Abstract
Over 50% of all human immunodeficiency virus type 1 (HIV-1) infections worldwide are caused by subtype C strains, yet most research to date focuses on subtype B, the subtype most commonly found in North America and Europe. The HIV-1 trans-acting regulatory protein (Tat) is essential for regulating productive replication of HIV-1. Tat is secreted by HIV-infected cells and alters several functions of uninfected bystander cells. One such function is that, by acting at the cell membrane, subtype B Tat stimulates the production of tumor necrosis factor (TNF) and chemokine (C-C motif) ligand 2 (CCL2) from human monocytes and can act as a chemoattractant. In this study, we show that the mutation of a cysteine to a serine at residue 31 of Tat commonly found in subtype C variants significantly inhibits the abilities of the protein to bind to chemokine (C-C motif) receptor 2 (CCR2), induce intracellular calcium flux, stimulate TNF and CCL2 production, and inhibit its chemoattractant properties. We also show that TNF is important in mediating some effects of extracellular Tat. This report therefore demonstrates the important functional differences between subtype C and subtype B Tat and highlights the need for further investigation into the different strains of HIV-1.
The human immunodeficiency virus type 1 (HIV-1) trans-acting regulatory protein (Tat) is an 86- to 101-residue regulatory protein (9 to 11 kDa) produced early in HIV-1 infection whose primary role is in regulating productive and processive transcription from the HIV-1 long terminal repeat (LTR) (4, 44). Most studies analyzing the effect of Tat on either HIV-1 or host gene expression have used a truncated 86-residue HIV-1 subtype B Tat, although HIV-1 subtype C is the most prevalent globally (38) and its Tat protein has 101 residues (57, 58, 66). These studies have shown that Tat is secreted from unruptured, HIV-1-infected lymphoid (21, 30) and myeloid (81) cells and is found in the sera of infected individuals (90, 92) where it has a variety of effects on a number of different cell types (reviewed in reference 43). Among the defined effects, subtype B Tat has been shown to be chemotactic for monocytes (2, 3, 50) and microglial cells (26) and to induce the production of tumor necrosis factor (TNF), chemokine (C-C motif) ligand 2 (CCL2) (previously known as monocyte chemotactic protein 1 [MCP-1]), and interleukin-6 (IL-6) from monocytes (25, 59, 87). However, recent research has shown that the different genetic subtypes and recombinant forms of HIV-1 have biological differences with respect to transmission, replication, and disease progression (14, 47, 74, 85). Moreover, different subtypes of Tat have different in vitro effects (17, 18, 27, 28, 57, 62, 66, 67, 76).
The influence of Tat on the transcription of TNF from monocytes and microglial cells is particularly important in HIV-1 pathogenesis. TNF binds to its cognate receptors, triggering signal transduction pathways and the production of numerous cytokines and chemokines as well as the activation of microglial cells, macrophages, and monocytes (12). Under normal conditions, TNF expression is at a constitutive level in various brain cells, suggesting a physiological role for this cytokine under normal conditions (63). However, in subtype B HIV-1-infected patients, activated macrophages and monocytes in both the white matter of brain tissue and sera show increased expression of TNF and TNF receptors, suggesting an important role in the pathogenesis of HIV-1 and HIV-1-associated dementia (HAD) (15, 36, 70, 89). HAD is characterized histopathologically by the infiltration of monocytes and macrophages into the central nervous system (CNS), gliosis, pallor of myelin sheaths, abnormalities of dendritic processes, and neuronal apoptosis (1, 35). TNF may play a critical role in this process. TNF has been shown to open a paracellular route for HIV invasion across the blood-brain barrier (BBB) (31). It also induces the expression of adhesion molecules on astrocytes and endothelial cells (16) and the release of chemokine factors from monocytes and microglial cells (23, 26), allowing HIV-1-infected monocytes and macrophages to transmigrate into the CNS. However, TNF also has neuroprotective effects, such as upregulating the production of RANTES from astrocytes and Bcl-2 from neurons (15), illustrating the multifactorial cause of the disease. In individuals with HAD, the level of anti-TNF antibodies is also elevated and positively correlates with viral load, CD8+ T-cell count, and antibodies against Tat (19). CD40, IL-1β, quinolinic acid, and CCL2 are also upregulated (15, 26, 76), whereas IL-6, transforming growth factor β, and gamma interferon are not (36, 70, 89). Tat is secreted by HIV-1-infected cells within the CNSs of infected individuals (41, 42, 54, 91), and extracellular Tat in serum has been shown to enter the CNS across the BBB (7). In both cases, it is taken up by CNS cells with toxic consequences (reviewed in reference 49). Indeed, in mice, a single intraventricular injection of Tat leads to pathological findings observed in HAD, namely, macrophage infiltration, progressive glial activation, and neuronal apoptosis (46, 59, 64, 68).
Mutational analysis of Tat has identified the cysteine-rich and core domains of Tat as being responsible for the trans-activational properties of Tat (44). Aligning this region of HIV-1 subtype B Tat with several β-chemokines indicates positioning and similarity of key residues critical for certain β-chemokine receptor binding and signal transduction (2). These include a CCF/Y motif at positions 30 to 32, a strongly conserved isoleucine at position 39, and an SYXR motif at positions 46 to 49. Interestingly, regardless of the geographic origin of the virus, subtype-specific variations have arisen. More than 90% of HIV-1 subtype C Tat sequenced to date have a C31→S mutation (66), whereas the circulating recombinant form AE (CRF_AE) has acquired the F/Y32→W change (67). The alteration in CRF_AE has been shown to result in lower Tat-induced TNF expression from T cells (67).
In this study, we compared the abilities of HIV-1 subtype C Tat and the full-length form of the HIV-1 subtype B Tat most commonly used (18, 44) to bind to chemokine receptors and induce transient calcium flux in monocytes, monocyte chemotaxis, and expression of inflammatory cytokines and chemokines. We found that the C31→S mutation found in subtype C Tat results in failure to bind to chemokine (C-C motif) receptor 2 (CCR2) and a marked reduction in the induction of transient calcium flux in monocytes. Moreover, these changes are correlated with a lack of chemotactic activity and a reduction in the ability to induce inflammatory mediators.
MATERIALS AND METHODS
Cells and reagents.
Human peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood samples from healthy donors using density centrifugation over Ficoll-Hypaque (Amersham Biosciences, Piscataway, NJ). Monocytes were isolated from PBMCs using the monocyte isolation kit from Miltenyi Biotec using MidiMACS with LS columns (Miltenyi Biotec, Auburn, CA).
The following cell lines were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: HeLa-CD4-LTR-β-gal cells from Michael Emerman (48) and GHOST cell transfectants from Vineet N. Kewal Ramani and Dan R. Littman (53). Human microglia cells were from Sciencell Research Laboratories (San Diego, CA) and propagated as indicated by the supplier.
The calcium signaling inhibitors nimodipine, xestospongin C, and 3,4,5-trimethyloxybenzoic acid 8-(diethylamino)octyl ester (TMB-8) were from Calbiochem (La Jolla, CA). FS-2 from Dendroaspis polylepis polylepis venom was from Sigma Aldrich (St. Louis, MO). A putative cytotoxic effect of the different inhibitors was tested by the trypan blue dye exclusion assay, and none was found to be cytotoxic (viability was >99%) at the concentrations used. Anti-TNF neutralizing antibody (clone 6401), mouse immunoglobulin G1 (IgG1) isotype control, and recombinant CCL2 were purchased from R&D Systems (Minneapolis, MN), BD Pharmingen (San Diego, CA), and Biosource (Camarillo, CA), respectively.
Tat proteins were synthesized in solid phase using Fast Fmoc (9-fluorenylmethoxy carbonyl) chemistry by the method of Barany and Merrifield (8) using 4-hydroxymethyl-phenoxymethyl-copolystyrene-1% divinylbenzene (HMP) preloaded resin (0.5 mmol) (Perkin Elmer, Applied Biosystem Inc., Forster City, CA) on an automated synthesizer (ABI 433A; Perkin Elmer, Applied Biosystem Inc.) as previously described (62). Purification and analysis using high-performance liquid chromatography (HPLC) were carried out as previously described (18, 62). Amino acid analysis was performed on a model 6300 Beckman analyzer, and mass spectrometry was carried out using an Ettan matrix-assisted laser desorption ionization time-of-flight apparatus (Amersham Biosciences, Uppsala, Sweden).
trans-Activation with HIV LTR-transfected cells.
The trans-activation activities of the synthetic Tat proteins were analyzed by monitoring the production of β-galactosidase after activation of lacZ expression in HeLa-CD4-LTR-β-gal cells using a previously described protocol (18).
Chemotaxis.
The cell migration assay was conducted using 24-well Transwell migration chambers with 5-μm-pore-size polyvinylpyrrolidone-free polycarbonate filters (Corning) as previously described (59). Data are expressed as the ratio of directed movement (chemotaxis) to random movement and are representative of the results of three independent experiments where each point represents the mean of three wells ± 1 standard deviation.
Calcium mobilization.
Freshly isolated PBMCs or purified monocytes (2 × 106/ml) were loaded with 3 μM Fluo-4 acetoxymethylester (Fluo-4/AM) and 3 μM Fura Red/AM (Molecular Probes, Carlsbad, CA) in loading buffer (Hanks' balanced salt solution without Ca2+ or Mg2+ [Invitrogen] supplemented with 0.5% [wt/vol] bovine serum albumin [Sigma] and 1 mM probenecid [Molecular Probes, Carlsbad, CA] at pH 7.4) according to the manufacturer's instructions. Cells were then stained with anti-CD14 conjugated to allophycocyanin (APC) at 4°C, washed, and resuspended at a density of 2 × 106 cells/ml in calcium buffer supplemented with 1 mM probenecid at pH 7.4 and kept on ice until required. In some cases, cells were also incubated with 1 μM nimodipine, 100 μM TMB-8, or 100 nM FS-2 at this stage. Just before the Ca2+ flux assay, cells were heated to 37°C in a water bath. Changes in dye fluorescence after stimulation with 50 nM Tat over time at 37°C were determined by flow cytometry (FACScan; Becton Dickinson, Mississauga, Ontario, Canada). Calcium mobilization is reported as the ratio of Fluo-4 to Fura Red fluorescence intensity over time as calculated using FCSPress v1.4 software (by Ray Hicks, Flow Cytometry Laboratory, Department of Medicine, University of Cambridge, Cambridge, United Kingdom).
Equilibrium competition binding assays.
GHOST cells were cultured in high-glucose Dulbecco's modified Eagle's medium supplemented with 10% (vol/vol) fetal bovine serum, 500 μg/ml G418, 100 μg/ml streptomycin, 100 U/ml penicillin, and 100 μg/ml hygromycin (all from Invitrogen, Carlsbad, CA). Transfected cell media also contained 1 μg/ml puromycin (Mediatech, Herndon, VA). Cells were harvested using Cellstripper (Mediatech), washed twice, and resuspended in binding buffer (RPMI 1640 [Invitrogen] supplemented with 1% [wt/vol] bovine serum albumin and 20 mM HEPES) at 1 × 104 cells/ml. For 125I-labeled chemokine binding to whole cells, 100 μl/well GHOST cell suspension was placed in 96-well microtiter plates. A 100-μl solution containing 0.1 nM 125I-labeled chemokines (Perkin Elmer) and increasing concentrations of Tat or buffer was then added to the cells. The radiolabeled ligands used were 125I-CCL2 for CCR2+ cells and 125I-CXCL12 for CXCR4+ cells. Specific activity for each chemokine was 2,200 Ci/mmol, as indicated by the manufacturer. Tat concentration varied between 0.01 nM and 10 μM. Cells were incubated at 20°C for 40 min with gentle agitation and then harvested onto a filter mat using an automatic cell harvester (Skatron Inc., Sterling, VA) and subjected to liquid scintillation counting in a Beckman LS-6000.
Determination of cytokine production. (i) Flow cytometry.
PBMCs (1 × 106/ml) were incubated in 24-well flat-bottomed plates (Falcon) at 37°C, 5% CO2 in a 2:1 mix of AIM-V and Iscove's media (Invitrogen) supplemented with 1% (vol/vol) fetal calf serum (Invitrogen) and 2 μM monensin (Calbiochem, La Jolla, CA) and stimulated with 50 nM Tat or CCL2. After 4 h of stimulation, cells were stained with monoclonal antibodies against CD14 (conjugated to fluorescein isothiocyanate [FITC]), TNF (conjugated to APC), and IL-6 (conjugated to phycoerythrin) (all from BD Pharmingen, San Diego, CA), using the commercially available Cytofix/Cytoperm according to the manufacturer's instructions (BD Pharmingen, San Diego, CA). Stained cells were then analyzed by flow cytometry (FACScan; Becton Dickinson, Mississauga, Ontario, Canada). Cytogram analysis was performed with Cell Quest Pro software (BD Bioscience, Mississauga, Ontario, Canada).
(ii) Real-time PCR.
mRNA expression in monocytes was measured by real-time PCR. Freshly purified monocytes (1 × 106/ml) were incubated in six-well flat-bottomed plates (Falcon) at 37°C and 5% CO2 in a 2:1 mix of AIM-V and Iscove's media (Invitrogen) supplemented with 1% (vol/vol) fetal calf serum (Invitrogen) and stimulated with 50 nM Tat. In some wells, anti-TNF neutralizing antibodies were added at 1 μg/ml. Controls included cultures with either isotype-matched monoclonal antibodies (BD Pharmingen, San Diego, CA) or with FcR blocking reagent (Miltenyi Biotec, Auburn, CA). At 2 h and 4 h posttreatment, cells were harvested, and total cellular RNA was prepared with the RNeasy Mini kit in accordance with the manufacturer's directions (QIAGEN, Valencia, CA). TNF and IL-6 mRNA quantification was determined using the LightCycler system and the LightCycler RNA amplification kit HybProbe (Roche Applied Science). PCRs were carried out in a 20-μl mixture composed of 3 mM MgCl2, 0.5 μM of each primer, 0.2 μM of each probe, 2 μl sample, and 1-fold LightCycler RNA amplification kit HybProbe. Primers used (Idaho Biochem, Salt Lake City, UT) were as follows: for TNF, 5′-CCTGGTATGAGCCCAT-3′ (sense) and 5′-AGGGGGTAATAAAGGGATT-3′ (antisense); for IL-6, 5′-CTTCAGAACGAATGGACAA-3′ (sense) and 5′-TTTTCACCAGGCAAGT-3′ (antisense). The sensor and anchor probe sequences (Idaho Biochem, Salt Lake City, UT) were as follows: TNF fluorescein probe, 5′-TGGAGAAGGGTGACCGACT-FITC; TNF LCRed 640 probe, LCRed 640-GCGCTGAGATCAATCGGCC-C3 blocker; IL-6 fluorescein probe, 5′-GTACATCCTCGACGGCATA-FITC; IL-6 LCRed 640 probe, LCRed 640-AGCCCTGAGAAAGGAGACA-C3 blocker. The reaction mixtures were initially incubated at 55°C for 30 min to reverse transcribe the RNA. The sample was then heated to 95°C for 10 min to denature the cDNA. CCL2, indoleamine 2,3-dioxygenase (IDO), CD40, and RNA polymerase II (RPII) mRNA quantification was determined using the FastStart RNA Master SYBR Green I kit (Roche Applied Science). PCRs were prepared using 0.3 μM of each primer (except CD40, for which 0.15 μM was used) according to the manufacturer's instructions. Primers used (Integrated DNA Technologies) were as follows: for CCL2, 5′-GATCTCAGTGCAGAGGCTCG-3′ (sense) and 5′-TGCTTGTCCAGGTGGTCCAT-3′ (antisense); for IDO, 5′-TGCTAAAGGCGCTGTT-3′ (sense) and 5′-TGGTAGCTCCTCAGGG-3′ (antisense); for CD40, 5′-CAGCCAGGACAGAAACTGGTGAGT-3′ (sense) and 5′-CTTCTTCACAGGTGCAGATGGTGTC-3′ (antisense); and for RPII, 5′-GCACCACGTCCAATGACAT-3′ (sense) and5′-GTGCGGCTGCTTCCATAA-3′ (antisense). The reaction mixtures were initially incubated at 61°C for 20 min to reverse transcribe the RNA. The sample was then heated to 95°C for 30 s to denature the cDNA. Amplification for all was performed for 45 cycles, with the following cycle parameters: for TNF, 10 s of denaturation at 95°C, 10 s of primer annealing at 53°C, and 8 s of fragment elongation at 72°C; for IL-6, 10 s of denaturation at 95°C, 10 s of primer annealing at 54°C, and 8 s of fragment elongation at 72°C; for CCL2, 5 s of denaturation at 95°C, 15 s of primer annealing at 62°C, and 16 s of fragment elongation at 72°C; for IDO, 5 s of denaturation at 95°C, 18 s of primer annealing at 56°C, and 16 s of fragment elongation at 72°C; and for CD40, 5 s of denaturation at 95°C, 10 s of primer annealing at 62°C, and 10 s of fragment elongation at 72°C. Conditions for RPII were the same as for the target gene. Target gene mRNA expression was normalized using both the LightCycler h-b2M housekeeping gene set with the LightCycler RNA Master HybProbe used according to the manufacturer's instructions (Roche Applied Science) and against RPII mRNA expression. All results are expressed as the ratio between the normalized expression of the target gene in treated cells and the normalized expression of the target gene in untreated or control cells.
(iii) ELISA.
For the secreted cytokine measurements, the supernatants from the cells examined by real-time PCR were measured using a sensitive sandwich enzyme-linked immunosorbent assay (ELISA) kit (Biosource, Camarillo, CA).
Statistics.
All P values were for a two-sample t test. A P value of 0.05 was considered statistically significant.
RESULTS
trans-Activation activity of HIV-1 subtype B Tat versus subtype C Tat.
In this study, we used only the full-length form of Tat. The HIV-1 subtype B Tat HXB2 was described by K. T. Jeang et al. (44). HIV-1 subtype C Tat 93In, carrying the C31→S mutation, was sequenced from the macrophage-tropic isolate 93In905 from India (51), and subtype C Tat 96Bw, lacking the C31→S mutation, was from Botswana (58). All were obtained using Fast Fmoc chemistry and, after purification using HPLC, were homogeneous by both mass spectrometry and HPLC as previously described (18, 62).
We assessed the abilities of these Tat variants to trans-activate the stably transfected HIV-1 LTR in HeLa-CD4-LTR-β-gal cells. We observed a dose-dependent response in the levels of β-galactosidase being quantified. We also observed that at all concentrations tested, there was no significant difference between the three variants except at 400 nM, the highest concentration tested where both subtype C Tat proteins had a higher activity (data not shown). This difference agrees with previously published data using different cell lines (28, 44, 57, 66).
Tat 93In does not induce a calcium flux in human monocytes.
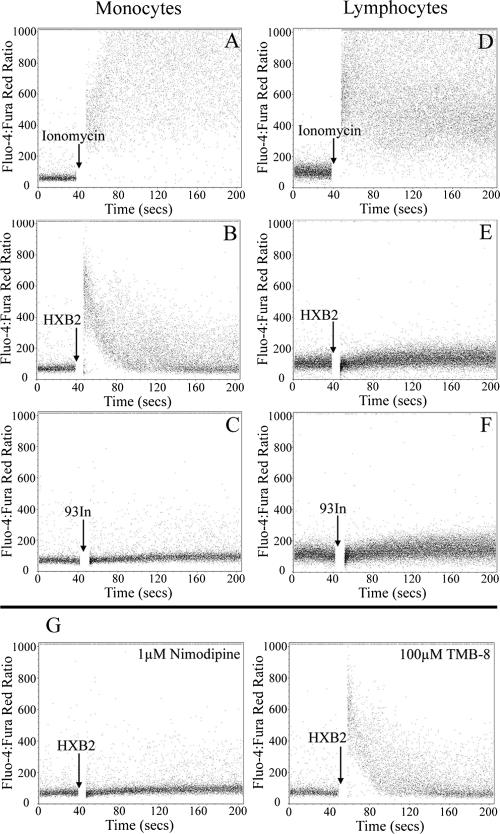
Previous studies showed that both HIV-1 subtype B Tat and a peptide spanning residues 24 to 51 of subtype B Tat are effective in mediating both monocyte chemotaxis and an increase in monocyte cytoplasmic Ca2+ concentration ([Ca2+]i) (3). Mutation of the CCF sequence in the peptide to SSG abrogated its ability to induce Ca2+ flux, indicating a critical role for these residues. To determine whether the mutations present in Tat 93In played a role in inducing a transient cytoplasmic Ca2+ flux in monocytes, PBMCs from healthy blood donors were loaded with the cell-permeant calcium fluorescent probes Fluo 4/AM and Fura Red/AM and stimulated with Tat. The variations in [Ca2+]i were measured using flow cytometry. Both Tat HXB2 (Fig. 1B) and Tat 96Bw (data not shown) elicited a transient increase in [Ca2+]i in monocytes that returned to baseline 1 minute after the initial flux. In both cases, this transient effect was not observed in lymphocytes (Fig. 1E). Interestingly, Tat 93In failed to induce an increase in [Ca2+]i (Fig. 1C) and was comparable to the vehicle control (data not shown). Increasing the concentration of subtype C Tat 93In failed to show any mobilization of calcium (data not shown). Using purified monocytes, we observed a profile similar to that of whole PBMCs (data not shown). For a positive control, ionomycin (1 μM), a calcium ionophore, was used in all our experiments (Fig. 1A and D).
FIG. 1.
Analysis of the intracellular variation of calcium concentration by flow cytometry. PBMCs were loaded with Fluo-4/AM and Fura Red/AM and stained with monoclonal anti-CD14 antibodies conjugated to APC and were treated with 50 nM Tat at the time points indicated. (A to F) Both CD14+ cells (predominantly monocytes) and CD14− cells (predominantly lymphocytes) were analyzed for calcium mobilization. Only HIV-1 subtype B Tat HXB2 was able to induce calcium mobilization in monocytes. (G) Cells were pretreated with either nimodipine or TMB-8. Data are shown for CD14+ cells. Nimodipine caused the marked inhibition of calcium flux in monocytes treated with subtype B Tat HXB2, whereas TMB-8 did not substantially affect the [Ca2+]i.
The origin of the calcium increase in Tat HXB2-stimulated monocytes is extracellular.
The source of the transient increase in [Ca2+]i in human monocytes can be either intracellular (released from intracellular stores) or extracellular (it enters the cell across the plasma membrane). Both processes often occur either simultaneously or sequentially. In some excitable cells, an important initiating step is intracellular release of Ca2+ from internal stores by the binding of a second messenger to its receptor in the endoplasmic reticulum. Commonly, this messenger is inositol 1,4,5-trisphosphate (13). Therefore, we sought to delineate the source of the [Ca2+]i by measuring the calcium flux by flow cytometry in monocytes loaded with Fluo 4/AM and Fura Red/AM and incubated with xestospongin C and TMB-8, inhibitors of inositol-1,4,5-trisphosphate and ryanodine, respectively. Neither TMB-8 (Fig. 1G) or xestospongin C (data not shown) had any effect on Tat-induced calcium flux in human monocytes, suggesting that the increased [Ca2+]i due to Tat was not from intracellular stores. Furthermore, when monocytes were incubated in the calcium-free loading buffer instead of the calcium buffer, Tat was unable to induce a transient [Ca2+]i flux, suggesting that extracellular calcium was required for this process, agreeing with previously published data (24; also data not shown).
Recent research has shown that L-type Ca2+ (CaL) channels are present in human monocytes and that they are implicated in the Tat-mediated transient increase in [Ca2+]i (25). Therefore, the calcium flux was measured by flow cytometry in monocytes treated with 1 μM nimodipine, a CaL channel inhibitor. HIV-1 subtype B Tat HXB2 was no longer able to induce the transient increase in [Ca2+]i in monocytes, confirming the report that the CaL channel is involved in the transient Tat-mediated increase in [Ca2+]i (Fig. 1G).
Tat 93In does not act as a chemoattractant.
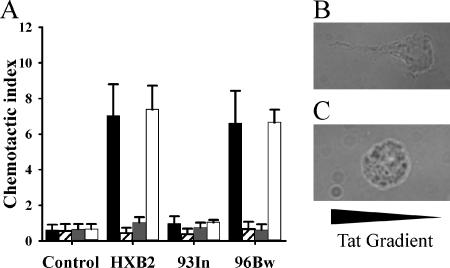
The increase in [Ca2+]i induced by HIV-1 subtype B Tat in macrophages/monocytes is accompanied by a strong macrophage/monocyte-specific chemoattractant activity (3). Although the three-dimensional structure of Tat shows no structural and little sequence similarity to the chemokine fold of CCL2, this chemoattractant ability is not without precedent; for example, gp120 is able to bind CCR5 and induce chemotaxis of T cells (88). The mutation of the CCF sequence in Tat peptides to SSG abrogates its ability to induce both a [Ca2+]i flux and monocyte chemotaxis. As Tat 93In was unable to induce a transient [Ca2+]i flux and has the mutation Cys31→Ser31, we tested its ability as a chemoattractant for monocytes. When cells encounter a chemotactic gradient, they respond by acquiring a migratory phenotype which includes the expression of leading and trailing edges. We show here that, compared with 93In Tat-treated cells, monocytes treated with Tat HXB2 exhibit morphological changes typical of the migratory phenotype (Fig. 2B and C). This was further assessed using a Transwell assay. Figure 2A shows that monocytes migrated toward Tat HXB2 and Tat 96Bw but not Tat 93In. The response to Tat 93In was not concentration dependent, with different concentrations of Tat 93In having no effect on monocyte migration as opposed to subtype B Tat (data not shown). Interestingly, when we specifically blocked the CaL channels, we observed an abrogation of monocyte chemotaxis toward subtype B Tat.
FIG. 2.
Monocyte migration induced by Tat. (A) Monocyte migration induced by 50 nM Tat proteins by themselves or in the presence of calcium channel and calcium store inhibitors. Cells were treated with Tat by itself (black bars), Tat in the presence of 1 μM nimodipine (striped bars), Tat in the presence of 100 nM FS-2 (dark gray bars), and Tat in the presence of 100 μM TMB-8 (white bars). The control wells contained 10 nM subtype B Tat in each compartment. The error bars represent the standard deviations measured in three independent experiments carried out in triplicate. Tat 93In (93In) showed no chemotactic activity, as it failed to induce significant chemotaxis of monocytes. Both nimodipine and FS-2 caused the marked inhibition of chemotaxis to HIV-1 subtype B Tat HXB2 (HXB2) and subtype C Tat 96Bw (96Bw), whereas TMB-8 did not affect migration. (B and C) Morphological changes induced by Tat HXB2 (B) and Tat 93In (C) along a gradient treatment.
Tat 93In does not bind CCR2.
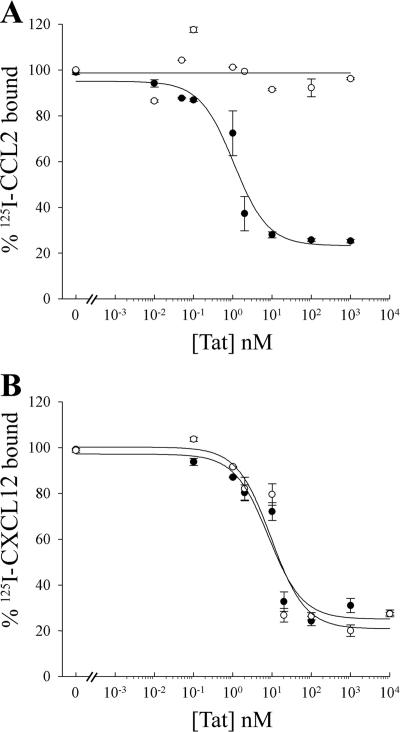
HIV-1 subtype B Tat shares some sequence similarities with β-chemokines, and previous studies demonstrated that a radiolabeled peptide spanning residues 24 to 51 of subtype B Tat is able to specifically bind the β-chemokine receptors CCR2 and CCR3 (3). As most subtype C Tat have a C31→S mutation, we wished to confirm the specific interactions of this Tat with CCR2 and CXCR4. GHOST cells expressing either CXCR4 or CCR2 were separately equilibrated with 125I-labeled ligands (CXCL12 or CCL2) with or without escalating amounts of Tat HXB2 or Tat 93In. Subtype B Tat but not subtype C Tat competed efficiently for 125I-CCL2 binding to CCR2+ cells (Fig. 3A). Conversely, both Tat variants competed efficiently for 125I-CXCL12 binding to CXCR4+ cells with approximately the same 50% inhibitory concentration (Fig. 3B).
FIG. 3.
Equilibrium competition of 125I-radiolabeled cytokines binding to monocytes. Radiolabeled cytokines were mixed with unlabeled Tat proteins and incubated with transfected cells for 40 min at 20°C. 125I-CCL2 and CCR2+ cells (A) and 125I-CXCL12 and CXCR4+ cells (B) were used. HIV-1 subtype C Tat 93In is represented by the open circles, and HIV-1 subtype B Tat HXB2 is represented by the closed circles. Cells were washed with binding buffer and fixed to a filter mat, and the amount of radiolabeled cytokine bound was determined by liquid scintillation. Error bars indicate standard deviations of two experiments carried out in duplicate. Both Tat proteins displaced CXCL12 from CXCR4 with the same 50% inhibitory concentration, while only Tat HXB2 displaced CCL2 from CCR2.
Tat 93In is less effective at upregulating TNF, IL-6, CCL2, and IDO than both HIV-1 subtype B Tat and 96Bw Tat.
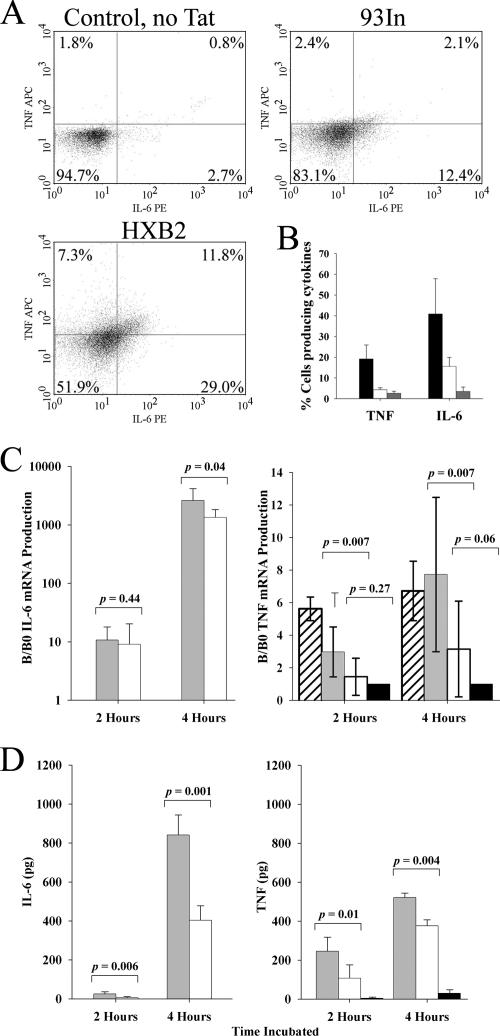
Tat-induced production of TNF by monocytes has been shown to be dependent on the presence of calcium in the extracellular medium (24), and blocking the CaL channel function with nimodipine results in a significant inhibition of Tat-induced TNF production from monocytes (25). In order to assess the ability of Tat 93In to induce TNF production in monocytes, we incubated PBMCs for 4 hours in the presence of either 50 nM Tat 93In or 50 nM Tat HXB2 and then analyzed them by flow cytometry. After gating on monocytes, we found that 19.1% of the subtype B Tat HXB2-stimulated monocytes produced TNF, whereas Tat 93In induced significantly less TNF production (4.5%; P = 0.0001; Fig. 4). We also found a difference in the number of cells producing IL-6. Subtype B Tat HXB2 induced 40.8% of cells to produce IL-6, whereas Tat 93In induced just 14.5% of cells to produce IL-6 (P = 0.0052; Fig. 4A).
FIG. 4.
TNF and IL-6 expression in Tat-stimulated monocytes. (A) PBMCs were treated with 2 μM monensin and Tat and left to incubate for 4 hours. Only cells bearing the CD14 receptor (predominantly monocytes) were analyzed for the presence of these cytokines (APC-conjugated TNF [TNF APC] and phycoerythrin-conjugated IL-6 [IL-6 PE]). Examples of fluorescence-activated cell sorting analysis of monocytes stained for IL-6 and TNF are shown. The percentages in each quadrant are the average seen for each quadrant from three different experiments using PBMCs from three different donors carried out in triplicate. (B) Results of the three independent experiments are quantified in a histogram. Cells were treated with Tat HXB2 (black bars), Tat 93In (white bars), and buffer alone (gray bars). The error bars represent the standard deviations measured in three independent experiments carried out in triplicate. Tat HXB2 induces significantly more monocytes to produce both TNF and IL-6 than Tat 93In does (P = 0.0001 and 0.0052, respectively). Purified monocytes were also treated with 50 nM Tat HXB2 and 50 nM Tat 93In and incubated for 2 and 4 hours. (C) mRNA was extracted from treated cells and subjected to quantification using the Roche LightCycler. Results are represented as histograms of three independent experiments. IL-6 mRNA production is represented as a log scale, while TNF mRNA production is depicted on a linear scale. Cells were treated with Tat HXB2 (light gray bars), Tat 93In (white bars), Tat 96Bw (hatched bars) and buffer alone (black bars). The error bars represent the standard deviations of three independent experiments carried out in triplicate. B/B0, quantity of mRNA measured by the LightCycler/quantity of background mRNA expressed by monocytes in a 2:1 mixture of AIM-V and Iscove's media supplemented with 1% (vol/vol) fetal calf serum without Tat. Tat HXB2 induces significantly more TNF mRNA and IL-6 mRNA than Tat 93In does. (D) The supernatants of cultured monocytes were analyzed by ELISA for the presence of IL-6 and TNF. Results of three independent experiments are represented by histograms. Cells were treated with HXB2 (light gray bars), Tat 93In (white bars), and by buffer alone (black bars). The error bars represent the standard deviations measured in three independent experiments carried out in triplicate. Tat HXB2 induces significantly more secreted TNF and IL-6 than Tat 93In does.
To confirm these results, purified monocytes were treated with 50 nM Tat. At 2 and 4 hours postincubation, cells and supernatants were harvested and analyzed for the presence of TNF mRNA, IL-6 mRNA, and the secreted forms of TNF and IL-6. We found that both Tat HXB2 and Tat 96Bw induced significantly more TNF mRNA and secreted TNF at both 2 and 4 hours than both the buffer alone and Tat 93In (Fig. 4C and D) and that Tat 93In was unable to induce significantly more TNF mRNA than buffer alone at both time points. However, the secreted cytokine quantity induced by Tat 93In was more than that induced by the negative control. Tat HXB2 also significantly upregulated the transcription of IL-6 mRNA at 4 hours posttreatment compared with 93In (P = 0.04), but at 2 hours, there was no significant difference (P = 0.44; Fig. 4C).
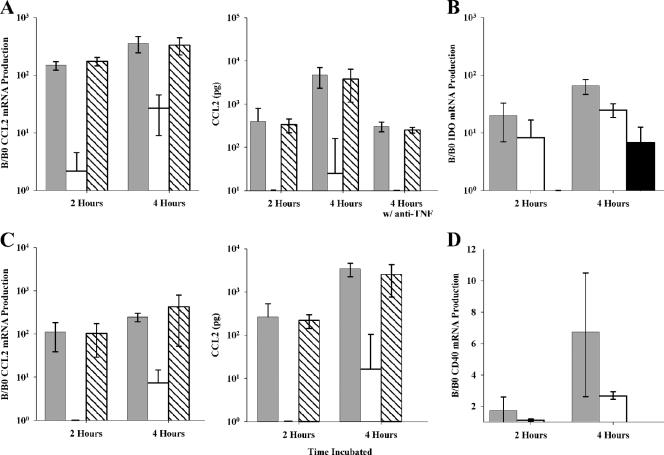
We then assessed the presence of CCL2 mRNA and secreted CCL2. Although Tat 93In induced CCL2 expression after 4 h, Tat HXB2 and Tat 96Bw both induced significantly more CCL2 at both time points tested (Fig. 5A; P < 0.05). We further tested these samples for the presence of IDO mRNA. IDO is the first enzyme of the kynurenine pathway that has several neurologically active metabolites, including quinolinic acid. Quinolinic acid is a neurotoxin that acts at N-methyl-d-aspartate receptors increasing intracellular calcium and cell death (79) and is found at elevated concentrations in the cerebrospinal fluid (CSF) and brains of individuals suffering from HAD (39). Previous in vitro experiments have shown that both HIV-1 subtype B Tat and Nef induce IDO (77), but no study to date has assessed the effect of subtype C Tat. We show that both Tat HXB2 and Tat 93In upregulate the expression of IDO mRNA, but that Tat 93In upregulates significantly less than Tat HXB2 does (Fig. 5B). In the presence of neutralizing anti-TNF antibodies, the synthesis of both CCL2 and IDO from monocytes is greatly reduced (P < 0.05), suggesting a role for this cytokine (Fig. 5A; also data not shown). Isotype-matched monoclonal antibodies did not have any significant effect on CCL2 or IDO production.
FIG. 5.
CCL2 expression in Tat-stimulated monocytes and microglia. (A) Tat HXB2 and Tat 96Bw treatment of purified monocytes causes a significant upregulation of the expression of CCL2, whereas Tat 93In upregulated significantly less CCL2 (P = 0.0001). In the presence of anti-TNF neutralizing antibodies, there was significantly less upregulation after 4 h for both Tat 96Bw and Tat HXB2 (P values of 0.049 and 0.017, respectively). Controls included cultures with either isotype-matched monoclonal antibodies (BD Pharmingen, San Diego, CA) or with FcR blocking reagent (Miltenyi Biotec, Auburn, CA). No significant difference was observed between these cells and those with no reagents (control). (B) Both Tat 93In and Tat 96Bw were able to upregulate the expression of indoleamine 2,3-dioxygenase. (C) Tat HXB2 and Tat 96Bw treatment of microglia causes a significant upregulation of the expression of CCL2, whereas Tat 93In upregulated significantly less (P < 0.05). (D) Tat HXB2 treatment significantly upregulated the expression of CD40 mRNA. Cells were treated with Tat HXB2 (light gray bars), Tat 93In (white bars), Tat 96Bw (hatched bars), and buffer alone (black bars). B/B0, quantity of mRNA measured by the LightCycler/quantity of background mRNA expressed by monocytes in a 2:1 mixture of AIM-V and Iscove's media supplemented with 1% (vol/vol) fetal calf serum without Tat. The error bars represent the standard deviations measured in three independent experiments carried out in triplicate.
Tat HXB2 activates and upregulates the expression of CD40 and CCL2 from microglial cells.
Microglia are the resident phagocytes of the brain and participate in various physiological functions. When microglia become activated, they upregulate major histocompatibility complex class II, CD40, B7, and chemokine/cytokine expression. Previous studies have shown that HIV-1 subtype B Tat induces CCL2 production and upregulates CD40 on microglial cells (26). We show that both Tat HXB2 and Tat 93In are able to upregulate mRNA for these proteins, but that Tat HXB2 induces significantly greater quantities (Fig. 5C and D). When secreted CCL2 was quantified, we found that the profile of CCL2 induced by the different variants of Tat was similar to that by monocytes, with subtype B Tat inducing significantly more than 93In Tat (Fig. 5C).
DISCUSSION
Monocyte infiltration and inflammatory responses in the brain, combined with the breakdown of the BBB, appear to be the main pathological features of HAD. Indeed, the abundance of macrophages and monocytes in the brain has been suggested to be a better correlate of HAD than the presence or extent of HIV-1 infection in the brain (33). Moreover, in the simian immunodeficiency virus animal model, the extent of macrophage infiltration into the white matter of the brain correlates with the severity of CNS lesions (93). Soluble factors produced by HIV-1-infected cells, such as Tat, TNF, and MCP-1 may be responsible, at least in part, for this finding (15, 26, 49, 52, 77).
The role of Tat in the development of neurocognitive impairment remains controversial. Tat has been detected in postmortem HIV encephalitic CNS tissue in various infected cells (41, 42, 54, 84, 91) as well as in uninfected oligodendrocytes (84), supporting in vitro findings that secreted Tat from infected cells can be localized in neighboring uninfected cells (81). Nevertheless, despite extensive in vitro research and in vivo animal studies demonstrating a potential role for Tat in HIV-related CNS impairment (reviewed in references 49 and 52 and references therein), because Tat is rapidly taken up by cells, no study to date has directly quantified the in vivo levels of secreted Tat in the CNS (42, 54). Moreover, in a mouse model of brain toxicity after a single intraventricular injection of Tat, pathological changes were observed over several days, while within 6 h Tat was undetectable, further highlighting the problem of detecting extracellular Tat in vivo (46). Thus, the biologically relevant levels of Tat in the brain that might be associated with HAD are unknown.
In vitro exposure of both glial cells and macrophages to Tat leads to their activation (6, 55). Additionally, virus-free supernatants of HIV-infected lymphoid and myeloid cells are able to trans-activate HIV-1 gene expression in a paracrine fashion, suggesting that the secreted extracellular Tat is functionally active (30). However, although we and others have immunoaffinity purified Tat to homogeneity from HIV supernatants, attempts to purify large quantities of biologically active Tat have been largely unsuccessful; Tat binds to heparin sulfate proteoglycans present in the cell membrane and extracellular matrix, rendering it inactive (21; also unpublished observations). Additionally, Tat can become easily inactivated, as its principal functional region contains 6/7 cysteines which are easily oxidized/reduced (62, 76), requiring the presence of reducing agents during the anaerobic purification process which would reduce its effect in neurotoxicity and functional tests (54). For these reasons, we have used functionally active synthetic Tat proteins for the experiments described.
Tat has been associated with many different functions, including apoptosis (17, 18, 90), stimulation or inhibition of cytokine production (6, 55), calcium mobilization, and chemotactic properties (3). We confirm previously published findings that HIV-1 subtype B Tat is able to bind CCR2 and induce a transient calcium flux in and chemoattract monocytes. We also show that the C31→S mutation found in the majority of subtype C Tat renders it unable to act in this manner. Moreover, we find that this variation possibly renders Tat less pathogenic, as monocytes treated with subtype B Tat produced significantly more TNF and CCL2 than those treated with Tat 93In. Interestingly, we were able to detect a significant increase in Tat 93In-induced TNF production only by ELISA and not by flow cytometry or reverse transcription-PCR. Tat 93In, as expected, also induced significantly less IL-6, CCL2, and CD40, as TNF induces the production of proinflammatory molecules, such as granulocyte colony-stimulating factor, CCL2, granulocyte-macrophage colony-stimulating factor, IL-6, IL-8, and nitric oxide. Indeed, neutralizing TNF antibodies led to a reduction in the Tat-induced production of CCL2 and CD40. Importantly, although subtype C Tat is unable to induce calcium flux and chemotaxis and has reduced capacity to induce TNF, its ability to perform in its primary role of trans-activating the HIV-1 LTR is unaffected, as we and others have shown that subtype C Tat with a C31→S mutation does not have reduced trans-activation ability (28, 44, 57, 66).
It is difficult to extrapolate these in vitro observations to what may happen within the HIV-infected human CNS where soluble factors, such as gamma interferon, gp120, and others produced by highly active antiretroviral therapy, modulate the activity of monocytes and microglia. Also, although Tat 93In is unable to induce significant levels of TNF, individuals infected with HIV-1 subtype C might still have elevated levels, as many host or viral factors, such as gp120, likely contribute to TNF production (22). Moreover, TNF may play a neuroprotective role, so conclusions about the role that Tat-induced TNF may play are limited.
When CCL2 binds to CCR2, it results in increased arachidonic acid release and influx of extracellular calcium and chemotaxis, which is inhibited by Bordetella pertussis toxin treatments and is dependent upon the calcium-dependent protein kinase Cβ (20). HIV-1 subtype B Tat has been shown to bind CCR2 and induce both a transient [Ca2+]i flux and chemotaxis. However, treatment with pertussis toxin only partially blocks the subtype B Tat-induced transient [Ca2+]i flux (3). Conversely, blocking the CaL channels completely abrogates subtype B Tat-induced Ca2+ flux and chemotaxis of monocytes. Treatment of monocytes with CCL2 does not result in the production of TNF (45; also unpublished observations). Therefore, although Tat binds to CCR2 and induces a calcium flux, this is not sufficient to induce the production of TNF from this cell type, suggesting that Tat elicits the production of TNF through a CCR2-independent mechanism.
Monocyte migration can also be mediated through adhesion molecule receptors, mainly integrins, such as α5β1. HIV-1 subtype B Tat can bind and activate α5β1 and αvβ3 through its RGD region (9, 10) and mediate migration of monocytes (11). As Tat 93In has a mutation of R78→Q and is unable to induce monocyte chemotaxis, it therefore cannot induce chemotaxis through either CCR2 or integrins.
Activation of α5β1 integrins in rat brain and smooth muscle acutely potentiates CaL channels (37), leading to an increase in [Ca2+]i. Studies using serum-deprived vascular smooth muscle cells stimulated with platelet-derived growth factor have shown that specifically inhibiting CaL channels leads to an abrogation of both calcium flux and associated chemotaxis (61). In general, published data do not support a requirement for calcium signals in chemotaxis mediated by G-protein-coupled receptors. However, recent studies have shown a calcium-dependent role in some situations (20, 60). This is the first study, to our knowledge, that examines the role of CaL channels in the chemotaxis of monocytes. By specifically blocking CaL channels of freshly isolated monocytes, we observed similar results to those targeting vascular smooth muscle cells, suggesting a role for CaL in Tat-induced chemotaxis.
The pathogenesis of HAD also involves astrocyte dysfunction and apoptosis. Astrocytes have important neuroprotective functions (82). They decrease neurotoxin levels (32), clear excess excitatory amino acids (56), produce neurotrophic factors (89), and maintain the BBB (16). Examination of postmortem brain tissue revealed that astrocyte apoptosis is higher in HIV-1-infected patients than in HIV-negative patients (82). Furthermore, the extent of astrocyte apoptosis was correlated with the rate of HAD progression. Postmortem brain tissue examinations reveal that during HAD, astrocytes upregulate CD95 expression (29), and in vitro studies show that soluble CD178 released from HIV-1-infected macrophages triggers apoptosis of uninfected astrocytes (4). Moreover, individuals with HAD have higher levels of soluble CD178 in both their serum and cerebrospinal fluid and higher levels of soluble CD95 in their cerebrospinal fluid than HIV-1-infected nondemented controls (69, 78, 83). Together, this suggests that the CD95-CD178 pathway may be involved. Tat is known to induce T-cell apoptosis through this same pathway (17, 18, 90).
Current estimates place the prevalence of HAD in the United States and Western Europe at between 10 and 20% (reviewed in reference 34). In India, although systematic studies have just been initiated in various centers, three recent independent studies have put the prevalence of HAD at just over 1% (72, 75, 86). It is possible that the low levels of reported HAD in economically developing societies like India are due to underdiagnosis, shorter life expectancy, or other factors (40, 80). However, it is interesting to note that the major HIV-1 subtype circulating in India is subtype C (65, 71, 73), in contrast to the United States and Western Europe, where infections are primarily caused by subtype B (38). As Tat is necessary for HIV-1 replication and is involved in a number of physiopathological effects, therapeutic approaches targeting Tat could be particularly effective in reducing the serious consequences of HIV-1 infection. Differing rates of disease progression correlate with different HIV-1 subtypes (47, 74) and different isotypes of Tat have been shown to induce different effects on T-cell function and viability (17, 18, 27, 62, 67). This study further highlights the importance of studying the pathological effects of different subtypes of HIV-1 and their proteins.
Acknowledgments
We thank Carol Mundy, Rodney Trout, Dennis Young, and Cynthia Bolovan-Fritts for skillful technical assistance.
This work was supported by the National Institute of Allergy and Infectious Diseases (grants AI41089 and AI-36214 [to the Virology Core, University of California San Diego Center for AIDS Research]).
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Adle-Biassette, H., F. Chretien, L. Wingertsmann, C. Héry, T. Ereau, F. Scaravilli, M. Tardieu, and F. Gray. 1999. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 25:23-133. [DOI] [PubMed] [Google Scholar]
- 2.Albini, A., R. Benelli, D. Giunciuglio, T. Cai, G. Mariani, S. Ferrini, and D. M. Noonan. 1998. Identification of a novel domain of HIV Tat involved in monocyte chemotaxis. J. Biol. Chem. 273:15895-15900. [DOI] [PubMed] [Google Scholar]
- 3.Albini, A., S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. Proudfoot, S. Alouani, T. N. Wells, G. Mariani, R. L. Rabin, J. M. Farber, and D. M. Noonan. 1998. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 95:13153-13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aquaro, S., S. Panti, M. C. Caroleo, E. Balestra, A. Cenci, F. Forbici, G. Ippolito, A. Mastino, R. Testi, V. Mollace, R. Calio, and C. F. Perno. 2000. Primary macrophages infected by human immunodeficiency virus trigger CD95-mediated apoptosis of uninfected astrocytes. J. Leukoc. Biol. 68:429-435. [PubMed] [Google Scholar]
- 5.Arya, S. K., B. Beaver, L. Jagodzinski, B. Ensoli, P. J. Kanki, J. Albert, E. M. Fenyo, G. Biberfeld, J. F. Zagury, F. Laure, M. Essex, E. Norby, F. Wong-Staal, and R. C. Gallo. 1987. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature 328:548-550. [DOI] [PubMed] [Google Scholar]
- 6.Badou, A., Y. Bennasser, M. Moreau, C. Leclerc, M. Benkirane, and E. Bahraoui. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J. Virol. 74:10551-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks, W. A., S. M. Robinson, and A. Nath. 2005. Permeability of the blood-brain barrier to HIV-1 Tat. Exp. Neurol. 193:218-227. [DOI] [PubMed] [Google Scholar]
- 8.Barany, G., and R. B. Merrifield. 1980. Solid phase peptide synthesis, p. 1-284. In E. Gross and J. Meinhofer (ed.), The peptide: analysis, synthesis, biology, vol. 2. Academic Press, New York, NY. [Google Scholar]
- 9.Barillari, G., R. Gendelman, R. C. Gallo, and B. Ensoli. 1993. The Tat protein of human immunodeficiency virus type 1, a growth factor for AIDS Kaposi sarcoma and cytokine-activated vascular cells, induces adhesion of the same cell types by using integrin receptors recognizing the RGD amino acid sequence. Proc. Natl. Acad. Sci. USA 90:7941-7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barillari, G., C. Sgadari, V. Fiorelli, F. Samaniego, S. Colombini, V. Manzari, A. Modesti, B. C. Nair, A. Cafaro, M. Stürzl, and B. Ensoli. 1999. The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the alpha5beta1 and alphavbeta3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94:663-672. [PubMed] [Google Scholar]
- 11.Benelli, R., R. Mortarini, A. Anichini, D. Giunciuglio, D. M. Noonan, S. Montalti, C. Tacchetti, and A. Albini. 1998. Monocyte-derived dendritic cells and monocytes migrate to HIV-Tat RGD and basic peptides. AIDS 12:261-268. [DOI] [PubMed] [Google Scholar]
- 12.Benveniste, E. N., V. T. Nguyen, and D. R. Wesemann. 2004. Molecular recognition of CD40 expression in macrophages and microglia. Brain Behav. Immun. 18:7-12. [DOI] [PubMed] [Google Scholar]
- 13.Berridge, M. J. 1993. Inositol trisphosphate and calcium signalling. Nature 361:315-325. [DOI] [PubMed] [Google Scholar]
- 14.Bhoopat, L., T. S. Rithaporn, S. Khunamornpong, T. Bhoopat, C. R. Taylor, and P. S. Thorner. 2006. Cell reservoirs in lymph nodes infected with HIV-1 subtype E differ from subtype B: identification by combined in situ polymerase chain reaction and immunohistochemistry. Mod. Pathol. 19:255-263. [DOI] [PubMed] [Google Scholar]
- 15.Brabers, N. A., and H. S. Nottet. 2006. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur. J. Clin. Investig. 36:447-458. [DOI] [PubMed] [Google Scholar]
- 16.Brack-Werner, R. 1999. Astrocytes. HIV cellular reservoirs and important participants in neuropathogenesis. AIDS 13:1-22. [DOI] [PubMed] [Google Scholar]
- 17.Campbell, G. R., E. Pasquier, J. Watkins, V. Bourgarel-Rey, V. Peyrot, D. Esquieu, P. Barbier, J. de Mareuil, D. Braguer, P. Kaleebu, D. L. Yirrell, and E. P. Loret. 2004. The glutamine-rich region of the HIV-1 Tat protein is involved in T-cell apoptosis. J. Biol. Chem. 279:48197-48204. [DOI] [PubMed] [Google Scholar]
- 18.Campbell, G. R., J. D. Watkins, D. Esquieu, E. Pasquier, E. P. Loret, and S. A. Spector. 2005. The C-terminus of HIV-1 Tat modulates the extent of CD178 mediated apoptosis of T cells. J. Biol. Chem. 280:38376-38382. [DOI] [PubMed] [Google Scholar]
- 19.Capini, C. J., M. W. Richardson, H. Hendel, A. Sverstiuk, J. Mirchandani, E. G. Régulier, K. Khalili, J. F. Zagury, and J. Rappaport. 2001. Autoantibodies to TNFalpha in HIV-1 infection: prospects for anti-cytokine vaccine therapy. Biomed. Pharmacother. 5:23-31. [DOI] [PubMed] [Google Scholar]
- 20.Carnevale, K. A., and M. K. Cathcart. 2003. Protein kinase C beta is required for human monocyte chemotaxis to MCP-1. J. Biol. Chem. 278:25317-25322. [DOI] [PubMed] [Google Scholar]
- 21.Chang, H. C., F. Samaniego, B. C. Nair, L. Buonaguro, and B. Ensoli. 1997. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421-1431. [DOI] [PubMed] [Google Scholar]
- 22.Clouse, K. A., L. M. Cosentino, K. A. Weih, S. W. Pyle, P. B. Robbins, H. D. Hochstein, V. Natarajan, and W. L. Farrar. 1991. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J. Immunol. 147:2892-2901. [PubMed] [Google Scholar]
- 23.Colotta, F., A. Borre, J. M. Wang, M. Tattanelli, F. Maddalena, N. Polentarutti, G. Peri, and A. Mantovani. 1992. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J. Immunol. 148:760-765. [PubMed] [Google Scholar]
- 24.Contreras, X., Y. Bennasser, N. Chazal, and E. Bahraoui. 2003. HIV-1 Tat induces TNF-alpha production by human monocytes: involvement of calcium and PKC pathways. J. Soc. Biol. 197:267-275. [PubMed] [Google Scholar]
- 25.Contreras, X., Y. Bennasser, N. Chazal, M. Moreau, C. Leclerc, J. Tkaczuk, and E. Bahraoui. 2005. Human immunodeficiency virus type 1 Tat protein induces an intracellular calcium increase in human monocytes that requires DHP receptors: involvement in TNF-alpha production. Virology 332:316-328. [DOI] [PubMed] [Google Scholar]
- 26.D'Aversa, T. G., E. A. Eugenin, and J. W. Berman. 2005. NeuroAIDS: contributions of the human immunodeficiency virus-1 proteins Tat and gp120 as well as CD40 to microglial activation. J. Neurosci. Res. 81:436-446. [DOI] [PubMed] [Google Scholar]
- 27.de Mareuil, J., M. Carre, P. Barbier, G. R. Campbell, S. Lancelot, S. Opi, D. Esquieu, J. D. Watkins, C. Prevot, D. Braguer, V. Peyrot, and E. P. Loret. 2005. HIV-1 Tat protein enhances microtubule polymerization. Retrovirology 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desfosses, Y., M. Solis, Q. Sun, N. Grandvaux, C. Van Lint, A. Burny, A. Gatignol, M. A. Wainberg, R. Lin, and J. Hiscott. 2005. Regulation of human immunodeficiency virus type 1 gene expression by clade-specific Tat proteins. J. Virol. 79:9180-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elovaara, I., F. Sabri, F. Gray, I. Alafuzoff, and F. Chiodi. 1999. Upregulated expression of Fas and Fas ligand in brain through the spectrum of HIV-1 infection. Acta Neuropathol. (Berlin) 98:355-362. [DOI] [PubMed] [Google Scholar]
- 30.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiala, M., D. J. Looney, M. Stins, D. D. Way, L. Zhang, X. Gan, F. Chiappelli, E. S. Schweitzer, P. Shapshak, M. Weinand, M. C. Graves, M. Witte, and K. S. Kim. 1997. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol. Med. 3:553-564. [PMC free article] [PubMed] [Google Scholar]
- 32.Giulian, D., J. Li, S. Bartel, J. Broker, X. Li, and J. B. Kirkpatrick. 1995. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J. Neurosci. 15:7712-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glass, J., H. Fedor, S. Wesselingh, and J. McArthur. 1995. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 38:755-762. [DOI] [PubMed] [Google Scholar]
- 34.Grant, I., N. Sacktor, and J. McArthur. 2005. HIV neurocognitive disorders, p. 357-373. In H. E. Gendelman, I. Grant, I. Everall, S. A. Lipton, and S. Swindells (ed.), The neurology of AIDS, 2nd ed. Oxford University Press, London, United Kingdom.
- 35.Gray, F., H. Adle-Biassette, F. Chrétien, G. Lorin de la Grandmaison, G. Force, and C. Keohane. 2001. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin. Neuropathol. 20:146-155. [PubMed] [Google Scholar]
- 36.Griffin, D. E. 1997. Cytokines in the brain during viral infection: clues to HIV-associated dementia. J. Clin. Investig. 100:2948-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gui, P., X. Wu, S. Ling, S. C. Stotz, R. J. Winkfein, E. Wilson, G. E. Davis, A. P. Braun, G. W. Zamponi, and M. J. Davis. 2006. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J. Biol. Chem. 281:14015-14025. [DOI] [PubMed] [Google Scholar]
- 38.Hemelaar, J., E. Gouws, P. D. Ghys, and S. Osmanov. 2006. Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20:W13-W23. [DOI] [PubMed] [Google Scholar]
- 39.Heyes, M. P., B. J. Brew, A. Martin, R. W. Price, A. M. Salazar, J. J. Sidtis, J. A. Yergey, M. M. Mouradian, A. E. Sadler, J. Keilp, D. Rubinow, and S. P. Markey. 1991. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann. Neurol. 29:202-209. [DOI] [PubMed] [Google Scholar]
- 40.Hira, S. K., G. J. Dore, and T. Sirisanthana. 1998. Clinical spectrum of HIV/AIDS in the Asia-Pacific region. AIDS 12(Suppl. B):S145-S154. [PubMed] [Google Scholar]
- 41.Hofman, F. M., M. M. Dohadwale, A. D. Wright, D. R. Hinton, and S. M. Walker. 1994. Exogenous tat protein activates central nervous system-derived endothelial cells. J. Neuroimmunol. 54:19-28. [DOI] [PubMed] [Google Scholar]
- 42.Hudson, L., J. Liu, A. Nath, M. Jones, R. Raghavan, O. Narayan, D. Male, and I. Everall. 2000. Detection of the human immunodeficiency virus regulatory protein tat in CNS tissues. J. Neurovirol. 6:145-155. [DOI] [PubMed] [Google Scholar]
- 43.Huigen, M. C., W. Kamp, and H. S. Nottet. 2004. Multiple effects of HIV-1 trans-activator protein on the pathogenesis of HIV-1 infection. Eur. J. Clin. Investig. 34:57-66. [DOI] [PubMed] [Google Scholar]
- 44.Jeang, K. T., H. Xiao, and E. A. Rich. 1999. Multifaceted activities of the HIV-1 transactivator of transcription, Tat. J. Biol. Chem. 274:28837-28840. [DOI] [PubMed] [Google Scholar]
- 45.Jiang, Y., D. I. Beller, G. Frendl, and D. T. Graves. 1992. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J. Immunol. 148:2423-2428. [PubMed] [Google Scholar]
- 46.Jones, M., K. Olafson, M. R. Del Bigio, J. Peeling, and A. Nath. 1998. Intraventricular injection of human immunodeficiency virus type 1 (HIV-1) tat protein causes inflammation, gliosis, apoptosis, and ventricular enlargement. J. Neuropathol. Exp. Neurol. 57:563-570. [DOI] [PubMed] [Google Scholar]
- 47.Kaleebu, P., A. Ross, D. Morgan, D. Yirrell, J. Oram, A. Rutebemberwa, F. Lyagoba, L. Hamilton, B. Biryahwaho, and J. Whitworth. 2001. Relationship between HIV-1 Env subtypes A and D and disease progression in a rural Ugandan cohort. AIDS 15:293-299. [DOI] [PubMed] [Google Scholar]
- 48.Kimpton, J., and M. Emerman. 1992. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J. Virol. 66:2232-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King, J. E., E. A. Eugenin, C. M. Buckner, and J. W. Berman. 2006. HIV tat and neurotoxicity. Microbes Infect. 8:1347-1357. [DOI] [PubMed] [Google Scholar]
- 50.Lafrenie, R. M., L. M. Wahl, J. S. Epstein, I. K. Hewlett, K. M. Yamada, and S. Dhawan. 1996. HIV-1-Tat protein promotes chemotaxis and invasive behavior by monocytes. J. Immunol. 157:974-977. [PubMed] [Google Scholar]
- 51.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minghetti, L., S. Visentin, M. Patrzio, L. Franchini, M. A. Ajmone-Cat, and G. Levi. 2004. Multiple actions of the human immunodeficiency virus type-1 Tat protein on microglial cell functions. Neurochem. Res. 29:965-978. [DOI] [PubMed] [Google Scholar]
- 53.Mörner, A., A. Bjorndal, V. KewalRamani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyo, and E. Bjorling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nath, A., J. D. Geiger, M. P. Mattson, D. S. K. Magnuson, M. Jones, and J. R. Berger. 1998. Role of viral proteins in HIV-1 neuropathogenesis with emphasis on Tat. NeuroAIDS 1:6. http://aidscience.com/neuroaids/articles/Neuro1(6).asp. [Google Scholar]
- 55.Nath, A., K. Conant, P. Chen, C. Scott, and E. O. Major. 1999. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J. Biol. Chem. 274:17098-17102. [DOI] [PubMed] [Google Scholar]
- 56.Nicholls, D., and D. Attwell. 1990. The release and uptake of excitatory amino acids. Trends Pharmacol. Sci. 11:462-468. [DOI] [PubMed] [Google Scholar]
- 57.Opi, S., J. M. Péloponèse, Jr., D. Esquieu, G. Campbell, J. de Mareuil, A. Walburger, M. Solomiac, C. Grégoire, E. Bouveret, D. L. Yirrell, and E. P. Loret. 2002. Tat HIV-1 primary and tertiary structures critical to immune response against non-homologous variants. J. Biol. Chem. 277:35915-35919. [DOI] [PubMed] [Google Scholar]
- 58.Opi, S., J. M. Péloponèse, Jr., D. Esquieu, J. Watkins, G. Campbell, J. de Mareuil, K. T. Jeang, D. L. Yirrell, P. Kaleebu, and E. P. Loret. 2004. Full-length HIV-1 Tat protein necessary for a vaccine. Vaccine 22:3105-3111. [DOI] [PubMed] [Google Scholar]
- 59.Park, I. W., J. F. Wang, and J. E. Groopman. 2001. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood 97:352-358. [DOI] [PubMed] [Google Scholar]
- 60.Partida-Sánchez, S., S. Goodrich, K. Kusser, N. Oppenheimer, T. D. Randall, and F. E. Lund. 2004. Regulation of dendritic cell trafficking by the ADP-ribosyl cyclase CD38: impact on the development of humoral immunity. Immunity 20:279-291. [DOI] [PubMed] [Google Scholar]
- 61.Patel, M. K., G. F. Clunn, J. S. Lymn, O. Austin, and A. D. Hughes. 2005. Effect of serum withdrawal on the contribution of L-type calcium channels (CaV1.2) to intracellular Ca2+ responses and chemotaxis in cultured human vascular smooth muscle cells. Br. J. Pharmacol. 145:811-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Péloponèse, J. M., Jr., Y. Collette, C. Grègoire, C. Bailly, D. Campèse, E. F. Meurs, D. Olive, and E. P. Loret. 1999. Full peptide synthesis, purification, and characterization of six Tat variants. Differences observed between HIV-1 isolates from Africa and other continents. J. Biol. Chem. 274:11473-11478. [DOI] [PubMed] [Google Scholar]
- 63.Perry, S. W., S. Dewhurst, M. J. Bellizzi, and H. A. Gelbard. 2002. Tumor necrosis factor-alpha in normal and diseased brain: conflicting effects via intraneuronal receptor crosstalk? J. Neurovirol. 8:611-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pu, H., J. Tian, G. Flora, Y. W. Lee, A. Nath, B. Hennig, and M. Toborek. 2003. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol. Cell. Neurosci. 24:224-237. [DOI] [PubMed] [Google Scholar]
- 65.Ramalingam, S., R. Kannangai, T. S. Vijayakumar, D. Mathai, O. C. Abraham, S. Subramanian, P. Rupali, M. V. Jesudason, and G. Sridharan. 2005. Subtype and cytokine profiles of HIV infected individuals from south India. Indian J. Med. Res. 121:226-234. [PubMed] [Google Scholar]
- 66.Ranga, U., R. Shankarappa, N. B. Siddappa, L. Ramakrishna, R. Nagendran, M. Mahalingam, A. Mahadevan, N. Jayasuryan, P. Satishchandra, S. K. Shankar, and V. R. Prasad. 2004. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J. Virol. 78:2586-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranjbar, S., R. Rajsbaum, and A. E. Goldfeld. 2006. Transactivator of transcription from HIV type 1 subtype E selectively inhibits TNF gene expression via interference with chromatin remodeling of the TNF locus. J. Immunol. 176:4182-4190. [DOI] [PubMed] [Google Scholar]
- 68.Rappaport, J., J. Joseph, S. Croul, G. Alexander, L. Del Valle, S. Amini, and K. Khalili. 1999. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. J. Leukoc. Biol. 65:458-465. [DOI] [PubMed] [Google Scholar]
- 69.Sabri, F., A. De Milito, R. Pirskanen, I. Elovaara, L. Hagberg, P. Cinque, R. Price, and F. Chiodi. 2001. Elevated levels of soluble Fas and Fas ligand in cerebrospinal fluid of patients with AIDS dementia complex. J. Neuroimmunol. 114:197-206. [DOI] [PubMed] [Google Scholar]
- 70.Saha, R. N., and K. Pahan. 2003. Tumor necrosis factor-alpha at the crossroads of neuronal life and death during HIV-associated dementia. J. Neurochem. 86:1057-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sahni, A. K., V. V. Prasad, and P. Seth. 2002. Genomic diversity of human immunodeficiency virus type-1 in India. Int. J. STD AIDS 13:115-118. [DOI] [PubMed] [Google Scholar]
- 72.Satishchandra, P., A. Nalini, M. Gourie-Devi, N. Khanna, V. Santosh, V. Ravi, A. Desai, A. Chandramuki, P. N. Jayakumar, and S. K. Shankar. 2000. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989-96). Indian J. Med. Res. 111:14-23. [PubMed] [Google Scholar]
- 73.Sengupta, S., S. Jana, K. Sarkar, S. K. Bhattacharya, and S. Chakrabarti. 2005. Determination of gag subtypes of HIV type 1 detected among female sex workers in Calcutta, India. AIDS Res. Hum. Retrovir. 21:806-809. [DOI] [PubMed] [Google Scholar]
- 74.Senkaali, D., R. Muwonge, D. Morgan, D. Yirrell, J. Whitworth, and P. Kaleebu. 2004. The relationship between HIV type 1 disease progression and V3 serotype in a rural Ugandan cohort. AIDS Res. Hum. Retrovir. 20:932-937. [DOI] [PubMed] [Google Scholar]
- 75.Shankar, S. K., A. Mahadevan, P. Satishchandra, R. U. Kumar, T. C. Yasha, V. Santosh, A. Chandramuki, V. Ravi, and A. Nath. 2005. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J. Med. Res. 121:468-488. [PubMed] [Google Scholar]
- 76.Siddappa, N. B., M. Venkatramanan, P. Venkatesh, M. V. Janki, N. Jayasuryan, A. Desai, V. Ravi, and U. Ranga. 2006. Transactivation and signaling functions of Tat are not correlated: biological and immunological characterization of HIV-1 subtype-C Tat protein. Retrovirology 3:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smith, D. G., G. J. Guillemin, L. Pemberton, S. Kerr, A. Nath, G. A. Smythe, and B. J. Brew. 2001. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J. Neurovirol. 7:56-60. [DOI] [PubMed] [Google Scholar]
- 78.Sporer, B., U. Koedel, F. D. Goebel, and H. W. Pfister. 2000. Increased levels of soluble Fas receptor and Fas ligand in the cerebrospinal fluid of HIV-infected patients. AIDS Res. Hum. Retrovir. 16:221-226. [DOI] [PubMed] [Google Scholar]
- 79.Stone, T. W. 1993. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol. Rev. 45:309-379. [PubMed] [Google Scholar]
- 80.Suh, G. H., and A. Shah. 2001. A review of the epidemiological transition in dementia-cross-national comparisons of the indices related to Alzheimer's disease and vascular dementia. Acta Psychiatr. Scand. 104:4-11. [DOI] [PubMed] [Google Scholar]
- 81.Tardieu, M., C. Hery, S. Peudenier, O. Boespflug, and L. Montagnier. 1992. Human immunodeficiency virus type 1-infected monocytic cells can destroy human neural cells after cell-to-cell adhesion. Ann. Neurol. 32:11-17. [DOI] [PubMed] [Google Scholar]
- 82.Thompson, K. A., J. C. McArthur, and S. L. Wesselingh. 2001. Correlation between neurological progression and astrocyte apoptosis in HIV-associated dementia. Ann. Neurol. 49:745-752. [DOI] [PubMed] [Google Scholar]
- 83.Towfighi, A., R. L. Skolasky, C. St. Hillaire, K. Conant, and J. C. McArthur. 2004. CSF soluble Fas correlates with the severity of HIV-associated dementia. Neurology 62:654-656. [DOI] [PubMed] [Google Scholar]
- 84.Valle, L. D., S. Croul, S. Morgello, S. Amini, J. Rappaport, and K. Khalili. 2000. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 6:221-228. [DOI] [PubMed] [Google Scholar]
- 85.Vasan, A., B. Renjifo, E. Hertzmark, B. Chaplin, G. Msamanga, M. Essex, W. Fawzi, and D. Hunter. 2006. Different rates of disease progression of HIV type 1 infection in Tanzania based on infecting subtype. Clin. Infect. Dis. 42:843-852. [DOI] [PubMed] [Google Scholar]
- 86.Wadia, R. S., S. N. Pujari, S. Kothari, M. Udhar, S. Kulkarni, S. Bhagat, and A. Nanivadekar. 2001. Neurological manifestations of HIV disease. J. Assoc. Physicians India 49:343-348. [PubMed] [Google Scholar]
- 87.Weiss, J. M., A. Nath, E. O. Major, and J. W. Berman. 1999. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J. Immunol. 163:2953-2959. [PubMed] [Google Scholar]
- 88.Weissman, D., R. L. Rabin, J. Arthos, A. Rubbert, M. Dybul, R. Swofford, S. Venkatesan, J. M. Farber, and A. S. Fauci. 1997. Macrophage-tropic HIV and SIV envelope proteins induce a signal through the CCR5 chemokine receptor. Nature 389:981-985. [DOI] [PubMed] [Google Scholar]
- 89.Wesselingh, S. L., C. Power, J. D. Glass, W. R. Tyor, J. McArthur, J. M. Farber, J. W. Griffin, and D. E. Griffin. 1993. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann. Neurol. 33:576-582. [DOI] [PubMed] [Google Scholar]
- 90.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 91.Wiley, C. A., M. Baldwin, and C. L. Achim. 1996. Expression of HIV regulatory and structural mRNA in the central nervous system. AIDS 10:843-847. [DOI] [PubMed] [Google Scholar]
- 92.Xiao, H., C. Neuveut, H. L. Tiffany, M. Benkirane, E. A. Rich, P. M. Murphy, and K. T. Jeang. 2000. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. USA 97:11466-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zink, M. C., K. Suryanarayana, J. L. Mankowski, A. Shen, M. Piatak, Jr., J. P. Spelman, D. L. Carter, R. J. Adams, J. D. Lifson, and J. E. Clements. 1999. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J. Virol. 73:10480-10488. [DOI] [PMC free article] [PubMed] [Google Scholar]