were frequently recognized even when these were not present among the autologous virus populations. Thus, T-cell clonotypes capable of cross-reactivity exist within the memory/effector pool, despite not having encountered the variants previously. These findings have wider implications in relation to vaccine immunogenicity, suggesting that vaccine-induced T cells may be able to recognize epitopes both in and outside the subtype represented in the vaccine.
The use of multiple peptide variants for variable epitopic domains, as in the PTE peptide set, provided important insights into TCR cross-reactivity, but it also raised important questions. T-cell responses that reacted with multiple epitope variants often showed significant differences in the magnitudes of their responses to the variants. Importantly, reductions in magnitude were not always accompanied by reductions in the avidity of the T-cell responses. In subject 1188, for example, while the magnitude of the response to the WF9 variant-R6S versus the WF9 epitope was nearly 1 log-fold lower, the avidity of the response was 4-fold greater (Table 2). It is possible that the immune response constitutes several T-cell clonotypes, both dominant and subdominant (39), and that different sets of clonotypes may react with the epitope and its variants. Studies to interrogate the constituent T-cell clonotypes within the memory T-cell pools are currently in progress.
The quality of the virus-specific T-cell response is variously determined by the avidity of the TCR-peptide MHC interaction, the duration and intensity of antigen stimulation, and the presence of CD4 help. The relative avidity of the TCR-peptide MHC interaction may influence the cytokine response profiles (25, 39). Epitope variants may deliver negative signals to T cells specific for the agonist peptide-MHC complexes. For example, altered class II anchor residues but identical TCR contact residues can induce T-cell anergy in an antigen-specific manner (32). Thus, while we detected IFN-γ-producing T-cell responses to multiple epitope variants, further investigation is needed to define the quality of the response to the different epitope variants. Such information will be insightful for the strategic design of vaccines that generate T-cell populations with optimal effector activities.
Furthermore, our data provide evidence for enhanced detection of T-cell responses with the Nef PTE peptides compared to that of CON peptides in subjects with early HIV-1 subtype B infection. The data support the model for which the per-epitope magnitude of response is comparable across both peptide sets but the PTE set provides greater numbers of reactive epitopes. Thus, the gain is primarily in the enhanced detection of breadth, with the increased magnitude of the total response being a secondary effect. Since the PTE peptides are designed to maximally cover known circulating viral sequences, they are especially well suited for the analysis of potentially protective immune responses in recipients of HIV-1 preventive vaccines and for objective comparisons of T-cell responses to various vaccine candidates. We are currently using the PTE peptides to screen vaccine candidates in HIV vaccine trials (phase I-IIB), and we continue to observe enhanced detection of responses with the PTE compared to those of CON peptides to gene products other than HIV-1 Nef as well. In addition, the use of these peptides has been associated with substantial cuts in peptide synthesis costs. The PTE peptide sets are also an affordable alternative to autologous sequence-based peptides for the evaluation of immune responses in HIV-1-infected subjects.
Overall, an additional 29 epitopic domains not detected with the CON peptide set were detected with the PTE peptides. The greater breadth of T-cell responses detected with the PTE than with the CON peptide set can be attributed to several factors. Most importantly, the PTE peptide set is larger and specifically optimized for the representation of viral population diversity. The PTE peptide set contains several peptide variants corresponding to the more variable domains. Such variants differed from those of the CON sequence by one or more amino acids, either within the presumed epitope or in the flanking sequences. Of note, 18 of the 29 epitopic domains detected with the PTE, but not the CON, peptides contained one or more amino acid substitutions. In the remaining 11, the position of the domain within the 15-mer differed between the PTE and CON peptides. Here, differences in T-cell recognition may be related to the position of the epitope within the 15-mer peptides, which may influence binding to the HLA molecule and complexing with the TCR. However, when differences attributable to epitope location are excluded from the analysis, the breadth of responses detected with the PTE peptide set still remains significantly higher than that detected with the CON peptide set (two-tailed paired nonparametric t test, P = 0.013). Importantly, the 15-mer PTE peptide set is designed to efficiently capture the most prevalent CTL epitopes among circulating HIV-1 strains using the fewest numbers of peptides. In other words, the primary emphasis in the PTE peptide design is to encompass the maximal number of 9-mer PTE within as few 15-mer peptides as possible. Thus, while epitope location within the 15-mer may be important, the 9-mer PTE are not positioned in any preferred location within the 15-mer. Finally, we speculate that other differences may be due to some epitope lengths being greater than 11 amino acids, since such epitopes may not be represented at all in a CON peptide set that consists of 15-mers overlapping by 11 amino acids.
The decision to construct a clade B Nef PTE reagent with 70% coverage was arbitrary. The potential benefit of higher-percentage (80 to 90%) coverage is largely outweighed by the low probability of sufficient numbers of donor cells being available to complete the testing. Of note, since 220 peptides are needed for 80% and 740 for 90% coverage, the processes of synthesizing, quality controlling, and pooling are costly and labor intensive. Of our two options to design either a 9-mer or a 15-mer peptide set, constructing a 9-mer peptide set required the synthesis of several hundred peptides, which was neither practical nor economically feasible. Therefore, we designed a 15-mer peptide set with each peptide containing either one or more overlapping high-frequency PTE, such that gene sequence colinearity was preserved. The ability to capture PTE, both CD4+ and CD8+, that may be longer than 9-mers, is another advantage of this approach. CTL epitope length restrictions are clearly not strict, and many epitopes of 11 to 14 residues elicit dominant responses (8, 11, 17, 18, 40, 44, 45). The frequency at which long epitopes are recognized is likely to be underestimated since widely used algorithms to predict CTL epitopes typically include peptides of up to 10 amino acids in length (19, 41).
Our findings demonstrate a highly characteristic pattern of PTE peptide recognition among the subjects. The greatest number of subjects’ T cells recognized the highest ranking peptides, and few recognized the low-ranking peptides. In other words, the most recognized peptides were also the ones containing the PTE present at the greatest frequency among the virus isolates. This finding is not surprising, since the high-ranking PTE are also the ones that are the most conserved among the different virus sequences. However, not all the high-ranking peptides were recognized. For example, PTE peptides 5, 11, 12, 13, 16, 17, and 18 did not react with PBMC from any of the 23 subjects. We speculate that some of these peptides encompass domains that, although highly conserved, do not contain T-cell epitopes. These findings are in agreement with prior observations for a nonuniform epitope distribution in HIV with distinct clusters separated by regions where no epitopes have been identified (14, 47). Mechanisms proposed to account for the distribution of epitopes include the processing preferences for proteasomal cleavage (6, 10, 22), the frequency of C-terminal amino acids that inhibit HLA binding (3, 6) and C-terminal anchor amino acids (13), and finally, the constraints on the amino acids that can be accommodated in the peptide-binding groove of the HLA molecules (16, 30, 38). Thus, while we detected a significantly greater breadth and magnitude of T-cell response with the PTE peptide set than with the CON set, the maps of epitope distributions with the two peptides sets had a striking resemblance, with similar epitope-rich and epitope-sparse domains. Additionally, we speculate that the pattern of greater reactivity to the more conserved PTE is likely a function of studying infected subjects. Thus, the pattern may be different in HIV-1-uninfected vaccinees, in whom the antigen stimulating the immune response is fixed for all study participants.
During early HIV-1 infection, Nef-specific T cells are commonly induced that are capable of recognizing multiple variants within subtype B HIV-1. Our findings suggest that when induced by a given vaccine strain before HIV-1 exposure, the T-cell responses may be sufficiently broad to recognize many variants within subtype B HIV-1. Our study confirms the enhanced detection of immune responses using PTE peptides in comparison with that of the CON peptides, which to date has been the single best strategy put forward to maximize coverage. While in this study we have employed the PTE peptides to evaluate vaccine immunogenicity, the approach lays the framework for the design of a vaccine immunogen with expanded T-cell epitope coverage for protection against highly diverse and rapidly changing virus populations.
Supplementary Material
Acknowledgments
We thank the clinical staff, Janine Maenza and Claire Stevens, and Lawrence Corey and his laboratory for performing the viral load measurements. We thank T. Smith for data management, Phyllis Stegall for editorial assistance, and the study participants for their time and effort.
This work was supported by National Institutes of Health grants AI64061, AI27757, and AI57005 and awards from the HIV Vaccine Trials Network (U.M.) and Puget Sound Partners in Global Health (U.M.).
Footnotes
Published ahead of print on 28 February 2007.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., M. M. Addo, R. Shankarappa, P. K. Lee, T. M. Allen, X. G. Yu, A. Rathod, J. Harlow, K. O'Sullivan, M. N. Johnston, P. J. Goulder, J. I. Mullins, E. S. Rosenberg, C. Brander, B. Korber, and B. D. Walker. 2003. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J. Virol. 77:7330-7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beekman, N. J., P. A. van Veelen, T. van Hall, A. Neisig, A. Sijts, M. Camps, P. M. Kloetzel, J. J. Neefjes, C. J. Melief, and F. Ossendorp. 2000. Abrogation of CTL epitope processing by single amino acid substitution flanking the C-terminal proteasome cleavage site. J. Immunol. 164:1898-1905. [DOI] [PubMed] [Google Scholar]
- 4.Berrey, M. M., T. Schacker, A. C. Collier, T. Shea, S. J. Brodie, D. Mayers, R. Coombs, J. Krieger, T. W. Chun, A. Fauci, S. G. Self, and L. Corey. 2001. Treatment of primary human immunodeficiency virus type 1 infection with potent antiretroviral therapy reduces frequency of rapid progression to AIDS. J. Infect. Dis. 183:1466-1475. [DOI] [PubMed] [Google Scholar]
- 5.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T.-M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bochtler, M., L. Ditzel, M. Groll, C. Hartmann, and R. Huber. 1999. The proteasome. Annu. Rev. Biophys. Biomol. Struct. 28:295-317. [DOI] [PubMed] [Google Scholar]
- 7.Bunce, M., G. C. Fanning, and K. I. Welsh. 1995. Comprehensive, serologically equivalent DNA typing for HLA-B by PCR using sequence-specific primers (PCR-SSP). Tissue Antigens 45:81-90. [DOI] [PubMed] [Google Scholar]
- 8.Burrows, S. R., J. Rossjohn, and J. McCluskey. 2006. Have we cut ourselves too short in mapping CTL epitopes? Trends Immunol. 27:11-16. [DOI] [PubMed] [Google Scholar]
- 9.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 77:6867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardozo, C., and R. A. Kohanski. 1998. Altered properties of the branched chain amino acid-preferring activity contribute to increased cleavages after branched chain residues by the “immunoproteasome.” J. Biol. Chem. 273:16764-16770. [DOI] [PubMed] [Google Scholar]
- 11.Chen, Y., J. Sidney, S. Southwood, A. L. Cox, K. Sakaguchi, R. A. Henderson, E. Appella, D. F. Hunt, A. Sette, and V. H. Engelhard. 1994. Naturally processed peptides longer than nine amino acid residues bind to the class I MHC molecule HLA-A2.1 with high affinity and in different conformations. J. Immunol. 152:2874-2881. [PubMed] [Google Scholar]
- 12.Dewar, R. L., H. C. Highbarger, M. D. Sarmiento, J. A. Todd, M. B. Vasudevachari, R. T. Davey, Jr., J. A. Kovacs, N. P. Salzman, H. C. Lane, and M. S. Urdea. 1994. Application of branched DNA signal amplification to monitor human immunodeficiency virus type 1 burden in human plasma. J. Infect. Dis. 170:1172-1179. [DOI] [PubMed] [Google Scholar]
- 13.Draenert, R., C. Brander, X. G. Yu, M. Altfeld, C. L. Verrill, M. E. Feeney, B. D. Walker, and P. J. Goulder. 2004. Impact of intrapeptide epitope location on CD8 T cell recognition: implications for design of overlapping peptide panels. AIDS 18:871-876. [DOI] [PubMed] [Google Scholar]
- 14.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaschen, B., J. Taylor, K. Yusim, B. Foley, F. Gao, D. Lang, V. Novitsky, B. Haynes, B. H. Hahn, T. Bhattacharya, and B. Korber. 2002. Diversity considerations in HIV-1 vaccine selection. Science 296:2354-2360. [DOI] [PubMed] [Google Scholar]
- 16.Germain, R. N., and D. H. Margulies. 1993. The biochemistry and cell biology of antigen processing and presentation. Annu. Rev. Immunol. 11:403-450. [DOI] [PubMed] [Google Scholar]
- 17.Green, K. J., J. J. Miles, J. Tellam, W. J. van Zuylen, G. Connolly, and S. R. Burrows. 2004. Potent T cell response to a class I-binding 13-mer viral epitope and the influence of HLA micropolymorphism in controlling epitope length. Eur. J. Immunol. 34:2510-2519. [DOI] [PubMed] [Google Scholar]
- 18.Guo, H. C., T. S. Jardetzky, T. P. Garrett, W. S. Lane, J. L. Strominger, and D. C. Wiley. 1992. Different length peptides bind to HLA-Aw68 similarly at their ends but bulge out in the middle. Nature 360:364-366. [DOI] [PubMed] [Google Scholar]
- 19.Hakenberg, J., A. K. Nussbaum, H. Schild, H. G. Rammensee, C. Kuttler, H. G. Holzhutter, P. M. Kloetzel, S. H. Kaufmann, and H. J. Mollenkopf. 2003. MAPPP: MHC class I antigenic peptide processing prediction. Appl. Bioinformatics 2:155-158. [PubMed] [Google Scholar]
- 20.Henikoff, S., and J. G. Henikoff. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 89:10915-10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kern, F., I. P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, P. Walden, and H. D. Volk. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975-978. [DOI] [PubMed] [Google Scholar]
- 22.Kloetzel, P. M., and F. Ossendorp. 2004. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 16:76-81. [DOI] [PubMed] [Google Scholar]
- 23.Korber, B. T. M., C. Brander, B. F. Haynes, R. Koup, J. P. Moore, B. D. Walker, and D. I. Watkins (ed.). 2005. HIV molecular immunology 2005. Los Alamos National Laboratory, Theoretical Biology and Biophysics Group, Los Alamos, NM. LA-UR 06-0036.
- 24.Korber, B., M. Muldoon, J. Theiler, F. Gao, R. Gupta, A. Lapedes, B. H. Hahn, S. Wolinsky, and T. Bhattacharya. 2000. Timing the ancestor of the HIV-1 pandemic strains. Science 288:1789-1796. [DOI] [PubMed] [Google Scholar]
- 25.La Gruta, N. L., S. J. Turner, and P. C. Doherty. 2004. Hierarchies in cytokine expression profiles for acute and resolving influenza virus-specific CD8+ T cell responses: correlation of cytokine profile and TCR avidity. J. Immunol. 172:5553-5560. [DOI] [PubMed] [Google Scholar]
- 26.Leitner, T., B. Foley, B. Hahn, P. Marx, F. McCutchan, J. Mellors, S. Wolinsky, and B. Korber (ed.). 2005. HIV sequence compendium. Los Alamos National Laboratory, Theoretical Biology and Biophysics Group, Los Alamos, NM. LA-UR 06-0680.
- 27.Li, F., U. Malhotra, P. B. Gilbert, N. R. Hawkins, A. C. Duerr, J. M. McElrath, L. Corey, and S. G. Self. 2006. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine 24:6893-6904. [DOI] [PubMed] [Google Scholar]
- 28.Liu, S. L., T. Schacker, L. Musey, D. Shriner, M. J. McElrath, L. Corey, and J. I. Mullins. 1997. Divergent patterns of progression to AIDS after infection from the same source: human immunodeficiency virus type 1 evolution and antiviral responses. J. Virol. 71:4284-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malhotra, U., S. Holte, T. Zhu, E. Delpit, C. Huntsberry, A. Sette, R. Shankarappa, J. Maenza, L. Corey, and M. J. McElrath. 2003. Early induction and maintenance of Env-specific T-helper cells following HIV-1 infection. J. Virol. 77:2663-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura, M., D. H. Fremont, P. A. Peterson, and I. A. Wilson. 1992. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science 257:927-934. [DOI] [PubMed] [Google Scholar]
- 31.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9:874-880. [DOI] [PubMed] [Google Scholar]
- 32.Mirshahidi, S., L. C. Ferris, and S. Sadegh-Nasseri. 2004. The magnitude of TCR engagement is a critical predictor of T cell anergy or activation. J. Immunol. 172:5346-5355. [DOI] [PubMed] [Google Scholar]
- 33.Momburg, F., J. Roelse, G. J. Hammerling, and J. J. Neefjes. 1994. Peptide size selection by the major histocompatibility complex-encoded peptide transporter. J. Exp. Med. 179:1613-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mullins, J. I., and M. A. Jensen. 2006. Evolutionary dynamics of HIV-1 and the control of AIDS, p. 171-192. In E. Domingo (ed.), Current topics in microbiology and immunology. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 35.Nabel, G. J. 2001. Challenges and opportunities for development of an AIDS vaccine. Nature 410:1002-1007. [DOI] [PubMed] [Google Scholar]
- 36.Nickle, D. C., M. A. Jensen, G. S. Gottlieb, D. Shriner, G. H. Learn, A. G. Rodrigo, and J. I. Mullins. 2003. Consensus and ancestral state HIV vaccines. Science 299:1515-1518. [DOI] [PubMed] [Google Scholar]
- 37.Nolan, D., I. James, and S. Mallal. 2005. HIV/AIDS. HIV: experiencing the pressures of modern life. Science 307:1422-1424. [DOI] [PubMed] [Google Scholar]
- 38.Pamer, E., and P. Cresswell. 1998. Mechanisms of MHC class I-restricted antigen processing. Annu. Rev. Immunol. 16:323-358. [DOI] [PubMed] [Google Scholar]
- 39.Price, D. A., J. M. Brenchley, L. E. Ruff, M. R. Betts, B. J. Hill, M. Roederer, R. A. Koup, S. A. Migueles, E. Gostick, L. Wooldridge, A. K. Sewell, M. Connors, and D. C. Douek. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202:1349-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Probst-Kepper, M., H. J. Hecht, H. Herrmann, V. Janke, F. Ocklenburg, J. Klempnauer, B. J. van den Eynde, and S. Weiss. 2004. Conformational restraints and flexibility of 14-meric peptides in complex with HLA-B*3501. J. Immunol. 173:5610-5616. [DOI] [PubMed] [Google Scholar]
- 41.Rammensee, H., J. Bachmann, N. P. Emmerich, O. A. Bachor, and S. Stevanovic. 1999. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50:213-219. [DOI] [PubMed] [Google Scholar]
- 42.Robbins, K. E., P. Lemey, O. G. Pybus, H. W. Jaffe, A. S. Youngpairoj, T. M. Brown, M. Salemi, A. M. Vandamme, and M. L. Kalish. 2003. U.S. human immunodeficiency virus type 1 epidemic: date of origin, population history, and characterization of early strains. J. Virol. 77:6359-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schacker, T. W., J. P. Hughes, T. Shea, R. W. Coombs, and L. Corey. 1998. Biological and virologic characteristics of primary HIV infection. Ann. Intern. Med. 128:613-620. [DOI] [PubMed] [Google Scholar]
- 44.Speir, J. A., J. Stevens, E. Joly, G. W. Butcher, and I. A. Wilson. 2001. Two different, highly exposed, bulged structures for an unusually long peptide bound to rat MHC class I RT1-Aa. Immunity 14:81-92. [DOI] [PubMed] [Google Scholar]
- 45.Urban, R. G., R. M. Chicz, W. S. Lane, J. L. Strominger, A. Rehm, M. J. Kenter, F. G. UytdeHaag, H. Ploegh, B. Uchanska-Ziegler, and A. Ziegler. 1994. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc. Natl. Acad. Sci. USA 91:1534-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenzel, T., C. Eckerskorn, F. Lottspeich, and W. Baumeister. 1994. Existence of a molecular ruler in proteasomes suggested by analysis of degradation products. FEBS Lett. 349:205-209. [DOI] [PubMed] [Google Scholar]
- 47.Yusim, K., C. Kesmir, B. Gaschen, M. M. Addo, M. Altfeld, S. Brunak, A. Chigaev, V. Detours, and B. T. Korber. 2002. Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J. Virol. 76:8757-8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.