Abstract
Borna disease virus (BDV) is an enveloped virus with a nonsegmented negative-strand RNA genome whose organization is characteristic of Mononegavirales. BDV cell entry follows a receptor-mediated endocytosis pathway, which is initiated by the recognition of an as-yet-unidentified receptor at the cell surface by the virus glycoprotein G. BDV G is synthesized as a precursor (GPC) that is cleaved by the cellular protease furin to produce the mature glycoproteins GP1 and GP2, which have been implicated in receptor recognition and pH-dependent fusion events, respectively. BDV is highly neurotropic and its spread in cultured cells proceeds in the absence of detectable extracellular virus or syncytium formation. BDV spread has been proposed to be strictly dependent on the expression and correct processing of BDV G. Here we present evidence that cell-to-cell spread of BDV required neither the expression of cellular receptors involved in virus primary infection, nor the furin-mediated processing of BDV G. We also show that in furin-deficient cells, the release of BDV particles induced by the treatment of BDV-infected cells with hypertonic buffer was not significantly affected, while virion infectivity was dramatically impaired, correlating with the decreased incorporation of BDV G species into viral particles. These findings support the view that the propagation of BDV within the central nervous systems of infected hosts involves both a primary infection that follows a receptor-mediated endocytosis pathway and a subsequent cell-to-cell spread that is independent of the expression of the primary receptor and does not require the processing of BDV G into GP1 and GP2.
Borna disease virus (BDV) is the causative agent of Borna disease, an immune-mediated disease of the central nervous system (CNS), affecting mainly horses and sheep in central Europe (29, 36). However, current evidence indicates that the natural host range as well as the prevalence and geographic distribution of BDV may have been largely underestimated (29, 36). Experimentally, BDV has a wide host range from birds to rodents and nonhuman primates (15, 20, 29, 36). Both host and viral factors contribute to a variable period of incubation and heterogeneity in the symptoms and pathology associated with BDV infection (10, 14, 19, 28, 29).
BDV is an enveloped virus that has a nonsegmented negative-strand RNA genome with an organization characteristic of those of mononegaviruses (3, 31, 34). However, based on its unique genetics and biological features, BDV is considered to be the prototypic member of a new virus family, Bornaviridae, within the order Mononegavirales (5, 31). The BDV genome contains six major open reading frames in the order 3′-N-p10/P-M-G-L-5′ (4, 6, 34, 39). The polypeptides coded by these open reading frames correspond to the nucleoprotein (N), nonstructural p10 protein (p10), phosphoprotein (P), matrix (M), surface glycoprotein (G), and RNA-dependent RNA polymerase (L) found in other mononegaviruses.
The BDV G gene directs the synthesis of a precursor polypeptide, GPC, with a predicted molecular mass of 56 kDa, but due to its extensive glycosylation, GPC migrates with a mass of 84 to 94 kDa. GPC is posttranslationally cleaved by the cellular protease furin into GP1 (GPN) and GP2 (GPC), which correspond to the N- and C-terminal regions, respectively, of GPC (9, 27). GPC and GP2 are readily detected in both infected cells and cell-free virions (9, 10), whereas antibody (Ab) detection of GP1 has been complicated by its high content of N-glycans that shield antigenic sites (16).
BDV G plays a key role in receptor recognition and cell entry (1, 10, 26) and has been shown to be strictly required for the generation of infectious BDV virus-like particles (24). GP1 is sufficient for virus receptor recognition and cell entry (26), whereas GP2 appears to mediate the pH-dependent fusion event between viral and endosome membranes (9). This fusion event delivers the virus ribonucleoprotein (RNP) core to the cytoplasm, which is followed by its import into the nucleus, where both RNA replication and transcription occur (3).
At early times after intracerebral inoculation of rodents, BDV multiplication is restricted to neuronal cell populations (29) despite the fact that the virus inoculum had access to all brain cell types. These findings suggest a restricted expression pattern of an as-yet-unidentified virus receptor required for BDV infection de novo. The propagation of BDV within the CNS progresses relatively rapidly but in the absence of detectable mature virion particles or syncytium formation (13), and at later times, astrocytes and other nonneuronal cell populations harbor viral antigen (13, 37). These observations led to the proposal that a receptor-independent mechanism contributes to the cell-to-cell propagation of BDV. In addition, recent evidence has suggested that this type of propagation depends on the expression and correct processing of BDV G (1). In this work, we have examined whether infection de novo and cell-to-cell propagation of BDV require the same cell surface receptors. In addition, we have revisited the role of furin-mediated processing of BDV G in cell-to-cell virus propagation. Our results provide evidence that neither the presence of cell surface receptors that mediated entry of cell-free virus nor the furin-mediated processing of BDV G is required for cell-to-cell propagation of BDV. We discuss the implications of these findings for BDV propagation within the CNS.
MATERIALS AND METHODS
Cells and viruses.
Chinese hamster ovary (CHO) cells (ATCC CRL-10154), DF11 cells, CHO-green fluorescent protein (GFP) cells, and DF11-GFP cells as well as BDV-infected CHO cells expressing GFP (CHO-GFP) and BDV-infected DF11-GFP cells were maintained in alpha Eagle's minimal essential medium supplemented with 5% heat-inactivated fetal calf serum (FCS; Life Technologies). Vero E6 cells (ATCC CRL-1586) and Vero E6 BDV persistently infected (BDV-Pi) cells were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 10 mM HEPES, and 10% heat-inactivated FCS. Human embryonic kidney 293T cells (ATCC CRL-11268) were maintained in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine and 10% heat-inactivated FCS. To generate CHO and DF11 cell lines expressing GFP, we transduced the cells with a retrovirus expressing GFP and a neomycin-resistant gene (35). Transduced cells were selected in the presence of G418 (900 μg/ml). After several passages in the presence of G418, the cultures showed 100% GFP-positive cells.
The BDV strain utilized for the generation of BDV-infected cell lines and primary infections was He80 (33). The generation and characterization of the recombinant vesicular stomatitis virus (rVSV) expressing the BDV G instead of its own G (rVSVΔG*/BDVG) have been described elsewhere (M. M. Perez, R. Clemente, C. S. Robinson, H. R. Jayakar, M. A. Whitt, and J. C. de la Torre, submitted for publication). The recombinant vaccinia virus expressing BDV G (rVV/BDVG) has been described previously (10).
Antibodies.
The rabbit sera to BDV G, BDV N, and BDV P have been described elsewhere (10, 30). The rabbit serum to GP1N was generated by immunizing rabbits with a peptide corresponding to amino acid residues 204 to 218 of BDV G, conjugated to keyhole limpet hemocyanin.
Immunofluorescence (IF).
Cells grown on coverslips were fixed with 4% paraformaldehyde in phosphate-buffered saline and permeabilized with saponin (0.01%). After a blocking step with 10% normal goat serum for 30 min at room temperature, cells were reacted with a rabbit serum to BDV N. After several washes with phosphate-buffered saline, cells were reacted with goat anti-rabbit immunoglobulins G conjugated to Alexa 488 or Alexa 568 (Molecular Probes). Confocal images were captured with a Bio-Rad (Zeiss) Radiance 2100 Rainbow laser scanning confocal microscope attached to a Nikon TE2000-U microscope with infinity-corrected optics.
Cell release virus (CRV) preparation.
BDV cell-free virus was prepared from virally infected Vero, CHO, and CHO-derived cell lines as described previously (10).
Analysis of RNA by Northern blot hybridization.
RNA was isolated from cells and tissues by using TRI reagent (Molecular Research Center, Cincinnati) according to the manufacturer's instructions. RNA was analyzed by Northern blotting using a 32P-labeled DNA probe for BDV N.
Glycoprotein enrichment by WGA affinity resin.
Cell lysates of BDV-Pi cells were prepared by adding solubilization buffer (200 mM NaCl, NP-40 1%, 1.2 mM EDTA, 50 mM HEPES, pH 7.5) containing a complete protease inhibitor cocktail (Roche) and 1 mM phenylmethylsulfonyl fluoride. After incubation for 45 min at room temperature, cell lysates were clarified by centrifugation at 15,000 × g for 10 min at 4°C. Supernatants were incubated with whole-germ agglutinin (WGA)-agarose (Vector Laboratories) in the presence of 5 mM MgCl2 and 10 mM sodium pyrophosphate overnight at 4°C. After several washes in solubilization buffer supplemented with 5 mM MgCl2 and 10 mM sodium pyrophosphate, the resin-bound proteins were subjected to analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Plasmids.
Plasmid pC-BDVG-Furin(−) was generated using the QuikChange site-directed mutagenesis kit (Stratagene), utilizing as a template pC-BDVG (25) and the oligonucleotides CGGTCCAAGTTGGCAGCGGCGGCTAGGGATACTCAACAG and CTGTTGAGTATCCCTAGCCGCCGCTGCCAACTTGGACCG. The elimination of the furin recognition cleavage site in pC-BDVG-Furin(−) was confirmed by sequencing.
Detection of BDV proteins by Western blot analysis.
Samples from cells or virion-purified preparations were boiled and subjected to acrylamide gel electrophoresis (10%). The proteins were transferred to an Immobilon membrane (Millipore), and after blocking with blocking reagent (Roche), the membrane was treated with the primary antibodies diluted 1:1,000. The secondary Ab was diluted by following instructions from the provider, and the membrane was developed by using SuperSignal West Pico chemiluminescent substrate (Pierce).
RESULTS
Susceptibility of CHO cells to BDV G-mediated infection.
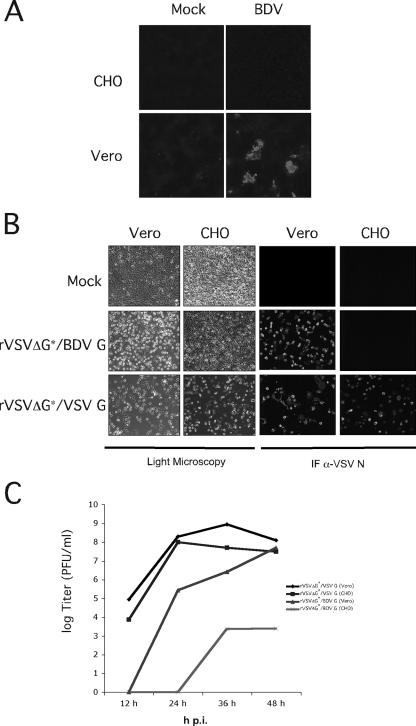
Early studies indicated that the entry of cell-free BDV into susceptible cells requires the presence of a specific cell surface receptor with a proteinaceous moiety (8). Subsequent studies showed that BDV G plays a central role in virus-receptor interaction and virus cell entry (1, 10, 26, 32). To further investigate the role of BDV G in virus tissue and cell tropism, we generated rVSVΔG*/BDVG (M. Perez, R. Clemente, C. S. Robinson, E. Jeetendra, H. R. Jayakar, M. A. Whitt, and J. C. de la Torre, submitted). Notably, rVSVΔG*/BDVG recreated the cell tropism and entry pathway of BDV. By using rVSVΔG*/BDVG, we identified, among others, CHO cells as being resistant to infection mediated by BDV G (Fig. 1). Vero cells, known to be susceptible to BDV, were readily infected by both rVSVΔG*/BDVG and rVSVΔG*/VSVG, a recombinant VSV wild type (Indiana strain) generated via reverse genetics. In contrast, CHO cells were resistant to BDV and rVSVΔG*/BDVG but fully susceptible to rVSVΔG*/VSVG. These findings indicated that the resistance of CHO cells to rVSVΔG*/BDVG infection was not caused by an intracellular blockade to VSV RNA replication or gene expression but rather by the lack of specific cell surface receptors required for BDV G-mediated cell entry.
FIG. 1.
Susceptibility of CHO cells to BDV G-mediated cell entry. (A) Susceptibility of CHO cells to BDV infection. Vero and CHO cells were infected with BDV (multiplicity of infection, 2 focus-forming units/cell), and at 48 h postinfection, cells were fixed and examined by IF using a rabbit serum to BDV N. (B) Susceptibility of CHO cells to rVSVΔG*/BDVG infection. Vero and CHO cells were infected with rVSVΔG*/VSVG or rVSVΔG*/BDVG (multiplicity of infection, 5 PFU/cell). At 48 h postinfection, cells were fixed and examined either by light microscopy to assess levels of cytopathic effect or by IF using a monoclonal Ab to VSV N. (C) Production of infectious progeny. Vero and CHO cells were infected with rVSVΔG*/VSVG and rVSVΔG*/BDVG (multiplicity of infection, 0.1 PFU/cell). Tissue culture supernatants were collected at the indicated hour postinfection (p.i.), and virus titers were determined by plaque assay.
Requirement of virus cell surface receptors for cell-to-cell propagation of BDV.
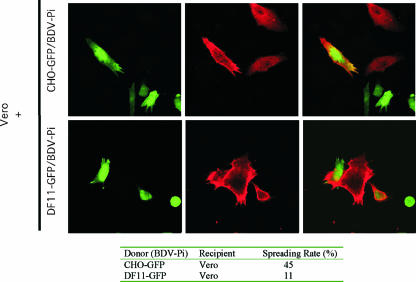
We used CHO cells to address the question of whether the cell-to-cell spread of BDV, characteristically observed in BDV-infected tissues and cell cultures (1), was strictly dependent on the presence of BDV receptors required for primary infection. To address this question, we first generated CHO-GFP (see Materials and Methods), which allowed us to distinguish between donor and recipient cells. We cocultured Vero/BDV-Pi cells (donor) with CHO-GFP (recipient), and at 144 h postcoculture, the cells were fixed and analyzed by IF using a rabbit serum to BDV N. We readily detected cells that expressed both GFP and BDV N (Fig. 2), which corresponded to CHO-GFP that became infected with BDV. Notably, CHO-GFP cells that became infected with BDV were always in close contact with Vero/BDV-Pi cells, which further supports the notion that BDV infection of CHO-GFP was mediated by cell-to-cell contact and was independent of the presence of the receptor that mediates BDV primary infection. Whether BDV G engagement of a different receptor is required for the cell-to-cell spreading of BDV remains to be determined.
FIG. 2.
BDV propagation from Vero cells to CHO and furin-deficient CHO (DF11) cells. Vero/BDV-Pi cells were cocultured with either CHO-GFP or DF11-GFP cells at a ratio of 1:50 (Vero to CHO or DF11). After 5 days, cocultured cells were fixed and examined by IF using a rabbit serum against BDV N. The red signal corresponds to cells expressing BDV N, and the green signal marks either CHO or DF11 cells expressing GFP. Cells positive for both signals correspond to CHO or DF11 cells that became infected with BDV. To assess quantitatively the spread rate of BDV from cell to cell, we mixed donor (Vero) and recipient (CHO-GFP or DF11-GFP) cells and, after 144 h of coculture, the monolayers were fixed and examined by IF to detect BDV N. We quantified the numbers of foci formed exclusively of Vero/BDV-Pi cells (BDV N positive and GFP negative) as well as those containing either BDV-infected CHO-recipient cells or BDV-infected DF11-recipient cells (BDV N positive and GFP positive). The table shows the percentage of foci where at least one spread event from donor to recipient cell was observed.
An analysis of cocultures of Vero E6 cells expressing red fluorescent protein (RFP) and CHO-GFP failed to identify cells coexpressing both GFP and RFP, a finding that argued against the possibility that the transfer of BDV antigen from Vero/BDV-Pi to CHO-GFP was mediated by nonspecific mechanisms unrelated to BDV infection (data not shown).
Expression of BDV G in furin-deficient CHO cells (DF11).
Previous studies have proposed that the processing of the BDV G by the cellular protease furin is required for cell-to-cell spread of BDV (1). This conclusion was based on results obtained using a furin inhibitor that could cross-inhibit other related proteases. These results led us to revisit this issue to gain a more detailed understanding of the requirement for furin-mediated processing of BDV G in virus propagation. We used the cell line DF11, derived from CHO cells, which is furin deficient (12). Similarly to CHO cells, DF11 cells were resistant to infection with both cell-free BDV and rVSVΔG*/BDVG but they became BDV infected upon cocultivation with Vero/BDV-Pi cells (not shown). These findings indicate that DF11 also lacked cell surface receptors that allowed for BDV primary infection.
We first confirmed that BDV G could not be processed in DF11 cells. CHO and DF11 cells were either transfected with a BDV G expression plasmid (pC-BDVG) (Fig. 3A) or infected with rVV/BDVG (Fig. 3B). The expression of GPC was readily detected in CHO and DF11 cells that were either transfected with pC-BDVG or infected with rVV/BDVG. We observed consistent, although modest, levels of GPC processing in CHO cells upon transfection with pC-BDVG or infection with rVV/BDVG as determined by the levels of GP1 expression detected by Western blot analysis. In contrast, we could not detect any level of GPC processing in pC-BDVG-transfected or rVV/BDVG-infected DF11 cells. These results indicated that DF11 cells do not express an alternative protease to furin capable of mediating processing of BDV G.
FIG. 3.
Expression of BDV G in CHO and DF11 cells. (A) BDV G expression pattern in transfected cells. Cells were transfected with the indicated plasmids, and at 48 h posttransfection, cell lysates were prepared and analyzed by Western blotting using the Ab-GP1N. The asterisk indicates the band corresponding to GP1. (B) Expression of BDV G in CHO and DF11 cells infected with rVV/BDVG. Cells were infected with rVV/BDVG (multiplicity of infection of 3), and at 24 h postinfection, cell lysates were prepared and analyzed by Western blotting as described for panel A. Cell lysates prepared from Vero/BDV-Pi cells were used as controls. −, absence of; +, presence of.
Cell-to-cell spread of BDV in furin-deficient cells.
To determine whether furin was required for cell-to-cell spreading of BDV, we examined whether CHO and DF11 cells, initially infected via cocultivation with Vero/BDV-Pi, were able to propagate BDV from cell to cell. For this examination, we took advantage of the G418 resistance gene present in the retroviral vector used to generate the CHO-GFP and DF11-GFP cell lines. CHO-GFP or DF11-GFP cells were cocultured with Vero/BDV-Pi, and after two passages, cultures were subjected to G418 selection (Fig. 4A). After three passages under selection conditions, all G418-susceptible Vero cells present in the original coculture were eliminated and the cultures of selected CHO or DF11 cells contained about 40% cells that were BDV positive, as determined by IF (Fig. 4Bi).
FIG. 4.
Expression of BDV G in CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi cells. (A) Scheme of the protocol used to generate CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi cell lines. CHO-GFP or DF11-GFP cells resistant to G418 were cocultured with Vero/BDV-Pi cells after several passages in the presence of G418 to eliminate Vero/BDV-Pi cells present in the original coculture population. (B) Characterization of CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi cell lines. IF analysis was used to estimate numbers of BDV-positive cells in each line. Panel i shows levels of BDV N, and P proteins (panel ii) and viral RNA (panel iii) were determined by Western blotting and Northern blotting, respectively, using cell samples normalized with respect to the numbers of BDV-positive cells. (C) Pattern of BDV G expression in CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi cell lines. Lysates of BDV-pi cells were subjected to purification by a WGA-based resin, and the glycoproteins attached to this resin were analyzed by Western blotting using the Ab-GP1N.
We next examined whether the lower rate of BDV propagation in CHO and DF11 cells correlated with altered levels of viral protein or RNA or both on a per cell basis. To address this question, we used the IF data to normalize the different cell lines with respect to the number of BDV-positive cells and prepared protein and RNA samples from equal numbers of BDV-infected cells. Results from Western blot and Northern blot assays (Fig. 4B, panels ii and iii) revealed that on a per cell basis, CHO/BDV-Pi, DF11/BDV-Pi, and Vero/BDV-Pi expressed similar levels of BDV N and P proteins as well as similar levels of viral RNA replication (genome RNA) and transcription (N mRNA).
The generation of DF11-GFP cells persistently infected with BDV (DF11-GFP/BDV-Pi) allowed us to analyze the expression pattern of BDV G in the context of the persistence of BDV but the absence of furin. Due to the low expression level of BDV G in DF11-GFP/BDV-Pi cells, we used a pull-down enrichment step using the lectin WGA that specifically retains oligosaccharides containing terminal N-acetylglucosamine. Consistent with our previous results (Fig. 3), BDV G was correctly proteolytically processed in CHO-GFP/BDV-Pi cells but not in DF11-GFP/BDV-Pi cells (Fig. 4C). We noticed that BDV G species exhibited different electrophoretic mobilities in DF11-GFP/BDV-Pi and CHO-GFP/BDV-Pi cells, which likely reflected differences in their respective glycosylation states. Fully glycosylated forms of BDV G species were almost undetectable in DF11-GFP cells, suggesting that furin might be involved in some steps of BDV G maturation in infected cells.
We next examined whether cocultures of DF11-GFP/BDV-Pi or CHO-GFP/BDV-Pi and uninfected Vero cells resulted in BDV infection and its propagation within the Vero cell population (Fig. 5). CHO-GFP/BDV-Pi cells were able to spread the virus into Vero cells within the first 72 h of cocultivation. Likewise, DF11-GFP/BDV-Pi cells were also able to spread the virus into Vero cells but at a lower rate, requiring about 144 h of cocultivation prior to the detection of BDV spread onto Vero cells. These data indicated that furin-mediated processing of BDV G was not required for cell-to-cell spreading of BDV, but the presence of furin enhanced virus spread, a finding consistent with previously reported results (1).
FIG. 5.
Spread of BDV from CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi to Vero cells. CHO-GFP and DF11-GFP cells, both Pi with BDV, were cocultured with Vero cells in a 20:1 ratio of CHO (or DF11) to Vero; at different times, cells were fixed and examined by IF. The spread of BDV from CHO-GFP/BDV-Pi to Vero cells was first detected at 72 h postcoculture, whereas the detection of virus spread from DF11-GFP/BDV-Pi to Vero cells required up to 144 h of coculture. The spread rate of BDV from either CHO or DF11 cells to Vero cells was quantified at 144 h of coculture as described in the legend for Fig. 2. The table shows the percentage of foci where at least one spread event from donor to recipient cell was observed.
Production and infectivity of BDV virions released from furin-deficient cells.
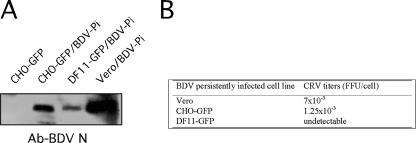
We used previously described methods (10) to prepare cell-free BDV (CRV) from CHO-GFP/BDV-Pi, DF11-GFP/BDV-Pi, and Vero/BDV-Pi cells. To assess the levels of total viral particles released from each cell type, we analyzed equal amounts of each CRV sample by Western blotting using a rabbit serum against BDV N (Fig. 6A). We normalized CRV samples with respect to the total number of particles as assessed by levels of BDV N detected by Western blot analysis and determined the corresponding infectivity associated with each CRV sample by using a focus-forming assay (Fig. 6B). Levels of BDV N associated with cell-free particles were reduced but readily detected in supernatants from DF11-GFP/BDV-Pi compared to those in supernatants from CHO-GFP/BDV-Pi or Vero/BDV-Pi cells. Virus recovered from CHO-GFP/BDV-Pi cells exhibited a modest (5- to 10-fold) reduction in specific infectivity compared to virus recovered from Vero/BDV-Pi, whereas we could not detect noticeable levels of infectivity associated with virions derived from DF11-GFP/BDV-Pi cells. These results indicated that furin-mediated processing of BDV G is not strictly required for the assembly and budding of BDV particles, but furin appears to play a critical role in the formation of infectious cell-free viral particles.
FIG. 6.
Infectivity associated with BDV particles released from DF11-GFP/BDV-Pi cells. BDV CRV was prepared from CHO-GFP (mock), Vero/BDV-Pi, CHO-GFP/BDV-Pi, and DF11-GFP/BDV-Pi cells. (A) Equal amounts of each preparation were analyzed by Western blotting using a serum to BDV N. (B) Infectivity associated with each CRV preparation was determined by using an immunofocus assay with Vero cells.
Characterization of BDV G species present in virions released from furin-deficient cells.
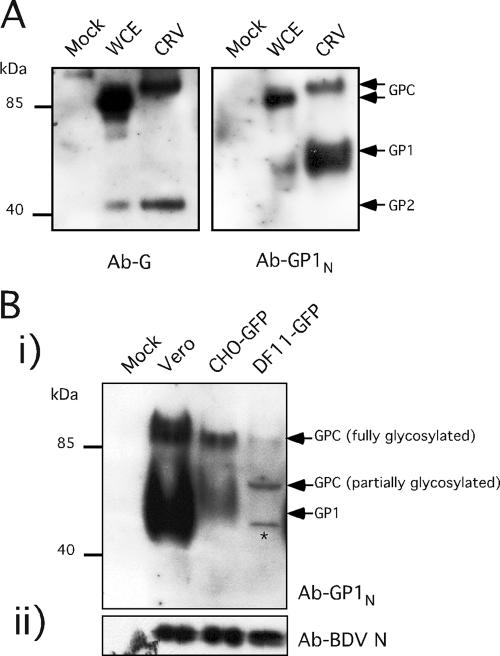
We next examined whether altered levels of virion-associated BDV G species were responsible for our observation that cell-free BDV virions released from cells that do not express furin lacked infectivity (Fig. 6). We first examined the BDV G composition of BDV virions released from Vero/BDV-Pi cells using (i) a previously described rabbit serum to BDV G (Ab-G) (10), which detects the glycosylated unprocessed GPC (mass, ca. 90 kDa) and GP2 (mass, ca. 43 kDa) species, and (ii) a newly generated rabbit serum to BDV G (Ab-GP1N) that recognizes GPC and GP1 (mass, ca. 55 to 60 kDa) species. Cell-free virions released from Vero/BDV-Pi contained BDV GPC and the two derived mature GP1 and GP2 species (Fig. 7A). We then used the Ab-GP1N to examine the pattern of BDV G species detected in virions derived from CHO-GFP/BDV-Pi and DF11-GFP/BDV-Pi cells (Fig. 7B). We used levels of BDV N to normalize the amounts of cell-released virions from each cell type. The incorporation of BDV G species was dramatically reduced in DF11-GFP/BDV-Pi and, to lesser extent, in CHO-GFP/BDV-Pi compared to that in Vero/BDV-Pi cells. Moreover, BDV G species associated with virions derived from DF11-GFP/BDV-Pi and CHO-GFP/BDV-Pi or Vero/BDV-Pi cells exhibited significant differences in their respective electrophoretic mobilities. Fully glycosylated GPC and GP1 were readily detected in virions obtained from Vero/BDV-Pi and CHO-GFP/BDV-Pi cells, whereas these BDV G species were barely detectable in virions derived from DF11-GFP/BDV-Pi cells. Virion preparations derived from DF11-GFP/BDV-Pi cells contained two other bands with masses of 70 to 75 kDa and ∼56 kDa that specifically immunoreacted with Ab-GP1N. These bands likely corresponded to uncleaved and partially glycosylated (70 to 75 kDa) BDV G species and unglycosylated GPC (56 kDa). In support of these assignments, we observed that cells transfected with a G expression plasmid where the furin cleavage site was eliminated expressed mainly a G species that migrated with a mass of 56 kDa, corresponding to the uncleaved and nonglycosylated GPC (Fig. 8).
FIG. 7.
BDV G species present in BDV particles released from Vero/BDV-Pi, CHO-GFP/BDV-Pi, and DF11-GFP/BDV-Pi cells. (A) Analysis of BDVG expression in cell lysates and CRV derived from Vero/BDV-Pi cells. Total cell lysates (whole-cell extracts [WCE]) or CRV was examined by Western blot analysis using Ab-BDVG and Ab-GP1N antibodies. (B) Analysis of BDVG expression in CRV derived from CHO-GFP/BDV-Pi and DF11-GFP/BV-Pi cells. CRV samples were concentrated and normalized by Western blot analysis detecting BDV N. The BDV G was detected by Ab-GP1N-detected BDV GPC and also a band of ∼56 kDa corresponding to unglycosylated GPC (*).
FIG. 8.
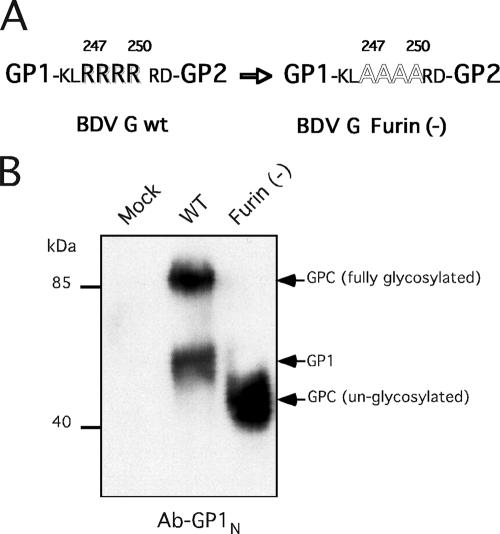
Expression in transfected cells of BDV G lacking the furin recognition cleavage site at positions 247 to 250. (A) Plasmid pC-BDV-G/Furin(−) that expresses BDV G lacking the furin cleavage site was generated by the mutation of four R residues (247 to 250) to A residues. (B) 293T cells were transfected with pC-BDVG or pC-BDVG/Furin(−) or with empty vector (mock), and 48 h later, lysates were prepared and analyzed by Western blotting using Ab-GP1N.
DISCUSSION
BDV propagates rapidly within the CNS and cultured cells, but attempts to detect mature infectious virion particles in the extracellular milieu have consistently failed. Previous studies have suggested that BDV G and its correct processing by the cellular protease furin are essential for virus propagation (1), but the underlying mechanisms remain unknown. BDV G plays a key role in the entry of BDV into susceptible cells (10, 26). BDV GP1 was found to be competent for receptor recognition and virus entry (26), whereas GP2 appears to be responsible for the pH-dependent fusion event required to release the virus RNP into the cytoplasm of infected cells (9). Here we have presented evidence that the cell-to-cell propagation of BDV requires neither the presence of the cellular receptor used by BDV for the primary infection nor the furin-mediated processing of BDV G.
Early studies provided evidence of a proteinaceous moiety as a key component of the cellular receptor used by BDV (8). Consistent with this observation, BDV-susceptible cells become refractory to infection with rVSVΔG*/BDVG upon treatment of cells with proteinase K (T. Gjoen and J. C. de la Torre, unpublished data). In this work, we have investigated whether the cell-to-cell spread of BDV was mediated by the same cellular receptors involved in the primary infection. For this investigation, we examined the propagation of BDV in cocultures of Vero and CHO cells, which are susceptible and resistant, respectively, to BDV infection. CHO cells were resistant to infection with rVSVΔG*/BDVG but permitted VSV replication when infected with rVSVΔG*/VSVG. Moreover, the CHO cells allowed BDV RNA replication and transcription upon transfection with BDV RNP or in a BDV minigenome rescue assay (data not shown). These findings unequivocally established that the restriction posed by CHO cells to infection with cell-free BDV was at the level of cell entry. Therefore, our observation that CHO cells became infected via cocultivation with Vero/BDV-Pi cells indicates that cell-to-cell infection of BDV is a process distinct from infection of cells by cell-free BDV. The mechanisms governing the cell-to-cell spread of BDV remain to be elucidated, but they do not require the same receptor molecules at the cell surface involved during BDV primary infection.
Admittedly, results obtained with CHO and DF11 cells may not reflect the complexity of the interactions and mechanisms underlying BDV propagation within the brain. Nevertheless, our findings support a scenario during BDV infection of the CNS where an initial receptor-mediated infection of a specific population of neurons could be followed by cell-to-cell viral spread without requiring the expression in these cells of the receptor used for the primary infection. Our results are conceptually similar to those described for CD46 transgenic mice infected with the Edmonston strain of measles virus (MV). In this system, the initial infection of neurons by MV depended strictly on the expression of CD46, one of the identified cellular receptors required for MV primary infection (7, 22), whereas the cell-to-cell transmission of MV was CD46 independent (18). Herpesvirus is one of the better-studied systems regarding alternative mechanisms of viral spread. In this system, the different spreading mechanisms are controlled by the activity of five different glycoproteins that participate in different steps of the viral spreading in a coordinate manner (21). Similarly, one could hypothesize that different domains of BDV G could have different contributions to the different steps of BDV spread.
For various viral GPs it has been shown that the posttranslational cleavage required for GP maturation could be mediated by more than one cellular protease (2). By comparing the expression patterns of BDV G in CHO and DF11 cells, we were able to determine that CHO and DF11 cells lacked proteases other than furin that were capable of mediating efficient processing of BDV GPC into GP1 and GP2. We observed cell-to-cell spread of BDV in both CHO and DF11, but BDV spread was significantly faster when using furin-positive cells than when using furin-negative cells as donor cells. These results suggest that furin-mediated maturation of GPC is not strictly required but plays an important role in the cell-to-cell spread of BDV.
We noticed that the only BDV G species consistently detected in DF11-GFP/BDV-Pi cells was a polypeptide of ca. 60 to 70 kDa (Fig. 4B), which can be incorporated into virions (Fig. 7B). This protein likely corresponds to a partially glycosylated GPC, suggesting that furin-mediated maturation of BDV G may be involved in the complete glycosylation of BDV G.
We have generated a novel Ab (Ab-GP1N) capable of detecting GP1 efficiently without the need of endoglycosidase treatments (16), which allowed us to examine the levels of GP1 in BDV-infected cells and virions. The use of Ab-GP1N, together with the previously described Ab-G (11), revealed that all three BDV G species, GPC, GP1, and GP2, are incorporated into virions. We consistently observed that GPC species present in virions migrated slightly slower than GPC species present in the lysates of BDV-infected cells (Fig. 7A). This finding raises the intriguing possibility that only a minor fraction, undetectable by Western blotting, of the GPC pool present in BDV-infected cells undergoes the additional posttranslational modification that is required for the incorporation of GPC into virions. The increased proportion of this GPC species in virions would explain its detection by Western blot analysis in cell-released viral preparations.
BDV G was shown to be strictly required for the formation of infectious BDV virus-like particles (24), but whether GPC processing into GP1 and GP2 was required for the infectivity associated with cell-free BDV virions remains unknown. The availability of CHO and DF11 cells infected with BDV allowed us to examine this question experimentally. Levels of BDV N were significantly reduced but readily detectable in cell-released virus preparations from DF11 compared to that from CHO BDV-infected cells (Fig. 6A). These findings indicate that the presence of furin enhances the assembly and release of BDV particles, a finding similar to that described for several other viruses, including Ebola virus (23) and arenavirus (17). However, the incorporation of BDV G species into virions was severely diminished in the absence of furin, which correlated with the lack of infectivity associated with these viral particles. Similar situations have been described for arenavirus (17) and respiratory syncytial virus (38). In contrast, unprocessed GPs of Ebola virus were incorporated efficiently into virions that exhibited very little impaired infectivity (23, 40).
Previous findings reported a decrease in infectivity of BDV preparations from infected cells that had been treated with a furin inhibitor (27). Consistent with this observation, here we have shown dramatic differences in specific infectivity between virions prepared from CHO cells and from furin-deficient DF11 cells (Fig. 6). DF11-derived virions exhibited a large reduction as well as altered glycosylation status of their associated BDV G species (Fig. 7), which likely determined the lack of detectable levels of infectivity associated with these virions. Whether BDV G species with altered glycosylation present in furin-deficient cells contribute to cell-to-cell viral spread remains to be determined.
Our findings have uncovered an additional level of complexity in the control of the propagation of BDV infection. The identification of cellular receptors required for BDV primary infection and the elucidation of the mechanisms underlying cell-to-cell spread of BDV will contribute significantly to a better understanding of the biology and neuropathogenesis of BDV.
Acknowledgments
We thank S. Kunz for his assistance with the use of WGA for the enrichment of BDV G species from virally infected cells, N. Nguyen for her technical assistance, S. H. Leppla for providing us with the DF11 cells, and H. M. Scobie for providing us with the retroviral GFP vector.
This work was supported by NIH grant R21 AI064820 to J.C.D.L.T.
This is publication 18477 from MIND.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Bajramovic, J. J., S. Munter, S. Syan, U. Nehrbass, M. Brahic, and D. Gonzalez-Dunia. 2003. Borna disease virus glycoprotein is required for viral dissemination in neurons. J. Virol. 77:12222-12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basak, A., M. Zhong, J. S. Munzer, M. Chretien, and N. G. Seidah. 2001. Implication of the proprotein convertases furin, PC5 and PC7 in the cleavage of surface glycoproteins of Hong Kong, Ebola and respiratory syncytial viruses: a comparative analysis with fluorogenic peptides. Biochem. J. 353:537-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cubitt, B., and J. C. de la Torre. 1994. Borna disease virus (BDV), a nonsegmented RNA virus, replicates in the nuclei of infected cells where infectious BDV ribonucleoproteins are present. J. Virol. 68:1371-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de la Torre, J. C. 2002. Molecular biology of Borna disease virus and persistence. Front. Biosci. 7:d569-579. [DOI] [PubMed] [Google Scholar]
- 5.de la Torre, J. C. 1994. Molecular biology of Borna disease virus: prototype of a new group of animal viruses. J. Virol. 68:7669-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de la Torre, J. C. 2006. Reverse-genetic approaches to the study of Borna disease virus. Nat. Rev. Microbiol. 4:777-783. [DOI] [PubMed] [Google Scholar]
- 7.Dörig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 8.Duchala, C. S., K. M. Carbone, and O. Narayan. 1989. Preliminary studies on the biology of Borna disease virus. J. Gen. Virol. 70:3507-3511. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Dunia, D., B. Cubitt, and J. C. de la Torre. 1998. Mechanism of Borna disease virus entry into cells. J. Virol. 72:783-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Dunia, D., B. Cubitt, F. A. Grasser, and J. C. de la Torre. 1997. Characterization of Borna disease virus p56 protein, a surface glycoprotein involved in virus entry. J. Virol. 71:3208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Dunia, D., C. Sauder, and J. C. de la Torre. 1997. Borna disease virus and the brain. Brain Res. Bull. 44:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon, V. M., K. R. Klimpel, N. Arora, M. A. Henderson, and S. H. Leppla. 1995. Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infect. Immun. 63:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 14.Hatalski, C. G., A. J. Lewis, and W. I. Lipkin. 1997. Borna disease. Emerg. Infect. Dis. 3:129-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikuta, K., M. S. Ibrahim, T. Kobayashi, and K. Tomonaga. 2002. Borna disease virus and infection in humans. Front. Biosci. 7:d470-d495. [DOI] [PubMed] [Google Scholar]
- 16.Kiermayer, S., I. Kraus, J. A. Richt, W. Garten, and M. Eickmann. 2002. Identification of the amino terminal subunit of the glycoprotein of Borna disease virus. FEBS Lett. 531:255-258. [DOI] [PubMed] [Google Scholar]
- 17.Kunz, S., K. H. Edelmann, J. C. de la Torre, R. Gorney, and M. B. Oldstone. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168-178. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence, D. M., C. E. Patterson, T. L. Gales, J. L. D'Orazio, M. M. Vaughn, and G. F. Rall. 2000. Measles virus spread between neurons requires cell contact but not CD46 expression, syncytium formation, or extracellular virus production. J. Virol. 74:1908-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipkin, W. I., C. G. Hatalski, and T. Briese. 1997. Neurobiology of Borna disease virus. J. Neurovirol. 3(Suppl. 1):S17-S20. [PubMed] [Google Scholar]
- 20.Ludwig, H., L. Bode, and G. Gosztonyi. 1988. Borna disease: a persistent virus infection of the central nervous system. Prog. Med. Virol. 35:107-151. [PubMed] [Google Scholar]
- 21.Milne, R. S., A. V. Nicola, J. C. Whitbeck, R. J. Eisenberg, and G. H. Cohen. 2005. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J. Virol. 79:6655-6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neumann, G., H. Feldmann, S. Watanabe, I. Lukashevich, and Y. Kawaoka. 2002. Reverse genetics demonstrates that proteolytic processing of the Ebola virus glycoprotein is not essential for replication in cell culture. J. Virol. 76:406-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez, M., and J. C. de la Torre. 2005. Identification of the Borna disease virus (BDV) proteins required for the formation of BDV-like particles. J. Gen. Virol. 86:1891-1895. [DOI] [PubMed] [Google Scholar]
- 25.Perez, M., A. Sanchez, B. Cubitt, D. Rosario, and J. C. de la Torre. 2003. A reverse genetics system for Borna disease virus. J. Gen. Virol. 84:3099-3104. [DOI] [PubMed] [Google Scholar]
- 26.Perez, M., M. Watanabe, M. A. Whitt, and J. C. de la Torre. 2001. N-terminal domain of Borna disease virus G (p56) protein is sufficient for virus receptor recognition and cell entry. J. Virol. 75:7078-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richt, J. A., T. Furbringer, A. Koch, I. Pfeuffer, C. Herden, I. Bause-Niedrig, and W. Garten. 1998. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J. Virol. 72:4528-4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richt, J. A., I. Pfeuffer, M. Christ, K. Frese, K. Bechter, and S. Herzog. 1997. Borna disease virus infection in animals and humans. Emerg. Infect. Dis. 3:343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 30.Sauder, C., A. Muller, B. Cubitt, J. Mayer, J. Steinmetz, W. Trabert, B. Ziegler, K. Wanke, N. Mueller-Lantzsch, J. C. de la Torre, and F. A. Grasser. 1996. Detection of Borna disease virus (BDV) antibodies and BDV RNA in psychiatric patients: evidence for high sequence conservation of human blood-derived BDV RNA. J. Virol. 70:7713-7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schneemann, A., P. A. Schneider, R. A. Lamb, and W. I. Lipkin. 1995. The remarkable coding strategy of Borna disease virus: a new member of the nonsegmented negative strand RNA viruses. Virology 210:1-8. [DOI] [PubMed] [Google Scholar]
- 32.Schneider, P. A., C. G. Hatalski, A. J. Lewis, and W. I. Lipkin. 1997. Biochemical and functional analysis of the Borna disease virus G protein. J. Virol. 71:331-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider, P. A., A. Schneemann, and W. I. Lipkin. 1994. RNA splicing in Borna disease virus, a nonsegmented, negative-strand RNA virus. J. Virol. 68:5007-5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider, U. 2005. Novel insights into the regulation of the viral polymerase complex of neurotropic Borna disease virus. Virus Res. 111:148-160. [DOI] [PubMed] [Google Scholar]
- 35.Scobie, H. M., G. J. Rainey, K. A. Bradley, and J. A. Young. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA 100:5170-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 37.Stitz, L., K. Noske, O. Planz, E. Furrer, W. I. Lipkin, and T. Bilzer. 1998. A functional role for neutralizing antibodies in Borna disease: influence on virus tropism outside the central nervous system. J. Virol. 72:8884-8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugrue, R. J., C. Brown, G. Brown, J. Aitken, and H. W. M. Rixon. 2001. Furin cleavage of the respiratory syncytial virus fusion protein is not a requirement for its transport to the surface of virus-infected cells. J. Gen. Virol. 82:1375-1386. [DOI] [PubMed] [Google Scholar]
- 39.Tomonaga, K., T. Kobayashi, and K. Ikuta. 2002. Molecular and cellular biology of Borna disease virus infection. Microbes Infect. 4:491-500. [DOI] [PubMed] [Google Scholar]
- 40.Wool-Lewis, R. J., and P. Bates. 1999. Endoproteolytic processing of the Ebola virus envelope glycoprotein: cleavage is not required for function. J. Virol. 73:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]