Abstract
Betanodaviruses are small RNA viruses that infect teleost fish and pose a considerable threat to marine aquaculture production. These viruses possess a small protein, termed B2, which binds to and protects double-stranded RNA. This prevents cleavage of virus-derived double-stranded RNAs (dsRNAs) by Dicer and subsequent production of small interfering RNA (siRNA), which would otherwise induce an RNA-silencing response against the virus. In this work, we have performed charged-to-alanine scanning mutagenesis of the B2 protein in order to identify residues required for dsRNA binding and protection. While the majority of the 19 mutated B2 residues were required for maximal dsRNA binding and protection in vitro, residues R53 and R60 were essential for both activities. Subsequent experiments in fish cells confirmed these findings by showing that mutations in these residues abolished accumulation of both the RNA1 and RNA2 components of the viral genome, in addition to preventing any significant induction of the host interferon gene, Mx. Moreover, an obvious positive correlation was found between dsRNA binding and protection in vitro and RNA1, RNA2, and Mx accumulation in fish cells, further validating the importance of the selected amino acid residues. The same trend was also demonstrated using an RNA silencing system in HeLa cells, with residues R53 and R60 being essential for suppression of RNA silencing. Importantly, we found that siRNA-mediated knockdown of Dicer dramatically enhanced the accumulation of a B2 mutant. In addition, we found that B2 is able to induce apoptosis in fish cells but that this was not the result of dsRNA binding.
Animal cells employ a multitude of defenses to prevent and combat viral infection. In recent years, a small number of interrelated biochemical pathways that form the intracellular innate immune response of animal cells have received considerable attention. These pathways include the double-stranded RNA (dsRNA)-inducible protein kinase PKR, oligoadenylate synthetase/RNase L, and Mx pathways (23, 35, 37) and the more recently described RNA interference pathway of innate immunity (7, 13, 26). Current data suggest that vertebrate animals use the interferon system as their primary intracellular innate immune response, while invertebrates, which for the most part appear to lack such a system, instead rely on a potent RNA interference response (33, 42). In both systems, it is dsRNA derived from the virus that appears to serve as the intracellular inducer of these responses.
Among animal viruses containing a single-stranded (positive strand) RNA (ssRNA) genome with no DNA stage, such as members of the families Flaviviridae, Nodaviridae, and Picornaviridae, the bulk of virus dsRNA is likely generated in the form of a replication intermediate comprised of the original positive-strand RNA and its complementary negative-strand RNA (43). In vertebrate cells, this long dsRNA molecule can act as a potent inducer of the interferon response, leading to global repression of translation and often apoptosis, which in turn prevents viral replication and transmission to other cells. Given that these viruses are clearly capable of replication and transmission, it is not surprising that many viruses have evolved a variety of strategies to block host interferon induction and proceed to replicate unhindered, and these strategies are reviewed in detail elsewhere (3, 23).
Animal cells also possess a second layer of defense in the form of the RNA silencing pathway, which for invertebrate positive-strand ssRNA viruses represents a major barrier to virus RNA accumulation (14, 24, 26, 30). Similar to the situation for RNA viruses of plants, several positive-strand ssRNA animal viruses, particularly members of the family Nodaviridae, possess small dsRNA-binding proteins that can suppress RNA silencing. The B2 proteins of several nodaviruses, namely the alphanodaviruses flock house virus (FHV) and Nodamura virus, and the fish betanodavirus greasy grouper nervous necrosis virus (GGNNV), bind long dsRNAs with high affinity to prevent Dicer cleavage and the subsequent creation of siRNAs (11, 28, 30, 40). In the case of FHV infection of insect cells, this blocks the creation of virus-derived small interfering RNAs (siRNAs) and has therefore established FHV B2 as a true suppressor of RNA silencing in invertebrates (25). Recently the structure of FHV B2 was solved by both crystallographic and nuclear magnetic resonance approaches, and it appears to consist of a four-helix bundle that binds to dsRNA in a sequence-independent manner (6, 28). While this data will no doubt facilitate further work on FHV B2, its virtually complete lack of sequence identity with betanodavirus B2 proteins (12) limits the usefulness of this structural data for betanodavirus studies.
For GGNNV, the true role of B2 as a host antagonist was somewhat complicated by the fact that fish host cells induce a potent interferon response following GGNNV infection (11). In vitro data clearly revealed that GGNNV B2 is a dsRNA binding protein that can block Dicer cleavage of dsRNA (11). Additionally, the finding that GGNNV B2 does not block induction of interferon in fish cells (11) makes this protein distinct from other small dsRNA binding proteins of vertebrate viruses such as NS1 of influenza virus and E3L of vaccinia virus, both of which block interferon induction (27).
Here we show that dsRNA binding and protection from RNase cleavage by GGNNV B2 requires several charged residues, the most important of which are residues R53 and R60. We go on to show that the intracellular accumulation of RNA1 and RNA2 and induction of the host interferon-inducible Mx gene are tightly controlled by the ability of B2 to bind and protect dsRNA. Moreover, we show that dsRNA binding activity of B2 determines the efficiency with which this protein can suppress RNA silencing.
MATERIALS AND METHODS
Cell lines.
SB cells (derived from Lates calcarifer) were cultured as described previously (17). HeLa cells (36) were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS). Unless otherwise indicated, SB cells were grown at 28°C in a humidified 5% CO2 incubator and HeLa cells were grown at 37°C in a humidified 5% CO2 chamber.
Protein expression and purification.
Recombinant GGNNV B2 fused to glutathione S-transferase (GST) was expressed in E. coli BL21(DE3) as described previously (11). Charged-to-alanine scanning mutagenesis of the B2 gene in pGEX-4T3 was performed by Pfu polymerase PCR and subsequent digestion by DpnI as described elsewhere (12). Mismatched oligonucleotides used for mutagenesis are shown in Table 1. Purification of the mutant proteins was performed under high-salt conditions using glutathione Sepharose 4B (Amersham Biosciences) as described elsewhere (11). Protein concentrations were determined by spectrometry with a NanoDrop ND-1000 instrument and calculated using the theoretical extinction coefficients for each protein, with results being confirmed by comparative analysis using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining.
TABLE 1.
Primers and synthetic RNAs used in this work
| Target | Primer name | Sequence (5′ to 3′)a |
|---|---|---|
| B2 E2A | B2 E2A FWD1 | CCCGGGTCGACATGGCACAAATCCAACAAGC |
| B2 E2A REV1 | GCTTGTTGGATTTGTGCCATGTCGACCCGGG | |
| B2 D9A | B2 D9A FWD1 | TCCAACAAGCGATCGCTCAGCACCTCGTCGA |
| B2 D9A REV1 | TCGACGAGGTGCTGAGCGATCGCTTGTTGGA | |
| B2 H11A | B2 H11A FWD1 | CAAGCGATCGATCAGGCCCTCGTCGAGCTCGA |
| B2 H11A REV1 | TCGAGCTCGACGAGGGCCTGATCGATCGCTTG | |
| B2 E14A | B2 E14A FWD1 | ATCAGCACCTCGTCGCGCTCGAGCAGCTCTT |
| B2 E14A REV1 | AAGAGCTGCTCGAGCGCGACGAGGTGCTGAT | |
| B2 E16A | B2 E16A FWD1 | ACCTCGTCGAGCTCGCGCAGCTCTTCCAGGT |
| B2 E16A REV1 | ACCTGGAAGAGCTGCGCGAGCTCGACGAGGT | |
| B2 D24A | B2 D24A FWD1 | TCCAGGTGATGATGGCCACGCGCGTCGCTCT |
| B2 D24A REV1 | AGAGCGACGCGCGTGGCCATCATCACCTGGA | |
| B2 R26A | B2 R26A FWD1 | GTGATGATGGACACGGCCGTCGCTCTCGGCGG |
| B2 R26A REV1 | CCGCCGAGAGCGACGGCCGTGTCCATCATCAC | |
| B2 E39A | B2 E39A FWD1 | CGATCCAGGTAAACGCGATGCGCACGTTCGT |
| B2 E39A REV1 | ACGAACGTGCGCATCGCGTTTACCTGGATCG | |
| B2 R41A | B2 R41A FWD1 | CAGGTAAACGAGATGGCCACGTTCGTGATAAG |
| B2 R41A REV1 | CTTATCACGAACGTGGCCATCTCGTTTACCTG | |
| B2 H48A | B2 H48A FWD1 | TTCGTGATAAGCGCCGCCGCCGCAGCTCGCCG |
| B2 H48A REV1 | CGGCGAGCTGCGGCGGCGGCGCTTATCACGAA | |
| B2 H55A | B2 H55A FWD1 | GCAGCTCGCCGCCTAGCCGTCCTGTCACGCCG |
| B2 H55A REV1 | CGGCGTGACAGGACGGCTAGGCGGCGAGCTGC | |
| B2 R52A | B2 R52A FWD1 | GCCCACGCCGCAGCTGCCCGCCTACACGTCCT |
| B2 R52A REV1 | AGGACGTGTAGGCGGGCAGCTGCGGCGTGGGC | |
| B2 R53A | B2 R53A FWD1 | CACGCCGCAGCTCGCGCCCTACACGTCCTGTC |
| B2 R53A REV1 | GACAGGACGTGTAGGGCGCGAGCTGCGGCGTG | |
| B2 R59A | B2 R59A FWD1 | CTACACGTCCTGTCAGCCCGGTTCCCACCCCT |
| B2 R59A REV1 | AGGGGTGGGAACCGGGCTGACAGGACGTGTAG | |
| B2 R60A | B2 R60A FWD1 | CACGTCCTGTCACGCGCGTTCCCACCCCTTCC |
| B2 R60A REV1 | GGAAGGGGTGGGAACGCGCGTGACAGGACGTG | |
| B2 E69A | B2 E69A FWD1 | TTCCAGCGGTGATCGCGGAACCGATGGAGAC |
| B2 E69A REV1 | GTCTCCATCGGTTCCGCGATCACCGCTGGAA | |
| B2 E70A | B2 E70A FWD1 | CAGCGGTGATCGAGGCACCGATGGAGACGGA |
| B2 E70A REV1 | TCCGTCTCCATCGGTGCCTCGATCACCGCTG | |
| B2 E73A | B2 E73A FWD1 | TCGAGGAACCGATGGCGACGGACTAGGCGGC |
| B2 E73A REV1 | GCCGCCTAGTCCGTCGCCATCGGTTCCTCGA | |
| B2 D75A | B2 D75A FWD1 | AACCGATGGAGACGGCCTAGGCGGCCGCATC |
| B2 D75A REV1 | GATGCGGCCGCCTAGGCCGTCTCCATCGGTT | |
| DicerHs | HS-DICER-FWD3 | CCTCAAGGACTCTTGGCCCCAATCC |
| HS-DICER-REV3 | CCTCATGCGCATCAGGGTAAGTGCA | |
| E3L | E3L-FWD1 | CTTAAGCTTCGAATTCTGCAGTCGACATGTCTAAGATCTATATTGAC |
| E3L-REV1 | AGTGGATCCTCAGAATCTAATGATGACGTAAC | |
| RNA2 | pBF012-rep1-FWD | GGAAAGGAGCAGCGTCTCACGTCACCTGGTCGGCTG |
| RNA2-REV2 | GGAGGATGGACTTGAAG |
Mismatched nucleotides are underlined.
Protein-RNA interaction analyses.
Electrophoretic mobility shift assays (EMSAs) were performed as described previously (11) using purified GST, GST-B2, or its mutants at a 1 μM concentration and a 40-bp dsRNA at 0.1 μM in buffer containing 50 mM Tris-HCl (pH 7.4) and 100 mM NaCl. Reaction mixtures were incubated at 25°C for 30 min, and the products were separated on 4% Tris-borate-EDTA (TBE)-polyacrylamide gels.
RNase III cleavage inhibition assays were performed using ShortCut RNase III (New England Biolabs). Reactions were performed in 15-μl volumes containing 100 ng of an RNA1-derived 600-bp dsRNA substrate (11), 1× RNase III buffer, 1 mM MnCl2, and 10 μM GST-B2 or its mutants. Following a 30-min preincubation at 25°C to allow B2 to bind to the dsRNA substrate, 0.03 U of RNase III was added and the reaction mixtures were incubated at 37°C for 20 min. This amount was found to be sufficient for complete dsRNA digestion while enabling substantial GST-B2 protection of the dsRNA (Fig. 1A and B). Reactions were terminated by the addition of 125 mM EDTA and stored at −80°C prior to electrophoresis. Reaction products were resolved by TBE-polyacrylamide gel electrophoresis on 4% gels, and the RNA was visualized by staining with ethidium bromide at 1 μg/ml. A Bio-Rad Gel-Doc system was used for imaging, and band quantitation was performed using the histogram function of Adobe Photoshop CS2.
FIG. 1.
Cleavage of virus-derived dsRNA by RNase III and protection by GGNNV B2. (A) Fixed quantities of purified GST-B2 were incubated with dsRNA in the presence of various amounts of RNase III, and the products were resolved by nondenaturing PAGE. Control reactions using GST are shown on the right. (B) Band quantitation of the image shown in panel A, with values shown as arbitrary units (AU). Increasing amounts of added RNase III resulted in a greater level of dsRNA digestion, though only 0.03 U of RNase III was required for complete digestion in the absence of recombinant B2. See Materials and Methods for experimental details.
Transfection of fish and human cells.
SB cells were transfected with synthetic m7G capped RNA1 and its B2 mutant derivatives using Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. Briefly, cells were grown in 24-well plates overnight in antibiotic-free medium containing 5% FBS to a final confluence of approximately 60%. Cells were then rinsed in serum-free medium and transfected with 100 ng of RNA1 or its mutants using 0.5 μl of Lipofectamine 2000 as suggested by the manufacturer. Cells were then incubated at 28°C for 48 h, and RNA was harvested using TRIzol reagent (Invitrogen).
RNA interference.
HeLa cells were transfected with siRNAs using Lipofectamine 2000. Cells were grown in antibiotic-free Dulbecco's modified Eagle's medium with 5% FBS to a confluence of 50 to 60% in a humidified CO2 incubator at 37°C. Cells were then transfected as described above with 10 pmol of either a specific Dicer siRNA (18) or egfp-441 siRNA (41) as a control. After a 24-h transfection period at 37°C, the cells were retransfected with siRNA plus a 100-ng aliquot of either RNA1 or RNA1ΔB2. Cells were incubated at 28°C (to enable RNA1 replication) for a further 24 h prior to RNA extraction. Cellular RNAs were extracted with TRIzol (Invitrogen), treated with DNase I (Roche), and stored at −80°C until required. Plasmid-based short hairpin RNA (shRNA) knockdown of enhanced green fluorescent protein (EGFP) and suppression by plasmid-expressed B2 were performed as described previously (12).
qRT-PCR.
Intracellular RNA1 from SB and HeLa cells was quantitated and normalized against cellular 18S rRNA using a previously described quantitative reverse transcription-PCR (qRT-PCR) assay (12). Interferon-inducible Mx from SB cells was measured as described elsewhere (11). human HeLa cell Dicer (DicerHs) mRNA was quantitated in the same way using primers HS-DICER-FWD3 and HS-DICER-REV3, while RNA2 was quantitated using primers and pBF012-rep1-FWD and RNA2-REV2 (Table 1).
Apoptosis analysis.
Subconfluent monolayers of SB cells were transfected with 2 μg of plasmids pEGFP-C1 (Clontech, CA), pEGFP-B2 (12) and its R53A and R60A derivatives, or pEGFP-E3L by electroporation as described previously (12). The latter plasmid was obtained by amplifying the E3L gene from vaccinia virus using primers E3L-FWD1 and E3L-REV1 (Table 1). Cells were monitored daily for the appearance of cellular blebbing in transfected cells by visualizing EGFP.
Immunofluorescence assays.
GGNNV alpha was detected in paraformaldehyde-fixed SB cells by using anti-alpha polyclonal antibody and fluorescein isothiocyanate-linked secondary antibody as described previously (10). Microscope images were captured using an Olympus IX71 microscope with fluorescent module and Nikon ACT-1 2.70 software.
RESULTS
Scanning mutagenesis of the GGNNV B2 protein.
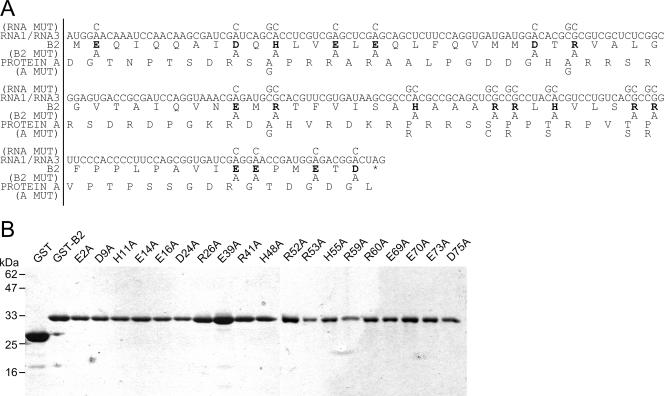
To better characterize the nature of dsRNA binding by B2, which contains no canonical dsRNA binding domains or other known functional domains, we performed charged-to-alanine scanning mutagenesis on each of the 19 charged residues of B2 using a previously described (11) GST-B2 expression vector as the template. Sequence analysis of the B2 coding region of GGNNV RNA1/RNA3 indicated that nine of these mutations would alter the amino sequence of the overlapping viral replicase, with the remainder being silent (Fig. 2A). Each of the B2 mutant proteins was expressed in E. coli with similar efficiency to the wild-type B2 protein and was subsequently purified for in vitro analyses (Fig. 2B).
FIG. 2.
Charged-to-alanine scanning mutagenesis of GGNNV B2. (A) Amino acid sequence of B2, with charged residues targeted for mutagenesis into alanine in boldface. (B) SDS-PAGE of purified GST-B2 mutants. Marker sizes are indicated on the left.
Charged residues are important for dsRNA binding and protection by B2.
Rapid determination of the effect of each charged-to-alanine mutant was made possible by performing gel shift assays with a previously described (11) synthetic 40-bp dsRNA, for which wild-type B2 has high affinity. These assays revealed a substantial difference between the different B2 mutants, as most bound substantially less dsRNA than the wild-type protein, while one mutant, R41A, formed a noticeable high-molecular-weight ladder, suggesting that changes to the mode of dsRNA binding had occurred in this mutant (Fig. 3A). The ability of the R26A, R53A, and R60A mutants to bind the 40-bp dsRNA appeared to be completely abolished (Fig. 3A and C), revealing an important role for these arginine residues in dsRNA binding by B2.
FIG. 3.
The dsRNA binding and protection activity of B2 is severely impaired by charged-to-alanine mutagenesis. (A) EMSA analysis of dsRNA binding by the mutant B2 proteins. A previously described (11) 40-bp dsRNA target at a concentration of 0.1 μM was incubated with GST (negative control), GST-B2 (wild type), or the mutant B2 proteins at 1 μM concentrations, and the products were separated by nondenaturing PAGE. The resulting mobility shift of the dsRNA was taken as a measure of the dsRNA affinity of the proteins. (B) Protection of long dsRNA by B2 and its mutants against RNase III digestion. (C) Quantitative analysis of the EMSA and RNase III protection results shown in panels B and C. Values obtained using wild-type B2 were normalized to 100%, with mutant protein values expressed relative to the wild type. (D) Correlation between 40-bp dsRNA binding and RNase III protection data for B2 mutants. Values shown in panel C were plotted as an XY scatter plot, and linear regression was calculated for the pool of mutants. Mutants selected for further analyses are indicated by stars. (E) Alignment of betanodavirus B2 proteins showing the conservation of important amino acid residues required for dsRNA binding and protection. Identical residues are indicated by asterisks, and dsRNA binding-related residues are boxed.
The ability of B2 to bind dsRNA enables it to protect long virus-derived dsRNAs from digestion by cellular RNases such as Dicer (11). However, it could not be assumed that the ability of B2 to bind a 40-bp dsRNA would correlate directly with its ability to protect long dsRNAs from enzymatic digestion to produce siRNA. With this in mind, we utilized another rapid assay for B2 activity using recombinant RNase III, against which B2 successfully protected a long (600 bp) GGNNV-derived dsRNA substrate against digestion (Fig. 1A and B). Again there was substantial variation between the mutants in their abilities to protect the long dsRNA from RNase III digestion (Fig. 3B). Strikingly, mutants E14A and D24A, though impaired in 40-bp dsRNA binding, appeared to protect the long dsRNA from digestion more than 50% better than wild-type B2 (Fig. 3C). Mutants R53A and R60A showed no detectable protective activity, while R26A retained 36% of the wild-type activity, despite being unable to bind the 40-bp dsRNA. Further analysis of these data revealed a positive correlation between the 40-bp dsRNA binding and 600-bp dsRNA protection assays (Fig. 3D), though several of the mutants, namely E2A, E16A, E39A, and R26A, did not behave predictably in both assays as they had lost essentially all 40-bp dsRNA binding or 600-bp dsRNA protective activity but retained the other activity. A most unexpected finding was that mutants E14A and D24A appeared to protect dsRNA substantially better than the wild-type protein but had lost 50% of their 40-bp dsRNA binding activity (Fig. 3C and D). This result is somewhat cryptic, but it may be the result of a change in the cooperative binding of B2 (11) during attachment to the 600-bp substrate, which did not occur when the proteins bound the 40-bp substrate.
Interestingly, an amino acid sequence alignment of B2 proteins from different betanodavirus strains revealed that residues important for dsRNA binding in GGNNV B2 were conserved (Fig. 3E), suggesting that the mode of binding is conserved between betanodavirus B2 proteins.
Betanodavirus RNA accumulation in fish cells is attenuated by B2 mutation.
For further selection of potentially useful attenuated mutants, we imposed two simple criteria: (i) the mutation should reduce the dsRNA binding or protective capacity of B2 to a level below that of the wild-type protein, and (ii) the mutation should not influence the amino acid sequence of the overlapping replicase protein encoded by RNA1. Using these criteria, we identified five mutants, namely E2A, D9A, E16A, E39A, and E73A (Fig. 2A and 3D). To determine the effect of these mutations on intracellular virus RNA accumulation, we created each mutation within our previously described (12) GGNNV RNA1 plasmid, pGGNNV1(2,0), and generated capped RNA transcripts by in vitro transcription. To validate our initial selection procedures, we also created the R53 and R60A mutations within RNA1 as these mutations completely abolished dsRNA binding and protection and were therefore expected to exhibit similar intracellular behavior to the ΔB2 mutant.
Following transfection of SB cells with the mutant RNA1s, we found that the accumulation of RNA1 was consistent with the ability of each of the B2 mutants to protect dsRNA from RNase digestion in vitro. Thus, the R53A and R60A mutants exhibited severely reduced RNA1 accumulation almost identical to the ΔB2 mutant, while mutants D9A and E73A yielded moderately reduced RNA1 levels compared to the wild type (Fig. 4A). Two mutants that were identified from our initial in vitro screen, E14A and D24A, appeared to protect dsRNA better than the wild-type protein (see Fig. 3A). However, RNA1 containing these mutations did not accumulate to levels beyond wild-type RNA when introduced into SB cells (data not shown), indicating that these mutations do not improve the ability of B2 to protect RNA1 in vivo.
FIG. 4.
GGNNV depends on the dsRNA binding activity of B2 for accumulation in SB cells. (A) Accumulation of RNA1 and its B2 mutants in SB cells. Subconfluent SB cells were cotransfected with 100 ng RNA1 or its B2 mutant derivatives, and RNA1 was measured by qRT-PCR at 48 h posttransfection. (B) Induction of the SB Mx gene by RNA1 or its B2 mutant derivatives. RNA samples were the same as for panel A. (C) RNA1-dependent accumulation of RNA2 in SB cells and the influence of B2 mutations on this process. RNA was prepared from SB cells at 48 h (filled bars) or 72 h (empty bars) posttransfection, and RNA2 was measured by qRT-PCR. Bars represent the means of at least three independent experiments, with the standard deviation indicated by error bars. (D) Mutation of GGNNV B2 attenuates viral pathogenicity and production of the alpha coat protein. Subconfluent SB cells were cotransfected with 100 ng of RNA2 and RNA1 or its B2 mutant derivatives. Mutant E2A is shown as an example, though the remaining mutants yielded results that were indistinguishable from those of this mutant (data not shown). Examples of cytopathic effects visible in cells cotransfected with RNA1/RNA2 are indicated with arrowheads. The upper row of images shows phase-contrast images of the transfected cells, while the lower row shows the same cells stained for alpha coat protein using immunofluorescence.
Host interferon-inducible Mx is poorly induced by B2 mutants.
We have previously used the interferon-inducible Mx gene expressed by SB cells as a marker for stimulation of the interferon system, and showed that this gene is induced by RNA1 accumulation in the presence of B2 (11). We again used Mx as a cellular marker to monitor the influence of the B2 mutants on interferon induction. As previously reported, the RNA1ΔB2 mutant did not induce Mx above background levels, while the B2 point mutants each induced Mx expression to a degree that was highly consistent with their levels of RNA1 accumulation (Fig. 4B). Consistent with its inability to bind dsRNA, mutant R60A failed to induce Mx beyond background levels, while mutants D9A and E73A, with intermediate dsRNA binding activities and RNA1 accumulation, also yielded intermediate levels of Mx induction (Fig. 4B). By correlating the level of Mx mRNA and RNA1 (Fig. 4A and B) for each of the B2 mutants, it appeared that Mx induction is dictated by RNA1 accumulation (r2 = 0.75).
B2 facilitates RNA2 accumulation.
The influence of the B2 mutations on RNA2 accumulation was assessed by cotransfecting SB cells with mutant RNA1 and wild-type RNA2 derived from pGGNNV2(1,0) (12) and measuring RNA2 levels. To eliminate potential interference derived from cells that were transfected with RNA2 but not RNA1, we performed a control transfection with only RNA2 and subtracted the resulting value from the total for each transfection. Thus, only RNA1-dependent RNA2 accumulation was being measured in these assays. As we anticipated, the RNA2 levels measured at 48 h posttransfection correlated very closely with RNA1 levels in cells transfected only with RNA1 and its derived B2 mutants (Fig. 4C, filled bars). However, when cotransfected cells were incubated for 72 h, only cells transfected with wild-type, E2A, and E73A RNA1 contained RNA2 levels beyond background (Fig. 4C, empty bars). Moreover, of these only the wild type and E2A yielded an increase in RNA2 levels, while RNA2 was being cleared from cells transfected with the E73A mutant. These results clearly indicate that sustained maintenance of a complete RNA1/RNA2 viral replication cycle was extremely sensitive to perturbations in the dsRNA binding activity of B2.
We next determined if the poor RNA accumulation exhibited by the B2 mutants was also reflected at the whole-cell level by analyzing cell morphology and performing immunofluorescence assays (IFAs) on the cotransfected SB cells. Transfection of SB cells with RNA2 alone did not produce any visible cytopathic effects (CPEs) or alpha protein IFA signals after 5 days, while cotransfection of wild-type RNA1 and RNA2 led to a pronounced CPE and an abundance of IFA signals (Fig. 4D). However, neither the ΔB2 mutant nor the other B2 mutants yielded any CPEs or IFA signals (Fig. 4D) (data not shown), further confirming the essential role of B2 and its associated dsRNA binding activity in GGNNV infection.
Cell death associated with B2 overexpression is not caused by dsRNA binding.
Previous work has indicated that betanodavirus B2, when overexpressed from a plasmid vector, can induce cell death in both plant tissues (19) and animal cells (39). It was suggested that this could potentially be caused by B2 binding to cellular micro-RNAs (miRNAs) that modulate cell growth and death (19). We have previously overexpressed B2 in SB cells using a cytomegalovirus (CMV) promoter-based plasmid but did not observe any obvious signs of cell death after 24 h (12). We decided to investigate this apparent discrepancy further by introducing EGFP, EGFP-B2, EGFP-B2R53A, and EGFP-B2R60A expression plasmids into SB cells and monitoring for signs of cell death over time. In addition to these plasmids, we also fused vaccinia virus E3L, which is a dsRNA binding protein that suppresses apoptosis and interferon induction (15, 21, 22), to EGFP as a control. While SB cells overexpressing EGFP appeared healthy and continued dividing over the 96-h observation period, cells transfected with EGFP-B2 or its EGFP-B2R53A and EGFP-B2R60A dsRNA binding mutant derivatives ceased dividing after transfection, with cell shrinkage and membrane blebbing characteristic of apoptosis becoming evident at 72 h (Fig. 5A). Counts of the total number of blebbing cells in each of the transfected SB cell populations did not reveal any distinct differences between wild-type and dsRNA binding mutants of B2 (Fig. 5B), indicating that this phenomenon is not related to the dsRNA binding activity of B2. Similar results were obtained in BSR T7/5 cells (4) at 37°C (data not shown), suggesting that this phenomenon is neither cell line nor temperature specific.
FIG. 5.
Cell death caused by B2 overexpression is not related to dsRNA binding. (A) SB cell blebbing is evident after transfection with an EGFP-B2 plasmid or its dsRNA binding mutants, but not after transfection with EGFP or EGFP-E3L plasmids. Subconfluent SB cells were transfected with 2 μg of each plasmid, and representative healthy or blebbing cells were photographed at 72 h. (B) Counts of blebbing SB cells from the experiment described in panel A. A minimum of 300 transfected (green) SB cells were counted and scored for blebbing. The results are expressed as a percentage of the total cells counted, with the error bars representing the standard deviation from three independent experiments.
Accumulation of B2 mutant RNA1 is enhanced in a Dicer knockdown background.
We have previously suggested that the accumulation defect of RNA1ΔB2 in SB and other cell lines was due to the action of Dicer, leading to the cleavage of virus dsRNAs and the production of siRNA (11), which would in turn lead to antiviral RNA silencing. If Dicer was responsible for the destruction of positive-strand RNA1ΔB2, then its elimination or down-regulation would enable RNA1ΔB2 to accumulate beyond the level seen in the presence of Dicer. As the Dicer nucleotide sequence for L. calcarifer and the derived SB cell line was unavailable, we instead used HeLa cells as a model for the RNA1ΔB2 accumulation defect. To reduce Dicer levels, we used an siRNA-mediated knockdown approach as reported previously (18) and monitored the level of Dicer knockdown using a qRT-PCR assay that targeted 165 bp in the 3′ region of the DicerHs gene. Amplification yielded a single band of the expected size (Fig. 6A), while melting curve analysis revealed a peak corresponding to a melting point of 84.4°C (Fig. 6B). We next used this assay to monitor the abundance of DicerHs mRNA following transfection of HeLa cells with 20 or 100 nM of a specific siRNA targeting DicerHs or a nonspecific control siRNA. A knockdown of approximately 80% was achieved with both concentrations of the specific siRNA (Fig. 6C). Surprisingly, however, transfection of the nonspecific control siRNA gave knockdowns of 30% at 20 nM and 50% at 100 nM (Fig. 6C), yielding effective specific knockdowns of approximately 75% at 20 nM and 60% at 100 nM. We thus used 20 nM siRNA concentrations in subsequent experiments.
FIG. 6.
A B2 mutant of RNA1 cannot accumulate in HeLa cells due to the action of the Dicer RNase. (A) Amplification of DicerHs mRNA by qRT-PCR. Total RNA from HeLa cells was extracted and subjected to RT-PCR as described in Materials and Methods. The amplicon size is 165 bp, corresponding to the observed band. (B) Melting curve analysis of the DicerHs qRT-PCR product. A single peak with an apparent melting temperature of 84.4°C was obtained. (C) RNA silencing of DicerHs mRNA in HeLa cells by siRNA transfection. Cells were left untransfected or were transfected with 10 or 50 pmol of a control siRNA or a specific siRNA. DicerHs mRNA was quantitated after 48 h by qRT-PCR. Values are expressed as a function of the amount of DicerHs mRNA present in untransfected HeLa cells. (D) Influence of siRNA-mediated Dicer knockdown on RNA1 and RNA1ΔB2 accumulation in transfected HeLa cells. Cells were transfected as described in Materials and Methods, and RNA1 was measured by qRT-PCR. Values shown are the means of three independent determinations, with the error bars indicating the standard deviations.
We next introduced in vitro-transcribed RNA1 and RNA1ΔB2 into HeLa cells that had 24 h previously been transfected with specific or control siRNA or left untransfected. At 24 h after this second transfection, we prepared total RNA from the cells and quantitated the amount of RNA1, with quite startling results. In the presence of Dicer siRNA, the level of RNA1ΔB2 was elevated by approximately eight times, increasing the amount of RNA1ΔB2 relative to RNA1 from 3% to 28% (Fig. 6D). Additionally, Dicer knockdown in cells transfected with wild-type RNA1 further increased RNA1 accumulation by threefold (Fig. 6D). This suggests that Dicer limits the accumulation of RNA1 in HeLa cells and that B2 plays a role in countering this process.
Suppression of RNA silencing by B2 requires dsRNA binding activity.
We next used HeLa cells as an RNA-silencing model to determine whether the previously described (12) RNA silencing suppression activity of B2 was related to its dsRNA binding activity. This was done by introducing the different mutations into a CMV promoter-driven B2 expression vector and introducing the resulting plasmids into HeLa cells with EGFP and anti-EGFP shRNA plasmids as before (12). As expected, wild-type B2 efficiently suppressed silencing of EGFP (Fig. 7), while the mutants exhibited decreases in their silencing suppression activity that correlated markedly with their accumulation defects in SB cells (compare Fig. 7 with Fig. 4A). In the case of mutants R53A and R60A, essentially all suppressor activity was lost, correlating with a complete loss of dsRNA binding activity. These data clearly support the idea that B2 blocks RNA silencing through its dsRNA binding activity.
FIG. 7.
The ability of B2 to suppress RNA silencing in HeLa cells is dependent on its dsRNA binding activity. HeLa cell transfections were performed using either pcDNA3.1(+) (vector) or pcDNA-B2 and its mutants, pEGFP-C1, and anti-EGFP shRNA vectors as described previously (12). Values shown are percentages of the amount of EGFP mRNA detected by qRT-PCR in the pcDNA-B2 transfection, with error bars indicating the standard deviation of at least three independent determinations.
Secondary structure predictions reveal a role for charged residues in B2 structure.
While the scanning mutagenesis experiments described above indicate that certain charged residues, such as R53 and R60, are required for B2 dsRNA binding and protection, their involvement in this process may be limited to maintaining the structural integrity of B2. We endeavored to address this possibility by modeling the structure of B2 using the PSIPRED method (20, 31). PSIPRED was chosen above other methods as its predictions proved most accurate using two relevant proteins of known structure, namely FHV B2 (28) and the dsRNA binding domain of Staufen (5). Using this method, we were able to predict the almost purely alpha-helical structure of FHV B2 to within 89% accuracy of the experimental solution structure (Fig. 8A), while an accuracy of 85% was obtained for the canonical αβββα structure of the Staufen dsRNA-binding domain (dsRBD) (Fig. 8B).
FIG. 8.
Charged amino acid mutations induce minor secondary structural changes to GGNNV B2 based on structural modeling. Comparisons of (A) FHV B2 and (B) Staufen dsRBD hypothetical (Hyp.) and experimental (Exp.) structures are shown in the top two panels, with alpha-helical regions indicated by a red α, beta sheets indicated by a blue β, and random coils indicated by dashes. Matches of 89% (64/72) and 85% (58/68) between hypothetical and experimental structures were obtained for FHV B2 and Staufen dsRBD, respectively. (C) GGNNV B2 is predicted to have a largely alpha-helical structure that is susceptible to minor structural changes caused by charged-to-alanine mutagenesis. A secondary structure model of wild-type (WT) B2 is shown aligned to models of each of the chosen mutants, with conserved positions shown as centered dots. The positions of each mutation are shown as asterisks. Modeling was performed using the PSIPRED method (20, 31).
Analysis of the predicted GGNNV B2 secondary structure suggested that this protein consists of alpha-helical regions at the N- and C-termini and beta sheets in the core (Fig. 8C). Interestingly, the N and C termini were very similar to the predicted FHV B2 structure, though the core beta sheet is absent in FHV B2. This suggested that the B2 proteins from GGNNV and FHV may have a limited degree of structural similarity, though this remains to be confirmed experimentally. We next analyzed structural predictions for each of the chosen GGNNV B2 mutants and compared them to the wild-type protein prediction (Fig. 8C). Surprisingly, all of the mutants underwent a minor structural change at S46, which changed from a beta sheet to an alpha helix. Moreover, mutants E39A, R53A, and R60A underwent additional changes in the same region. These changes may provide an explanation as to why R53A and R60A are unable to bind dsRNA.
DISCUSSION
While RNA silencing is now a well-established mechanism by which plants and invertebrates fight off viral infection, its role as an antiviral defense in vertebrates has remained somewhat controversial (7). Part of this controversy revolves around the idea that vertebrates, unlike plants and most invertebrates, have a potent interferon response that functions as a rapid, nonspecific defense mechanism that prevents viral replication and spread. This system would thus appear to be a more highly evolved and more effective innate response to viral infection than RNA silencing, which is often referred to as a type of primitive immune system (1). This notion is further supported by the abundance of vertebrate virus proteins that function to block the interferon response at numerous signaling steps through a series of highly specialized functional domains (3, 23, 35). However, unlike these proteins, the B2 protein of GGNNV cannot antagonize the interferon response and thus it seems that for nodaviruses the most important barrier to viral replication is RNA silencing. Interestingly, recent work suggests that interferon signaling only becomes important in fish during later stages of fish life cycle, with zebrafish larvae exhibiting a poor interferon response (29). This suggests that betanodaviruses need not antagonize the interferon response because it is inactive during the developmental stage at which they typically infect fish.
Scanning mutagenesis of the GGNNV B2 protein has revealed that amino acids from position 2 through to the end of the protein at position 73 play roles in dsRNA binding and protection, suggesting that the entire length of B2 functions as a dsRNA binding domain. Given that B2 is only 75 amino acids in length, it is probably not surprising that this protein possesses only a single biological function—that of binding and protecting dsRNA. It also contrasts with the substantially longer and more complex interferon antagonists of other animal viruses that usually contain several discrete domains responsible for blocking the interferon pathways as well as possibly the RNA silencing pathway through their common dsRNA binding activities (27). With this in mind, we speculate that other animal virus proteins that consist of only a dsRNA binding domain would also function as dedicated RNA silencing suppressors and not interferon antagonists, while those proteins with a dsRNA binding domain and additional domains would most likely function primarily as interferon antagonists and secondarily as silencing suppressors.
Of the 19 residues included in our mutagenesis scan of B2, residues R53 and R60 stood out as being critical for dsRNA binding and protection. This finding has recently been repeated for the B2 protein of a betanodavirus from orange-spotted grouper (32), confirming the importance of these residues. As structural information about betanodavirus B2 is not yet available, the roles of R53 and R60 in dsRNA binding can only be predicted, but it is of obvious significance that a number of other viral dsRNA binding proteins, including human immunodeficiency virus TRBP (8, 9, 16), vaccinia virus E3L (34), and FHV B2 (6, 28), employ basic arginine residues to mediate direct contact with guanine residues on the dsRNA substrate. However, results of our structural predictions suggest that our charged-to-alanine mutations may influence the structure of B2 and in turn negatively impact its ability to bind dsRNA. In the case of mutants E2A, D9A, E16A, E39A, and E73A (all acidic to neutral mutations), dsRNA binding or protection was still evident in vitro, which suggests that these mutants had retained sufficient structural integrity to bind to the substrate. Mutants R53A and R60A, however, have completely lost all binding activity, and this might be explained by a structural perturbation that prevented the protein from interacting with dsRNA. Experimental structural analyses of GGNNV B2 will be required to address this possibility.
The importance of B2 residues R53 and R60, along with several other residues, was further established by experiments that revealed that sustained GGNNV RNA1 and RNA2 accumulation in SB cells required full dsRNA binding and protection activities. Even slight decreases in these activities, such as in the case of mutant D9A, prevented long-term accumulation of RNA2. These findings suggest that for these mutants the rate of virus RNA degradation due to RNA silencing is greater than the rate of accumulation. In mutants E2A and E73A, where RNA2 persisted long enough for a viral replication cycle to take place, the difference in the accumulation and degradation rates was probably minor but still enough to prevent the massive RNA2 accumulation seen in the wild type. A similar phenomenon exists with FHV replication in mammalian cells, where B2 mutation prevents sustained RNA accumulation over several cell passages (2). Experiments in our laboratory are now under way to investigate the nature of the RNA-silencing response in SB cells in order to correlate this with the loss of B2 mutant RNA.
While some recently presented work (19, 38, 39) indicates that B2 can induce apoptosis or necrosis, data presented here indicate that in the case of SB cells this is not the result of dsRNA binding by B2. Mutations in residues R53 and R60 that abolish dsRNA binding did not significantly reduce the level of apoptosis, suggesting that this phenomenon is due to either overexpression of the protein beyond the levels that normally occur during infection, resulting in artifactual induction of apoptosis, or a secondary function of B2. However, the lack of apoptosis observed in our control plasmids expressing EGFP or EGFP-E3L suggests that there is indeed a degree of specificity in this process, and we believe this finding warrants further investigation.
Acknowledgments
This work was generously supported by Temasek Life Sciences Laboratory, the Agrifood and Veterinary Authority of Singapore, and the Singapore Millennium Foundation.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Bagasra, O., and K. R. Prilliman. 2004. RNA interference: the molecular immune system. J. Mol. Histol. 35:545-553. [DOI] [PubMed] [Google Scholar]
- 2.Ball, L. A. 1995. Requirements for the self-directed replication of flock house virus RNA 1. J. Virol. 69:720-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz, U. J., S. Finke, and K.-K. Conzelmann. 1999. Generation of bovine respiratory syncytial virus (BRSV) from cDNA: BRSV NS2 is not essential for virus replication in tissue culture, and the human RSV leader region acts as a functional BRSV genome promoter. J. Virol. 73:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bycroft, M., S. Grunert, A. G. Murzin, M. Proctor, and D. St Johnston. 1995. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 14:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao, J. A., J. H. Lee, B. R. Chapados, E. W. Debler, A. Schneemann, and J. R. Williamson. 2005. Dual modes of RNA-silencing suppression by Flock House virus protein B2. Nat. Struct. Mol. Biol. 12:952-957. [DOI] [PubMed] [Google Scholar]
- 7.Cullen, B. R. 2006. Is RNA interference involved in intrinsic antiviral immunity in mammals? Nat. Immunol. 7:563-567. [DOI] [PubMed] [Google Scholar]
- 8.Daviet, L., M. Erard, D. Dorin, M. Duarte, C. Vaquero, and A. Gatignol. 2000. Analysis of a binding difference between the two dsRNA-binding domains in TRBP reveals the modular function of a KR-helix motif. Eur. J. Biochem. 267:2419-2431. [DOI] [PubMed] [Google Scholar]
- 9.Erard, M., D. G. Barker, F. Amalric, K. T. Jeang, and A. Gatignol. 1998. An Arg/Lys-rich core peptide mimics TRBP binding to the HIV-1 TAR RNA upper-stem/loop. J. Mol. Biol. 279:1085-1099. [DOI] [PubMed] [Google Scholar]
- 10.Fenner, B. J., Q. Du, W. Goh, R. Thiagarajan, H. K. Chua, and J. Kwang. 2006. Detection of betanodavirus in juvenile barramundi, Lates calcarifer (Bloch), by antigen capture ELISA. J. Fish Dis. 29:423-432. [DOI] [PubMed] [Google Scholar]
- 11.Fenner, B. J., W. Goh, and J. Kwang. 2006. Sequestration and protection of double-stranded RNA by the betanodavirus B2 protein. J. Virol. 80:6822-6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenner, B. J., R. Thiagarajan, H. K. Chua, and J. Kwang. 2006. Betanodavirus B2 is an RNA interference antagonist that facilitates intracellular viral RNA accumulation. J. Virol. 80:85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritz, J. H., S. E. Girardin, and D. J. Philpott. 2006. Innate immune defense through RNA interference. Sci. STKE 2006:pe27. [DOI] [PubMed] [Google Scholar]
- 14.Galiana-Arnoux, D., C. Dostert, A. Schneemann, J. A. Hoffmann, and J. L. Imler. 2006. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7:590-597. [DOI] [PubMed] [Google Scholar]
- 15.Garcia, M. A., S. Guerra, J. Gil, V. Jimenez, and M. Esteban. 2002. Anti-apoptotic and oncogenic properties of the dsRNA-binding protein of vaccinia virus, E3L. Oncogene 21:8379-8387. [DOI] [PubMed] [Google Scholar]
- 16.Gatignol, A., C. Buckler, and K.-T. Jeang. 1993. Relatedness of an RNA-binding motif in human immunodeficiency virus type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and Drosophila Staufen. Mol. Cell. Biol. 13:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde, A., H. C. Teh, T. J. Lam, and Y. M. Sin. 2003. Nodavirus infection in freshwater ornamental fish, guppy, Poicelia reticulata—comparative characterization and pathogenicity studies. Arch. Virol. 148:575-586. [DOI] [PubMed] [Google Scholar]
- 18.Hutvágner, G., J. McLachlan, A. E. Pasquinelli, É. Bálint, T. Tuschl, and P. D. Zamore. 2001. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293:834-838. [DOI] [PubMed] [Google Scholar]
- 19.Iwamoto, T., K. Mise, A. Takeda, Y. Okinaka, K. Mori, M. Arimoto, T. Okuno, and T. Nakai. 2005. Characterization of striped jack nervous necrosis virus subgenomic RNA3 and biological activities of its encoded protein B2. J. Gen. Virol. 86:2807-2816. [DOI] [PubMed] [Google Scholar]
- 20.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195-202. [DOI] [PubMed] [Google Scholar]
- 21.Kibler, K. V., T. Shors, K. B. Perkins, C. C. Zeman, M. P. Banaszak, J. Biesterfeldt, J. O. Langland, and B. L. Jacobs. 1997. Double-stranded RNA is a trigger for apoptosis in vaccinia virus-infected cells. J. Virol. 71:1992-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwon, J. A., and A. Rich. 2005. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc. Natl. Acad. Sci. USA 102:12759-12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langland, J. O., J. M. Cameron, M. C. Heck, J. K. Jancovich, and B. L. Jacobs. 2006. Inhibition of PKR by RNA and DNA viruses. Virus Res. 119:100-110. [DOI] [PubMed] [Google Scholar]
- 24.Li, F., and S. W. Ding. 2006. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu. Rev. Microbiol. 60:503-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, H., W. X. Li, and S. W. Ding. 2002. Induction and suppression of RNA silencing by an animal virus. Science 296:1319-1321. [DOI] [PubMed] [Google Scholar]
- 26.Li, H. W., and S. W. Ding. 2005. Antiviral silencing in animals. FEBS Lett. 579:5965-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W. X., H. Li, R. Lu, F. Li, M. Dus, P. Atkinson, E. W. Brydon, K. L. Johnson, A. Garcia-Sastre, L. A. Ball, P. Palese, and S. W. Ding. 2004. Interferon antagonist proteins of influenza and vaccinia viruses are suppressors of RNA silencing. Proc. Natl. Acad. Sci. USA 101:1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lingel, A., B. Simon, E. Izaurralde, and M. Sattler. 2005. The structure of the flock house virus B2 protein, a viral suppressor of RNA interference, shows a novel mode of double-stranded RNA recognition. EMBO Rep. 6:1149-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu, M. W., Y. M. Chao, and J. L. Wu. 2006. The interferon response involved in nervous necrosis virus acute and persistent infection using zebrafish as an animal disease model, VNN 2006—First International Symposium on Viral Nervous Necrosis of Fish, Hiroshima, Japan, p. OP-17.
- 30.Lu, R., M. Maduro, F. Li, H. W. Li, G. Broitman-Maduro, W. X. Li, and S. W. Ding. 2005. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436:1040-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 32.Ou, M. C., Y. M. Chen, M. F. Jeng, H. L. Yang, and T. Y. Chen. 2006. Identification of orange-spotted grouper (Epinephelus coioides) nervous necrosis virus (OGNNV) B2-Arg53 as a critical residue for dsRNA binding activity, VNN 2006—First International Symposium on Viral Nervous Necrosis of Fish, Hiroshima, Japan, p. PP-7.
- 33.Robalino, J., T. Bartlett, E. Shepard, S. Prior, G. Jaramillo, E. Scura, R. W. Chapman, P. S. Gross, C. L. Browdy, and G. W. Warr. 2005. Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? J. Virol. 79:13561-13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romano, P. R., F. Zhang, S.-L. Tan, M. T. Garcia-Barrio, M. G. Katze, T. E. Dever, and A. G. Hinnebusch. 1998. Inhibition of double-stranded RNA-dependent protein kinase PKR by vaccinia virus E3: role of complex formation and the E3 N-terminal domain. Mol. Cell. Biol. 18:7304-7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scherer, W. F., J. T. Syverton, and G. O. Gey. 1953. Studies on the propagation in vitro of poliomyelitis viruses. IV. Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. J. Exp. Med. 97:695-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 38.Su, Y. C., H. L. Yang, J. L. Wu, and J. R. Hong. 2006. Cloning and characterization of a non-structural protein B2 as a death inducer from betanodavirus, VNN 2006—First International Symposium on Viral Nervous Necrosis of Fish, Hiroshima, Japan.
- 39.Su, Y. C., H. L. Yang, J. L. Wu, and J. R. Hong. 2004. A novel viral gene B2 induces host apoptotic cell death and death function knockdown by small interfering RNA (siRNA), p. 67. In H. N. Chou and Y. L. Wang (ed.), The Fisheries Society of Taiwan 2004 Annual Conference. National Taiwan University, Taipei.
- 40.Sullivan, C. S., and D. Ganem. 2005. A virus-encoded inhibitor that blocks RNA interference in mammalian cells. J. Virol. 79:7371-7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ui-Tei, K., Y. Naito, F. Takahashi, T. Haraguchi, H. Ohki-Hamazaki, A. Juni, R. Ueda, and K. Saigo. 2004. Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res. 32:936-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, X. H., R. Aliyari, W. X. Li, H. W. Li, K. Kim, R. Carthew, P. Atkinson, and S. W. Ding. 2006. RNA interference directs innate immunity against viruses in adult Drosophila. Science 312:452-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber, F., V. Wagner, S. B. Rasmussen, R. Hartmann, and S. R. Paludan. 2006. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 80:5059-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]