Abstract
Here, we study the temporal expression of the inhibitory receptor programmed death 1 (PD-1) on simian immunodeficiency virus (SIV) Gag-specific T cells following pathogenic SIV infection or following vaccination with a DNA/modified vaccinia virus Ankara (DNA/MVA) vaccine and simian/human immunodeficiency virus (SHIV) challenge in macaques. Following infection, the majority (>95%) of Gag-specific CD8 T cells expressed PD-1, and the level of PD-1 expression per cell increased over time. The level of PD-1 expression in lymph nodes and rectal mucosal tissue, the major sites of virus replication, was higher compared to blood. In vitro blockade of PD-1 resulted in enhanced proliferation of SIV-specific CD8 as well as CD4 T cells. In contrast, following vaccination, the majority of peak effector Gag-specific CD8 T cells expressed low levels of PD-1, and these levels decreased further as the cells differentiated into memory cells. In addition, following SHIV challenge of these vaccinated macaques, the level of PD-1 expression on Gag-specific CD8 T cells correlated positively with plasma viremia. These results demonstrate that SIV-specific CD8 T cells express PD-1 after exposure to antigen but downregulate expression under conditions of antigen clearance and enhance expression under conditions of antigen persistence. They also demonstrate that the level of PD-1 expression per cell rather than the presence or absence of expression plays an important role in regulating CD8 T-cell dysfunction in pathogenic SIV infection. In addition, they demonstrate that similar to HIV infection, the PD-1:PD-1 ligand inhibitory pathway is operational in pathogenic SIV infection, and the macaque/SIV model would be ideal to test the safety and therapeutic benefit of blocking this pathway in vivo.
Antiviral CD8 T cells play a critical role in the control of human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections as shown by viral reemergence during transient in vivo depletion of CD8 T cells in SIV-infected macaques (21, 26, 35). Consistent with this, contemporary vaccine strategies designed to elicit high frequencies of antiviral CD8 T cells have contained pathogenic simian/human immunodeficiency virus (SHIV) (4, 7, 37) and SIV (8, 22, 40) challenges in macaques. Antiviral CD4 T cells also have proven important in restricting viral replication, as suggested by a highly significant correlation between the magnitude of virus-specific CD4 T cells and the control of viremia in HIV type 1 (HIV-1)-infected humans (23, 33) and SIV-infected macaques (18).
Both the function and the frequency of antiviral CD8 T cells are crucial for the control of chronic viral infections (24, 27, 39). Effective antiviral CD8 T cells possess a number of functional properties, including the ability to produce different cytokines, cytotoxic potential, high proliferative potential, and low apoptosis. During chronic viral infection, virus-specific CD8 T cells undergo exhaustion that is associated with the loss of many of these functions (41). Similarly, HIV-specific CD8 T cells from individuals with progressive disease have been shown to be impaired in their function. These CD8 T cells can produce cytokines such as gamma interferon but are impaired for the production of interleukin-2, a cytokine that is important for T-cell proliferation and survival (1). They are also defective for expression of perforin (5, 27), a molecule that is critical for cytolytic function, and proliferative capacity, a property that has been implicated in the control of HIV replication (17, 20, 27).
Recent studies have shown that the coinhibitory receptor programmed death 1 (PD-1) is highly expressed by CD8 T cells during chronic lymphocytic choriomeningitis virus (LCMV) infection and that the PD-1:PD-1 ligand (PDL) pathway plays a major role in regulating T-cell exhaustion during this infection (6). A transient blockade of the interaction between PD-1 and PDL in vivo using an anti-PD-L1 or PD-1 blocking antibody restored CD8 T-cell function and enhanced control of chronic LCMV infection. More recent studies have extended these observations to HIV-specific CD4 and CD8 T cells in HIV-infected individuals (9, 13, 30, 38). These studies demonstrate that the HIV-specific T cells express high levels of PD-1, and this expression is higher in individuals with high viremia. A transient blockade of interaction between PD-1 and PDL in vitro restores HIV-specific T-cell function (9, 38) and promotes survival of HIV-specific CD8 T cells (30). These results strongly suggest that in vivo blockade of the PD-1:PDL pathway may restore HIV-specific T-cell function and thus may represent a novel therapeutic strategy to enhance control of HIV/AIDS.
The PD-1:PDL pathway within the B7:CD28 superfamily consists of the PD-1 receptor and its two ligands, PD-L1 and PD-L2. Engagement of PD-1 by its ligands inhibits immune responses. PD-1 was isolated as a gene upregulated in a T-cell hybridoma undergoing apoptotic cell death, hence the name, programmed death 1 (28). PD-1 is inducibly expressed on CD4 T cells, CD8 T cells, NK T cells, B cells, and monocytes upon activation (reviewed in references 15 and 28). PD-1 transduces a signal when engaged along with the T-cell receptor (TCR) but does not transduce a signal when cross-linked alone, similar to other CD28 family members (14). The cytoplasmic domain of PD-1 contains two tyrosine-signaling motifs, both of which may be phosphorylated upon receptor engagement. Phosphorylation of the second tyrosine, an immunoreceptor tyrosine-based switch motif, recruits SHP-2 and to a lesser extent SHP-1 to the PD-1 cytoplasmic domain (25, 29). Recruitment of these phosphatases leads to dephosphorylation of TCR-proximal signaling molecules, including ZAP70, PKCθ, and CD3ζ, leading to attenuation of the TCR/CD28 signal. PD-1 signaling prevents CD28-mediated activation of phosphatidylinositol 3-kinase, resulting in reduced Akt phosphorylation and glucose metabolism.
While the studies in HIV-infected people were mostly cross-sectional and conducted during the chronic phase of infection (>3 months), the kinetics of modulation of PD-1 expression on virus-specific CD8 T cells during the acute phase of infection (<3 months) is not known. Furthermore, all these studies have studied PD-1 expression on HIV-specific CD8 T cells in blood, and there are no data available on the effect of viremia on the virus-specific CD8 T cells that are present in lymph nodes and gut mucosal tissue that represent the preferential sites of viral replication (31). In addition, we submit that in light of the recent adverse events with an immunomodulatory antibody to CD28 (36), it will be imperative to thoroughly evaluate the safety and therapeutic efficacy of in vivo blockade of the PD-1:PDL immunomodulatory pathway using an appropriate subhuman primate model before this approach can be tested in HIV-infected people.
Here, using a macaque/SIV model, we studied the temporal expression of PD-1 on SIV Gag-specific CD8 T cells in blood, lymph nodes, and rectal mucosal tissue following infection with pathogenic SIV239 or SHIV. We also study the temporal expression of PD-1 on SIV-specific CD8 T cells in blood after vaccination with a replication-defective DNA/modified vaccinia virus Ankara (DNA/MVA) vaccine and after a pathogenic SHIV challenge of these vaccinated macaques to understand the relationship between PD-1 expression and viral control. In addition, we performed in vitro studies to evaluate the effect of blockade of the PD-1:PDL pathway on the function of SIV-specific CD8 and CD4 T cells during chronic infection.
MATERIALS AND METHODS
Study group.
Three groups of Indian rhesus macaques were studied: unvaccinated SIV-infected macaques, unvaccinated SHIV 89.6P-infected macaques, and DNA/MVA-vaccinated SHIV 89.6P-challenged macaques. Most of these macaques were described before (2-4, 34), and the information relevant to this study is outlined in Table 1. All macaques selected for this study express the macaque major histocompatibility complex I molecule Mamu A*01, and the selection was based on the availability of cells for analyses. These macaques were housed at the Yerkes National Primate Research Center and were cared for under guidelines established by the Animal Welfare Act and the NIH Guide for the Care and Use of Laboratory Animals using protocols approved by the Emory University Institutional Animal Care and Use Committee.
TABLE 1.
Study groups: vaccinations, challenge infections, and macaque ID numbers
| Group | Treatmenta (reference) | Macaque ID nos. |
|---|---|---|
| Unvaccinated, SIV infected | SIV239, i.v., 1,000 TCID50 (unpublished) | RCv7, RHr7, RNr7, RYi7, RGt9, RZr9, RJu9, REt9 |
| SIV251, intrarectal (unpublished) | RPw9, RQj9, RRk10, RYd9 | |
| Unvaccinated, SHIV 89.6P infected | SHIV 89.6P, intrarectal, 20 monkey ID50 (unpublished) | ROj8, RWf9, RQj8, RKe8, RSn9, REb9 |
| SHIV 89.6P (2, 4) | RGy5, RJn6 | |
| DNA/MVA vaccinated | DNA prime MVA boost expressing SHIV 89.6 Env (4) and/or SIV Gag-Pol (3) | RBr5, RQf5, RAj5, RJi5, RAl5, RDe5 |
| DNA prime MVA boost expressing SIV Gag-Pol and Env (unpublished) | ROw7, RLk7, RNi7, RGd8, RLs8 | |
| DNA/MVA vaccinated, SHIV 89.6P infected | DNA prime MVA boost, SHIV 89.6P, intrarectal, 20 monkey ID50 (4) | RBr5, RQf5, RAj5, RJi5, RAl5, RDe5, RIm5, RIi5, RKw5 |
| DNA prime MVA boost, SHIV 89.6P, intrarectal, 20 monkey ID50 (3) | ROv4, RAv4, RQk5, RDV4 |
Abbreviations: i.v., intravenous; TCID50, 50% tissue culture infective dose; ID50, 50% infectious dose.
Isolation of cells from blood, lymph nodes, and rectal biopsies.
Peripheral blood mononuclear cells (PBMC) from blood were isolated according to standard procedures as described before (3). Lymphocytes from peripheral lymph nodes were isolated as described before (4). Lymphocytes from pinch biopsies from the rectum were obtained by digestion with collagenase followed by a separation step using percoll gradients as described before (12). Briefly, 10 to 20 pinch biopsies were collected in complete RPMI and washed two times with ice-cold Hanks' balanced salt solution. Biopsies were digested with 200 units/ml of collagenase IV (Worthington, Lake wood, NJ) and DNase I (Roche, Indianapolis, IN), passed through decreasing sizes of needles (16-, 18-, and 20-gauge, five to six times with each needle), and filtered through a 100-μm filter. Cells were suspended in 35% percoll, underlayed with 60% percoll, and centrifuged at 2,500 rpm for 30 min. Cells from the interface were collected, washed, and resuspended in complete RPMI for analysis.
Measurement of T-cell responses.
For tetramer analyses, approximately 1 × 106 PBMC were surface stained with antibodies to CD3 (clone SP34-2; BD Biosciences, San Diego, CA), CD8 (SK1; Becton Dickinson, San Jose, CA), anti-human PD-1 (clone EH12) (10), and Gag-CM9 (CTPYDINQM)-Mamu-A*01 tetramer, each conjugated to different fluorochromes. For some experiments, polyclonal goat antibody against human PD-1 (AF1086; R&D Systems, Minneapolis, MN) was used. Comparative experiments using the monoclonal and polyclonal antibodies yielded very similar patterns of staining (data not shown). Following staining, cells were acquired using either a FACScalibur or LSRII apparatus (BD Biosciences, San Jose, CA). Initial experiments used an isotype control antibody (mouse immunoglobulin G1) to determine PD-1-positive and -negative cells. For proliferation assays, PBMC were prestained with carboxyfluorescein diacetate succinimidyl ester (CFSE) as described before (32), and approximately 1 × 106 PBMC were stimulated in 24-well plates in a volume of 1 ml in RPMI containing 10% human serum at 37°C under 5% CO2 for 6 days. Cells were stimulated with P11C peptide at a final concentration of 0.1 μg/ml or pooled peptides spanning the entire SIV Gag protein (single pool of 125 peptides; catalog number 6204; NIH AIDS Research and Reference Reagent Program) at a concentration of 1.0 μg/ml. Unstimulated cells in the presence and absence of blocking antibody served as negative controls. Where blocking antibody was used, duplicate cultures were set up and anti-human PD-1 antibody (clone EH12) was added to a final concentration of 10 μg/ml 30 min before the addition of the stimulus. Recombinant human interleukin-2 (Roche, Indianapolis, IN) was added on day 3 to a final concentration of 100 units/ml. At the end of 6 days in culture, the cells were stained on the surface for CD3-phycoerythrin, CD8-peridinin chlorophyll a protein, and Gag-CM9 tetramer-allophycocyanin, acquired on a FACSCalibur, and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). In some experiments, the cells were permeabilized and stained for intracellular perforin using anti-perforin-fluorescein isothiocyanate (clone Pf-344; MABTECH, Cincinnati, OH) and granzyme B-A700 (clone GB11; BD Biosciences, San Diego, CA). For these experiments, cells were not prestained with CFSE and acquired on LSRII.
Quantitation of SIV and SHIV copy number.
The SIV or SHIV copy number was determined using a quantitative real-time PCR as previously described (4, 19). All specimens were extracted and amplified in duplicates, with the mean results reported.
Statistical analyses.
The Wilcoxon rank sum test was used for comparison of PD-1-positive cells in blood of control versus vaccinated groups, because data failed to meet the normality assumption. The Bonferroni method was used to adjust P values for multiple comparisons. All the reported P values are after adjustments. The Student t test was used for comparison of PD-1-positive cells in tissues. A matched paired Student t test was used for comparison within the group. A two-sided P value of <0.05 was considered statistically significant. Spearman's rank correlation test was used to assess the relationship between viral load and percentage of PD-1-positive cells. Statistical analyses were performed using software programs SAS 9.1 and S-PLUS 7.0.
RESULTS
PD-1 expression is elevated on SIV Gag-specific CD8 T cells following pathogenic SIV or SHIV infection.
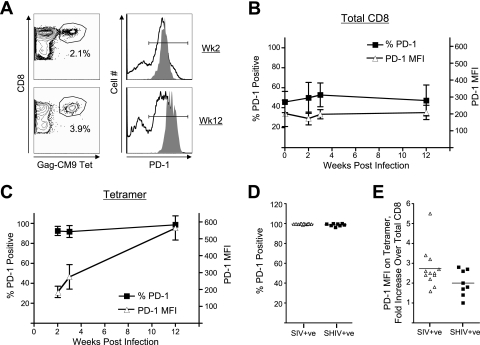
We studied the temporal expression of PD-1 on total and SIV-specific CD8 T cells at various times during the acute phase following infection with SIV239 in macaques to understand the role of PD-1 expression and its relationship with viremia. Prior to infection, a significant proportion (40 to 50%) of total CD8 T cells expressed PD-1 (Fig. 1A and B). PD-1 expression was predominantly restricted to memory cells and was absent on naïve CD8 T cells (data not shown). Following infection, the frequency of PD-1-positive total CD8 T cells did not change significantly over the initial 12 weeks (Fig. 1B). A similar pattern was also observed for the mean fluorescence intensity (MFI) of PD-1 expression on these CD8 T cells in blood. In contrast to PD-1 expression on total CD8 T cells, at 2 weeks after SIV infection the majority (>97%) of SIV Gag CM9 tetramer-specific CD8 T cells were positive for PD-1 expression (Fig. 1A and C). These cells not only remained PD-1 positive over the initial 2 weeks postinfection, but also the density of PD-1 expression per cell (as measured by the MFI) increased two- to threefold from 2 to 12 weeks (Fig. 1C). A similar pattern was also observed for macaques infected with pathogenic SHIV 89.6P (Fig. 1D and E). These results demonstrate that the majority of SIV-specific CD8 T cells express PD-1 following a pathogenic SIV or SHIV infection, and the expression of this marker on these antigen-specific cells increases with time.
FIG. 1.
PD-1 expression on total and SIV Gag-specific CD8 T cells following pathogenic SIV or SHIV infection. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from a SIV251-infected macaque. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells (CD3+, CD8+) and tetramer-positive cells were analyzed for expression of PD-1. The open and filled histograms represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. The numbers on the FACS plots represent the frequency of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of frequency of PD-1-positive cells and the MFI of PD-1 on total CD8 T cells at various times following SIV251 infection. (C) Summary of frequency of PD-1-positive cells and the MFI of PD-1 on tetramer-positive cells at various times following SIV251 infection. The data represent the mean values for four macaques. Error bars represent standard deviations. (D) Summary of the frequency of PD-1-positive cells as a percentage of tetramer-positive CD8 T cells at 12 weeks following infection. (E) Fold increase in the MFI of PD-1 on Gag-CM9 tetramer-positive cells over the MFI for total CD8 T cells in respective macaques at 12 weeks after infection. Each symbol represents an individual macaque.
Higher frequencies of PD-1-positive CD8 T cells in lymph nodes and rectal mucosal tissue compared to blood in SHIV-infected macaques.
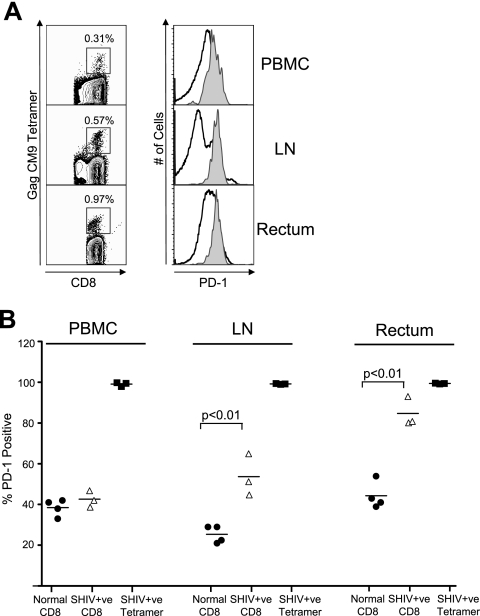
We next studied expression of PD-1 on total and SIV-specific CD8 T cells in lymph nodes and rectal tissue, the two major sites of SIV and HIV replication during the chronic phase of infection (31). These analyses were performed on four normal uninfected and three SHIV 89.6P-infected macaques (Fig. 2). In normal macaques about 20 to 30% of total CD8 T cells in the lymph nodes expressed PD-1. This frequency was lower than the frequency of PD-1-positive CD8 T cells in blood in these macaques (Fig. 2B). However, the frequency of PD-1-positive CD8 T cells in the rectal mucosal tissue was similar to the frequency of PD-1-positive cells in blood (Fig. 2B). In SHIV-infected macaques about 50% of the total CD8 T cells in lymph nodes and 80% of the total CD8 T cells in rectal mucosal tissue expressed PD-1 (Fig. 2B). These levels in the SHIV-infected macaques were significantly higher than the levels observed in the normal macaques at these sites (P < 0.01). Similar to blood, the majority (>99%) of SIV Gag-CM9 tetramer-specific CD8 T cells in lymph nodes and rectal mucosal tissue expressed PD-1. However, the MFI of PD-1 expression on tetramer-specific CD8 T cells in these tissue was about twofold higher than the MFI of tetramer-specific cells in blood of respective macaques (Fig. 2A and data not shown). These results demonstrate that PD-1 expression is markedly higher on SIV-specific CD8 T cells in lymph nodes and rectal mucosal tissue compared to blood. They also suggest that the pathogenic SHIV infection increases the frequency of PD-1-positive total CD8 T cells in lymph nodes and rectal mucosa but not in blood of infected macaques.
FIG. 2.
PD-1 expression on total and SIV Gag-specific CD8 T cells in lymph node and rectal mucosal tissue. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total (open histograms) and SIV Gag CM9 tetramer-specific (filled histograms) CD8 T cells in blood, lymph node, and rectal mucosal tissue from a SHIV-infected macaque. Cells were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The numbers on the FACS plots represent the frequencies of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of PD-1 expression on total and tetramer-positive CD8 T cells in blood, peripheral lymph node, and rectal mucosal tissue from normal and SHIV-infected macaques. For SHIV-infected macaques, analysis was performed at 12 weeks after infection. Each symbol represents an individual macaque.
Enhanced proliferation of SHIV-specific CD8 T cells following in vitro blockade of the PD-1:PDL pathway.
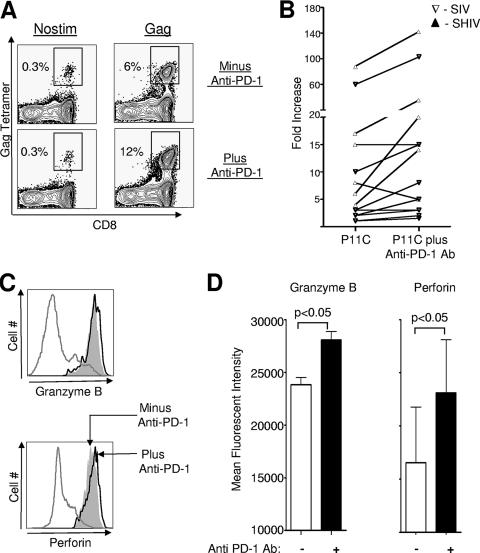
We next performed in vitro experiments to evaluate the effect of blockade of the PD-1:PDL pathway on the proliferative capacity of SIV- and SHIV-specific CD8 T cells. PBMC obtained at 12 weeks after infection were used for these analyses. Blocking experiments were performed using a blocking antibody to PD-1. Stimulation with P11C peptide resulted in a 5- to 90-fold enhancement in the frequency of Gag-CM9 tetramer-positive cells (Fig. 3A and B). Blockade of PD-1 resulted in further enhancement in the proliferative capacity of these SIV-specific CD8 T cells in 8 out of the 14 macaques tested (Fig. 3A and B). This enhancement ranged from two- to threefold over the enhancement observed in the absence of blocking antibody. Similar enhancement was also observed using a combination of antibodies to PD-L1 and PD-L2 (data not shown). No proliferation was observed in the absence of P11C peptide and the presence of blocking antibody alone (Fig. 3A and data not shown). The majority (>95%) of Gag-CM9 tetramer-positive cells that was present at the end of stimulation with P11C peptide expressed granzyme B and perforin (Fig. 3C). However, the MFI for granzyme B and perforin on tetramer-positive cells was marginally higher in the presence of blocking antibody (P < 0.05) (Fig. 3C and D). These results demonstrate that in vitro blockade of the PD-1:PDL pathway enhances the proliferative capacity of SIV-specific CD8 T cells from macaques chronically infected with a pathogenic SIV or SHIV.
FIG. 3.
Effect of in vitro blockade of PD-1 on SIV-specific CD8 T cells in SIV- or SHIV-infected macaques. (A) Fluorescence-activated cell sorter plots representing the frequency of Gag-CM9 tetramer-positive cells following stimulation in vitro. PBMC were prestained with CFSE and stimulated for 6 days with P11C peptide in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) in the presence and absence of blocking antibody served as negative controls. At the end of 6 days, cells were stained on the surface for CD3, CD8, and Gag-CM9 tetramer, acquired on a FACSCalibur, and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). Cells were gated on CD3 and analyzed for expression of CD8 and tetramer binding. The numbers on the plots represent the frequency of tetramer-positive cells as a percentage of total CD8 T cells. (B) Summary of proliferation data for SIV- or SHIV-infected macaques. Fold increase (frequency of tetramer-positive cells in stimulated cultures over unstimulated cultures) in the frequency of tetramer-positive cells is plotted for each macaque. Each symbol represents an individual macaque. (C) Granzyme B and perforin expression on tetramer-positive cells following in vitro stimulation in the presence and absence of blocking antibody. PBMC were stimulated for 6 days with P11C peptide in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) served as negative controls. At the end of 6 days cells, were stained on the surface for CD3, CD8, and Gag-CM9 tetramer. Cells were then fixed, permeabilized, and stained for intracellular perforin and granzyme B and acquired on an LSRII apparatus. Cells were gated on CD3, CD8, and tetramer and analyzed for expression of granzyme B or perforin. The gray filled histograms and black open histograms represent expression on P11C-stimulated cells in the absence and presence of anti-PD-1 blocking antibody, respectively. The gray open histograms represent expression on total CD8 T cells in unstimulated cultures. (D) Summary of granzyme B and perforin data for three SHIV-infected macaques.
Low levels of PD-1 expression on SIV-specific memory CD8 T cells elicited via immunization.
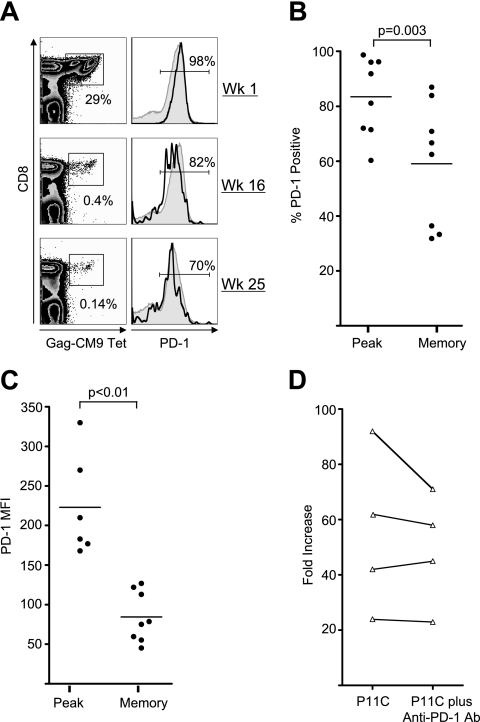
We next studied the temporal expression of PD-1 on SIV Gag-specific CD8 T cells at various times following vaccination to understand the relationship between antigen persistence and PD-1 expression in macaques. PD-1 expression on the SIV Gag CM9 tetramer-specific CD8 T cells was measured in a cohort of macaques that were vaccinated with a replication-defective DNA/MVA vaccine expressing SIV gag-pol and HIV env. PD-1 expression was measured at the peak (1 week after the MVA boost) and memory phases (2 to 6 months after the MVA boost) following vaccination. As shown in Fig. 4, the majority (60 to 98%, with a mean of 83% for the group) of vaccine-elicited peak effector Gag-specific CD8 T cells expressed PD-1. These levels were lower than the levels observed on SIV-specific CD8 T cells during chronic SIV or SHIV infection (Fig. 1). However, a lower proportion of memory cells (30 to 87%, with a mean of 59%) expressed PD-1 than the peak effector cells (P = 0.003) (Fig. 4A and B). Consistent with their PD-1 expression levels, in vitro blockade of PD-1 did not enhance the proliferative capacity of SIV Gag-specific memory cells in four out of the four tested macaques (Fig. 4C). These results demonstrate that the majority of SIV Gag-specific CD8 T cells elicited by a DNA/MVA vaccine express PD-1 during the peak effector phase, but a significant proportion of them downregulate PD-1 expression as they differentiate into memory.
FIG. 4.
PD-1 expression on total and SIV Gag-specific CD8 T cells following vaccination with a DNA/MVA SIV vaccine. (A) Fluorescence-activated cell sorter plots representing the expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from a macaque vaccinated with DNA/MVA vaccine. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The gray filled histograms and black open histograms represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. (B) Summary of PD-1 expression on tetramer-positive CD8 T cells at the peak (1 week) and memory (6 months) phases following the MVA boost. (C) Summary of MFI of PD-1 on tetramer-positive CD8 T cells at the peak (1 week) and memory (6 months) phases following the MVA boost. (D) Effect of in vitro blockade of PD-1 on the proliferative capacity of SIV Gag CM9 tetramer-specific memory cells. Cells were stimulated, stained, and analyzed as described for Fig. 3. Fold increase (frequency of tetramer-positive cells in stimulated cultures over unstimulated cultures) in the frequency of tetramer-positive cells is plotted for each macaque. Each symbol represents an individual macaque.
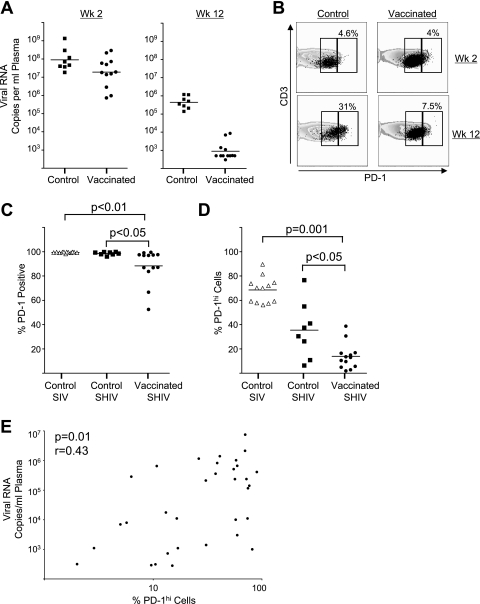
High level of PD-1 expression per cell correlates directly with viremia following pathogenic SHIV challenge.
We evaluated the PD-1 expression on SIV Gag-specific CD8 T cells in unvaccinated and DNA/MVA-vaccinated macaques following pathogenic SHIV infection to understand the relationship between PD-1 expression and viral control. Macaques challenged with SHIV 89.6P were used for these analyses. At 2 weeks following challenge, both vaccinated and unvaccinated macaques had high levels of viremia. Vaccinated macaques rapidly controlled the virus by 8 to 12 weeks, while unvaccinated controls failed to control viremia (Fig. 5A) (3, 4). PD-1 expression was measured at 2 and 12 weeks after the SHIV challenge. At 2 weeks following challenge, the majority (>95%) of SIV Gag CM9 tetramer-specific CD8 T cells from both vaccinated and unvaccinated macaques were positive for PD-1 expression (Fig. 5B and C). At this time only a small proportion of them (<5%) expressed elevated levels of PD-1 (PD-1hi) in both groups (Fig. 5B). However, by 12 weeks after challenge a marked difference was observed between vaccinated and unvaccinated monkeys: while most SIV Gag-specific cells expressed PD-1 in all monkeys, a significant difference in the frequencies of PD-1 expression was noted between vaccinated versus unvaccinated infected animals (Fig. 5C). In addition, more readily apparent was a markedly higher proportion of antigen-specific CD8 T cells that showed elevated expression of PD-1 (PD-1hi) in macaques that failed to control the challenge infection, whereas such PD-1hi cells were less frequent in vaccinated macaques (P < 0.05) (Fig. 5D). In 6 out of the 8 unvaccinated SHIV-infected macaques, greater than 25% of the total PD-1-positive Gag tetramer-specific cells were PD-1hi, whereas this was true for only 2 out of the 13 vaccinated SHIV-infected macaques. A similar situation was also observed in unvaccinated SIV-infected macaques. A significant positive correlation was observed between the frequency of PD-1hi cells and plasma viremia (P = 0.01; r = 0.43) (Fig. 5E). These results clearly demonstrate that the virus-specific CD8 T cells further elevate PD-1 expression in the presence of high levels of persistent viremia, and the level of PD-1 expression per cell rather than just PD-1 expression correlates with loss of viral control.
FIG. 5.
PD-1 expression following SHIV challenge in unvaccinated and DNA/MVA-vaccinated macaques. (A) Viral RNA levels in plasma following SHIV challenge. (B) Fluorescence-activated cell sorter plots representing expression of PD-1 on total and SIV Gag CM9 tetramer-specific CD8 T cells in blood from unvaccinated (control) and DNA/MVA-vaccinated macaques at 2 and 12 weeks after SHIV 89.6P challenge. PBMC were stained on the surface with antibodies to human CD3, CD8 and PD-1, and SIV Gag-CM9 tetramer. CD8-positive cells and tetramer-positive cells were analyzed for expression of PD-1. The contour plots and black dots represent PD-1 expression on total and tetramer-specific CD8 T cells, respectively. The boxes on the plots represent PD-1lo (left) and PD-1hi (right) cells. The numbers on the plots represent the frequency of PD-1hi cells as a percentage of total tetramer-positive cells. (C) Summary of the frequency of PD-1-positive cells as a percentage of tetramer-positive CD8 T cells at 12 weeks following SIV or SHIV infection in unvaccinated SIV-infected (control SIV), unvaccinated SHIV-infected (control SHIV), and DNA/MVA-vaccinated SHIV-infected (vaccinated SHIV) macaques. (D) Summary of frequency of PD-1hi cells on tetramer-positive CD8 T cells at 12 weeks after SIV or SHIV infection. (E) Correlation between the frequency of PD-1hi cells and plasma viral load at 12 weeks after infection in unvaccinated SIV-infected, unvaccinated SHIV-infected, and DNA/MVA-vaccinated SHIV-infected macaques. The MFI of PD-1 on tetramer-positive cells could not be used for comparisons between groups because these analyses were performed using different batches of antibody that resulted in differences in staining intensity. Each symbol represents an individual macaque.
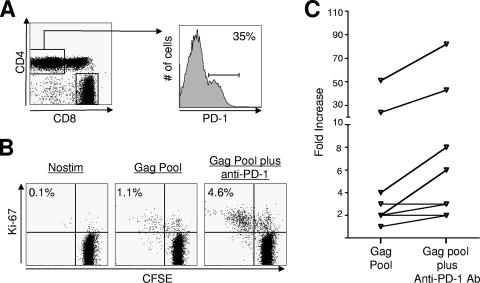
Enhanced proliferation of SIV-specific CD4 T cells following in vitro blockade of the PD-1:PDL pathway.
We next performed in vitro blockade experiments to evaluate the effect of blockade of the PD-1:PDL pathway on the proliferative capacity of SIV-specific CD4 T cells. PBMC obtained at 12 weeks after SIV infection were used for these analyses. A significant proportion of total CD4 T cells from SIV-infected macaques expressed PD-1 (Fig. 6A). Stimulation with a peptide pool specific for SIV Gag resulted in proliferation of Gag-specific CD4 T cells, and blockade of PD-1 enhanced the proliferative capacity of these cells in six out of the eight macaques tested (Fig. 6B and C). This enhancement ranged from two- to threefold over the proliferation observed in the absence of blocking antibody. These results demonstrate that in vitro blockade of the PD-1:PDL pathway enhances the proliferative capacity of SIV-specific CD4 T cells from macaques chronically infected with a pathogenic SIV.
FIG. 6.
Effect of in vitro blockade of PD-1 on SIV-specific CD4 T cells in SIV-infected macaques. (A) Fluorescence-activated cell sorter (FACS) plots representing the expression of PD-1 on total CD4 T cells in blood from a SIV-infected macaque. PBMC were stained on the surface with antibodies to human CD3, CD4, CD8, and PD-1. CD3+, CD4+, and CD8− cells were analyzed for expression of PD-1. (B) FACS plots representing the frequency of Gag-specific CD4 T cells following stimulation in vitro. PBMC were prestained with CFSE and stimulated for 6 days with Gag peptide pool in the absence and presence of anti-PD-1 blocking antibody. Unstimulated cells (Nostim) served as negative controls. At the end of 6 days cells were stained on the surface for CD3, CD8, and intracellular Ki-67, acquired on a FACSCalibur, and analyzed using FlowJo software (Treestar, Inc., San Carlos, CA). CD4 cells (CD3+, CD8−) were analyzed for CFSE dilution and Ki-67 expression. The numbers on the plots represent the frequency of CFSE−, Ki-67+ cells as a percentage of total CD4 T cells. (C) Summary of proliferation data for SIV-infected macaques. Fold increase (frequency of CFSE-negative, Ki-67-positive cells in stimulated cultures over unstimulated cultures) in the frequency of Gag-specific cells is plotted for each macaque. Each symbol represents an individual macaque.
DISCUSSION
As described above, there is compelling evidence that chronic LCMV and HIV infections cause upregulation of PD-1 on virus-specific T cells and that blockade of the PD-1:PD-1 ligand pathway markedly improves T-cell function in vivo and in vitro, respectively. Availing the unique model of pathogenic lentiviral infection in nonhuman primates, we were able to evaluate the temporal expression of PD-1 on SIV-specific T cells during acute and various stages of chronic SIV infection not only in blood but also at sites of preferential virus replication in the macaque model of AIDS. This enabled us to understand the kinetics and level of PD-1 expression on virus-specific T cells at different anatomic locations in the body and the relationship between PD-1 expression and viral control. Our results demonstrate that immediately following SIV infection, the majority of SIV Gag-specific CD8 T cells express PD-1 and further enhance the density of PD-1 expression per cell over time. In addition, the level of PD-1 expression on SIV-specific CD8 T cells in lymph node and gut mucosal tissue was markedly higher compared to the level on SIV-specific CD8 T cells in blood. Since lymph nodes and gut are the major sites for replication during chronic SIV and HIV infections (31), these results demonstrate a clear correlation between the density of PD-1 expression on virus-specific CD8 T cells and the site of predominant virus replication and likely point to a potential functional relation.
Furthermore, the frequency of PD-1-positive total CD8 T cells in blood did not increase significantly following SIV infection. Similarly, the frequency of PD-1-positive total CD8 T cells in blood did not differ between healthy uninfected and SIV- or SHIV-infected macaques (data not shown). However, the frequency of these cells in lymph nodes and rectal mucosal tissue was significantly higher in SHIV-infected macaques than in uninfected macaques. These results demonstrate that large numbers of PD-1-positive total CD8 T cells accumulate at sites of preferential virus replication but not in blood following SHIV infection as well, suggesting that these results were not restricted to specific lentiviral isolates but a generalized finding of pathogenic lentiviral infection. The presence of high levels of PD-1-positive total CD8 T cells in lymph nodes and rectal mucosal tissue could be due to hyperimmune activation of these cells at these sites.
The potential functional implication of this PD-1 upregulation was investigated, and the blockade of the PD-1:PDL pathway in vitro unequivocally enhanced the proliferative capacity of virus-specific CD8 T cells, demonstrating that the PD-1:PDL inhibitory pathway may be operational during chronic SIV infection. In vitro blockade of the PD-1:PDL pathway not only resulted in enhanced proliferation of SIV-specific CD8 cells but resulted in higher levels of granzyme B and perforin expression by these proliferating cells, suggesting that these cells may have better cytolytic potential. These results strongly suggest that the PD-1:PDL pathway may play an important role in regulating CD8 T-cell dysfunction during pathogenic SIV infection in macaques. Our results in SIV- and SHIV-infected macaques are similar to those that have recently been reported in HIV-infected people (9, 30, 38). Collectively, these results strongly suggest that in vivo blockade of the PD-1:PDL pathway may enhance the frequency and functional quality of HIV/SIV-specific CD8 T cells that may provide therapeutic benefit. Based on the higher levels of PD-1 expression on CD8 T cells in lymph node and gut mucosal tissue than in blood, our results also suggest that the inhibitory effects of the PD-1:PDL pathway may be more pronounced on virus-specific CD8 T cells that reside at these nonsystemic compartments, and our data underscore the added difficulty in having to deliver potential inhibitors of this pathway in vivo to these sites in addition to systemic compartments.
The mechanistic implications of PD-1 upregulation were highlighted further by the recognition that SIV Gag CM9 tetramer-specific CD8 T cells elicited by a replication-defective DNA/MVA vaccine, unlike similar cells analyzed during chronic infection, failed to exhibit such sustained and marked expression of PD-1. These results suggest that antigen persistence does play a role in this phenomenon and elimination of such high antigenemia results in a decrease of PD-1 expression on antigen-specific memory T cells. Interestingly, though, this downregulation was a slow process and occurred over several months, although MVA is known to persist only for a short period of time (16). These results demonstrate that SIV-specific CD8 T cells express PD-1 following exposure to antigen, and the level of antigen persistence determines the frequency of PD-1-positive cells as well as the density of PD-1 expression per cell. Following vaccination, as the antigen is being cleared, antigen-specific CD8 T cells downregulated PD-1 expression, whereas following infection, under continued persistence of antigen, they further enhanced PD-1 expression. Consistent with the level of PD-1 expression, in vitro blockade of the PD-1:PDL pathway enhanced the proliferative capacity of virus-specific CD8 T cells following chronic infection but not following vaccination. Thus, our results strongly suggest that the level of PD-1 expression per cell rather than the presence or absence of PD-1 expression may determine the inhibitory effects of the PD-1:PDL pathway.
Temporal expression studies following SHIV challenge in unvaccinated and DNA/MVA-vaccinated macaques revealed that soon after challenge (week 2) the majority of Gag-specific CD8 T cells express PD-1. However, the level of PD-1 expression on these cells was further elevated (PD-1hi) only in those macaques that failed to control the challenge infection by 6 to 12 weeks. A significant positive correlation was observed between the frequency of PD-1hi cells and plasma viremia but not between the frequency of total tetramer-specific PD-1-positive cells and plasma viremia (data not shown). These results again confirm the results that were obtained following chronic SIV infection, clearly demonstrating that in the presence of persisting levels of viral antigen/infection virus-specific CD8 T cells elevate the density of PD-1 expression per cell.
Of interest was also the finding that CD4 T cells from SIV-infected macaques express PD-1, and in vitro blockade of the PD-1:PDL pathway restores the function of SIV-specific CD4 T cells. These results suggest that in vivo blockade of the PD-1:PDL pathway may increase the frequency of virus-specific CD4 T cells and restore their function. Restoration of CD4 T-cell function during chronic SIV infection may have positive as well as negative effects. The enhanced CD4 help may enhance the CD8 T-cell function and affinity maturation of antibody that may help to improve control of virus replication. On the other hand, the enhanced CD4 response may provide more targets for virus replication, as HIV-specific CD4 T cells have been shown to be the preferential targets for virus replication (11). A balance of these positive and negative activities may determine the therapeutic benefit, and our results suggest that in vivo blockade approaches may need to be performed in combination with antiviral drug therapy.
In conclusion, our results demonstrate that the PD-1:PDL inhibitory pathway is operational during chronic SIV infection and suggest that in vivo blockade of the PD-1:PDL pathway may enhance the magnitude and functional quality of SIV-specific CD8 and CD4 T cells that may provide therapeutic benefit. They also suggest that the SIV/macaque model of AIDS would be an ideal model to test the safety and therapeutic benefit of in vivo blockade of the PD-1:PDL pathway. In addition, they suggest that the blockade may be more effective in the chronic rather than the acute phase of infection, e.g., between 6 and 12 weeks rather than 2 weeks after infection, by which time the level of PD-1 expression per cell is elevated.
Acknowledgments
We are indebted to Harriet Robinson for her support throughout these studies. We thank John Altman for providing Gag-CM9 tetramer and Emory Center for AIDS Research virology core for conducting viral load assays. We thank James G. Herndon for help with statistical analysis and Helen Drake-Perrow for outstanding administrative support. We are thankful to The Yerkes Division of Research Resources for the consistent excellence of pathology support. Also, we thank the NIH AIDS Research and Reference Reagent Program for the provision of peptides.
This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases grants R01 AI57029 to R.R.A., P01 AI49364 to H.R., RO1 HL075833 to F.V., P30 AI50409 to Emory Center for AIDS Research, and Yerkes National Primate Research Center base grant P51 RR00165 and by AI56299 and the Foundation for the NIH through the Grand Challenges in Global Health initiative to R.A. and G.J.F.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Akbar, A. N., N. J. Borthwick, R. G. Wickremasinghe, P. Panayoitidis, D. Pilling, M. Bofill, S. Krajewski, J. C. Reed, and M. Salmon. 1996. Interleukin-2 receptor common gamma-chain signaling cytokines regulate activated T cell apoptosis in response to growth factor withdrawal: selective induction of anti-apoptotic (bcl-2, bcl-xL) but not pro-apoptotic (bax, bcl-xS) gene expression. Eur. J. Immunol. 26:294-299. [DOI] [PubMed] [Google Scholar]
- 2.Amara, R. R., K. Patel, G. Niedziela, P. Nigam, S. Sharma, S. I. Staprans, D. C. Montefiori, L. Chenareddi, J. G. Herndon, H. L. Robinson, H. M. McClure, and F. J. Novembre. 2005. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides enhanced protection from simian/human immunodeficiency virus-induced disease. J. Virol. 79:15356-15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara, R. R., J. M. Smith, S. Staprans, D. Montefiori, F. Villinger, J. D. Altman, S. P. O'Neil, N. L. Kozyr, Y. Xu, L. Wyatt, P. L. Earl, J. G. Herndon, J. M. McNicholl, H. M. McClure, B. Moss, and H. L. Robinson. 2002. Critical role for Env as well as Gag-Pol for the control of a pathogenic SHIV challenge by a DNA/rMVA vaccine. J. Virol. 76:6138-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H.-L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 5.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8+ T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 7.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 8.Casimiro, D. R., F. Wang, W. A. Schleif, X. Liang, Z. Q. Zhang, T. W. Tobery, M. E. Davies, A. B. McDermott, D. H. O'Connor, A. Fridman, A. Bagchi, L. G. Tussey, A. J. Bett, A. C. Finnefrock, T. M. Fu, A. Tang, K. A. Wilson, M. Chen, H. C. Perry, G. J. Heidecker, D. C. Freed, A. Carella, K. S. Punt, K. J. Sykes, L. Huang, V. I. Ausensi, M. Bachinsky, U. Sadasivan-Nair, D. I. Watkins, E. A. Emini, and J. W. Shiver. 2005. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J. Virol. 79:15547-15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. Depierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 10.Dorfman, D. M., J. A. Brown, A. Shahsafaei, and G. J. Freeman. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30:802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douek, D. C., J. M. Brenchley, M. R. Betts, D. R. Ambrozak, B. J. Hill, Y. Okamoto, J. P. Casazza, J. Kuruppu, K. Kunstman, S. Wolinsky, Z. Grossman, M. Dybul, A. Oxenius, D. A. Price, M. Connors, and R. A. Koup. 2002. HIV preferentially infects HIV-specific CD4+ T cells. Nature 417:95-98. [DOI] [PubMed] [Google Scholar]
- 12.Evans, D. T., L. M. Chen, J. Gillis, K. C. Lin, B. Harty, G. P. Mazzara, R. O. Donis, K. G. Mansfield, J. D. Lifson, R. C. Desrosiers, J. E. Galan, and R. P. Johnson. 2003. Mucosal priming of simian immunodeficiency virus-specific cytotoxic T-lymphocyte responses in rhesus macaques by the Salmonella type III secretion antigen delivery system. J. Virol. 77:2400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman, G. J., E. J. Wherry, R. Ahmed, and A. H. Sharpe. 2006. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J. Exp. Med. 203:2223-2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grakoui, A., E. John Wherry, H. L. Hanson, C. Walker, and R. Ahmed. 2006. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J. Hepatol. 45:468-472. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald, R. J., G. J. Freeman, and A. H. Sharpe. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515-548. [DOI] [PubMed] [Google Scholar]
- 16.Hanke, T., A. J. McMichael, M. J. Dennis, S. A. Sharpe, L. A. Powell, L. McLoughlin, and S. J. Crome. 2005. Biodistribution and persistence of an MVA-vectored candidate HIV vaccine in SIV-infected rhesus macaques and SCID mice. Vaccine 23:1507-1514. [DOI] [PubMed] [Google Scholar]
- 17.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 18.Hel, Z., J. Nacsa, E. Tryniszewska, W. P. Tsai, R. W. Parks, D. C. Montefiori, B. K. Felber, J. Tartaglia, G. N. Pavlakis, and G. Franchini. 2002. Containment of simian immunodeficiency virus infection in vaccinated macaques: correlation with the magnitude of virus-specific pre- and postchallenge CD4+ and CD8+ T cell responses. J. Immunol. 169:4778-4787. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann-Lehmann, R., R. K. Swenerton, V. Liska, C. M. Leutenegger, H. Lutz, H. M. McClure, and R. M. Ruprecht. 2000. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res. Hum. Retrovir. 16:1247-1257. [DOI] [PubMed] [Google Scholar]
- 20.Iyasere, C., J. C. Tilton, A. J. Johnson, S. Younes, B. Yassine-Diab, R. P. Sekaly, W. W. Kwok, S. A. Migueles, A. C. Laborico, W. L. Shupert, C. W. Hallahan, R. T. Davey, Jr., M. Dybul, S. Vogel, J. Metcalf, and M. Connors. 2003. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J. Virol. 77:10900-10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, P. R., B. C. Schnepp, M. J. Connell, D. Rohne, S. Robinson, G. R. Krivulka, C. I. Lord, R. Zinn, D. C. Montefiori, N. L. Letvin, and K. R. Clark. 2005. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 79:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalams, S. A., S. P. Buchbinder, E. S. Rosenberg, J. M. Billingsley, D. S. Colbert, N. G. Jones, A. K. Shea, A. K. Trocha, and B. D. Walker. 1999. Association between virus-specific cytotoxic T-lymphocyte and helper responses in human immunodeficiency virus type 1 infection. J. Virol. 73:6715-6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6:873-879. [DOI] [PubMed] [Google Scholar]
- 25.Latchman, Y., C. R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A. J. Long, J. A. Brown, R. Nunes, E. A. Greenfield, K. Bourque, V. A. Boussiotis, L. L. Carter, B. M. Carreno, N. Malenkovich, H. Nishimura, T. Okazaki, T. Honjo, A. H. Sharpe, and G. J. Freeman. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2:261-268. [DOI] [PubMed] [Google Scholar]
- 26.Metzner, K. J., X. Jin, F. V. Lee, A. Gettie, D. E. Bauer, M. Di Mascio, A. S. Perelson, P. A. Marx, D. D. Ho, L. G. Kostrikis, and R. I. Connor. 2000. Effects of in vivo CD8+ T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Exp. Med. 191:1921-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 3:1061-1068. [DOI] [PubMed] [Google Scholar]
- 28.Okazaki, T., and T. Honjo. 2006. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 27:195-201. [DOI] [PubMed] [Google Scholar]
- 29.Parry, R. V., J. M. Chemnitz, K. A. Frauwirth, A. R. Lanfranco, I. Braunstein, S. V. Kobayashi, P. S. Linsley, C. B. Thompson, and J. L. Riley. 2005. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol. Cell. Biol. 25:9543-9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson, T., J. McArthur, and R. F. Siliciano. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 18:665-708. [DOI] [PubMed] [Google Scholar]
- 32.Robinson, H. L., and R. R. Amara. 2005. T cell vaccines for microbial infections. Nat. Med. 11:S25-S32. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 34.Sadagopal, S., R. R. Amara, D. C. Montefiori, L. S. Wyatt, S. I. Staprans, N. L. Kozyr, H. M. McClure, B. Moss, and H. L. Robinson. 2005. Signature for long-term vaccine-mediated control of a simian and human immunodeficiency virus 89.6P challenge: stable low-breadth and low-frequency T-cell response capable of coproducing gamma interferon and interleukin-2. J. Virol. 79:3243-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan, C. 2006. TeGenero fiasco prompts regulatory rethink. Nat. Biotechnol. 24:475-476. [DOI] [PubMed] [Google Scholar]
- 37.Shiver, J. W., T. Fu, L. Chen, D. Casimiro, M. E. Davies, R. K. Evans, Z.-Q. Zhang, A. Simon, W. L. Trigona, S. Dubey, L. Huang, V. A. Harris, R. S. Long, L. Xiaoping, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Iospi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 38.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, G. Wang, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 39.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 78:5535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]