Abstract
Sizes of most kinds of animal groups vary considerably within a population, with group size often causing direct effects on the fitness of group members. Although the consequences of varying group size have been well studied, the causes of variation in group size remain poorly known for most animals. Groups might vary in size because different individuals perform better in differently sized groups and thus have genetic predispositions to choose large or small groups. We examined whether heritable variation for choice of group size exists in the cliff swallow (Petrochelidon pyrrhonota), a colonial bird that nests in colonies ranging from 2 to 3,700 nests. Parent–offspring regressions showed significant heritabilities for choice of colony size under natural conditions. Partial cross-fostering experiments showed that individuals reared in colonies of sizes different from those of their birth returned to breed the next year in colonies that matched their birth colony in size and actively avoided those similar to their rearing colony, suggesting that choice of colony size is genetically based. Common environmental effects, maternal effects, and philopatry did not explain these results. Variation in group size probably results in part from a polymorphism in genetic preferences within the population, and the range in colony sizes is maintained by natural selection on the type of bird occupying each site.
Virtually all social animals occur in groups of different sizes, and often the largest and smallest groups within a population differ in size by several orders of magnitude. Variation in group size has been observed in foraging groups, leks, extended family groups, migratory herds and flocks, communal roosts, and breeding colonies (1–9). This natural variation in group size has been used to measure consequences of sociality, such as reproductive or foraging success and predator avoidance through vigilance, and to infer the selective advantages of grouping (10–16). However, the consequences of variation in group size are better understood than the causes. Despite considerable interest in the evolution of social living, it remains unclear in general why individuals choose to live in groups of different sizes, and the few hypotheses to explain variation in group size (8) have rarely been tested.
Groups have been suggested to vary in size for three possible reasons. One possibility is that a single group size is best, but individuals are constrained from achieving this ideal by the behavior of animals that seek to join the group. When a group achieves the optimal size, it cannot maintain that size, because other individuals receive a greater payoff by joining that group and inflating it slightly above the optimum than by avoiding the group and settling as a solitary (17–18). This process continues until the group reaches a point at which fitness is higher for a potential joiner if it avoids the group and settles as a solitary. Such a scenario requires a variety of largely untested assumptions about how fitness varies with group size, the degree of relatedness among group members, whether settlement can be regulated by despotic behavior, and the extent of collective decision making by settlers (19–21); thus, whether constraints on group size in fact generate variation in size is unknown.
Another explanation for variation in group size is that group size reflects local variation in resource abundance. Habitat patches that are productive can support more individuals (larger groups) than those with scarce resources. Population density may be regulated among habitat patches through a variety of mechanisms, including classical ideal-free processes, despotic behavior among unequal competitors, attraction to conspecifics, and direct assessment of habitat quality (22–27). Only a few studies, however, have demonstrated a strong causal link between local resource abundance and degree of aggregation by resource users (28–31).
Variation in group size may also reflect inherent differences among animals. Some individuals by virtue of their size, condition, age, experience, or genetic makeup may be superior at competing for resources in large groups, and therefore, these individuals settle in habitat patches that can support more animals. Other individuals may be less capable of competing in larger groups, and they realize higher fitness by settling as solitaries or in small groups (32). Individuals may also have different cost-benefit expectations from a given group size. For example, experienced animals may have less to gain from the social advantages of large groups and more to lose from the automatic costs such as increased probability of encountering diseases or parasites (15), and consequently, they avoid dense concentrations of conspecifics. Although preference for group sizes could be a conditional strategy that depends on age or nonheritable variation in condition (31–33), preferences could also reflect heritable variation in an individual's ability to function in different social environments. One way to infer the likelihood that choice of group size is genetically based is by estimating the heritability of individuals' choices.
Here, we estimate the extent of heritable variation for choice of breeding-colony size in cliff swallows (Petrochelidon pyrrhonota) of western North America by using parent–offspring regressions and a partial cross-fostering experiment in which young were exchanged between colonies of different sizes for rearing and their subsequent breeding-colony choices were monitored. In our study area in southwestern Nebraska, cliff swallows breed in colonies that range from 2 to over 3,000 nests. This study is the first on any taxa, to our knowledge, that has measured heritability of group-size preference.
Methods
Our research was conducted along the North and South Platte Rivers centered near Ogallala, NE, in Keith and Garden counties, from 1982 to 1999. In this area, cliff swallows nested on artificial structures such as bridges and highway culverts and on natural cliff faces, where their gourd-shaped mud nests are built on a vertical wall underneath a horizontal overhang. The total study area was approximately 150 × 50 km, and mean (±SEM) colony size (n = 1,282) was 356.5 (± 16.3) nests, ranging from birds that nested solitarily to colonies of 3,700 nests (15). Colony size was the maximum number of nests at a site containing one or more eggs.
Breeding colonies of individuals were determined by repeatedly capturing birds during mark-recapture sampling at colony sites. We rotated among all accessible colonies (30–35 per season) and on repeated visits mist-netted cliff swallows at each. Nets were usually strung across the entrances of highway culverts or along the sides of bridges that contained swallow colonies, and the birds were caught as they exited their nests. In some cases, we captured individuals inside their nests at night by entering the colony after dark and plugging the nest entrances with cotton. All birds were banded with U.S. Fish and Wildlife Service bands. Nestlings were banded before fledging. Nestlings not part of the cross-fostering experiment were removed briefly from their nests at 10 days of age for banding and then promptly returned to their natal nests.
We performed a partial cross-fostering experiment consisting of exchanging newly hatched chicks with similarly aged chicks in colonies of a different size. In broods of even numbers (two or four chicks), we exchanged exactly half of the nestlings and left the other half to be reared in their natal nest. Siblings were kept together and fostered to the same nest whenever two or more nestlings from a single brood were exchanged. In broods of three chicks, we exchanged either one or two nestlings per brood, alternating between each three-chick brood, so as to achieve roughly similar numbers of chicks transferred and not transferred for the colony as a whole. In broods of five chicks, we alternated between two and three nestlings transferred per brood. We maintained the same brood sizes for rearing that each chick was hatched in. Nestlings were transferred at approximately 5 days of age, the youngest age at which they could be banded for permanent identification; the nestling period for cliff swallows in our study area was approximately 25–26 days. During transfer, nestlings were placed in a cardboard box with dividers that isolated each bird from others being transferred at the same time.
Settlement at colony sites by these birds as breeders in subsequent years was monitored through systematic mist-netting. Cliff swallow colonies were defined as groups of nesting pairs, usually on the same bridge or culvert, that interacted at least occasionally (15). For our analyses, we converted all colony sizes each year to ranks, with 1 being the largest colony available that season. We used ranks, because they account for between-year variation in the number of colonies available and their size distribution, both of which depend on annual population size. Using colony ranks allowed us to combine data across years. In addition, ranks are probably more biologically meaningful than actual colony sizes, because actual colony size is subject to change that is beyond an individual's control. For example, a bird may settle in a colony of its target size, but later arrivals may join the group, increasing its size, or other birds may decide to vacate the site, reducing its size. However, an individual probably has greater control over simply choosing a relatively large or relatively small colony among those available in a given season.
Ranks were computed separately for five clusters of colony sites in the study area. Radio-tracking studies of cliff swallows early in the season showed that birds generally confine their prospecting for sites to total linear distances of 3 to 15 km along the North Platte River Valley (15). Based on these observations and mark-recapture data showing that most swallows move between colonies that are ≤3.5 km apart, we defined clusters of colonies as those in which a bird's colony selection likely occurred. The colonies farthest apart within these clusters were ≤13 km from each other; the mean (±SEM) distance between adjacent colony sites within a cluster (n = 55) was 0.99 km (±0.13), and the mean (±SEM) distance between clusters (n = 5) at their closest points was 10.72 km (±6.87). In each case, clusters were separated by expanses of habitat that were uniformly unsuitable for nesting, and thus, clusters seemed to be naturally bounded. We designated five clusters in our main study area, a region extending approximately 80 km between Lewellen and Paxton, NE, along the North and South Platte Rivers. These clusters contained 11, 12, 15, 20, and 25 separate colony sites, respectively. We ranked sites within a cluster that had active colonies each year. Ranks ranged from 1 up to 21 in some clusters in some years.
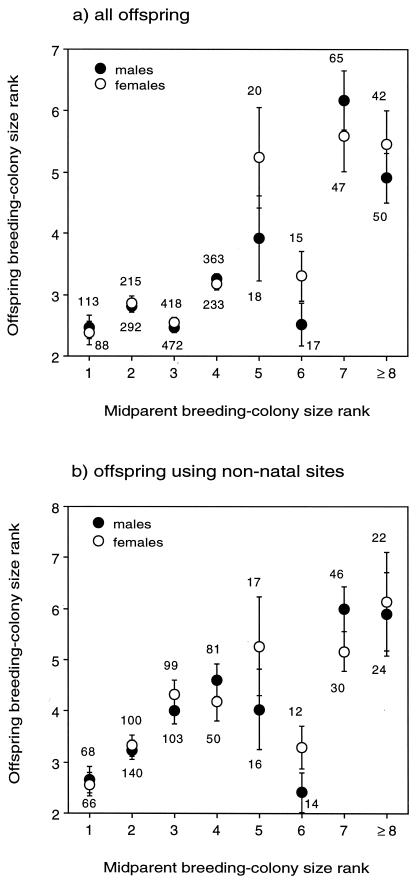
We included all five clusters in our mark-recapture sampling. Birds were caught at 72–88% of the colony sites in four of the clusters and at 45% of the colonies in the fifth (smallest) cluster. Our netting effort within a cluster was done roughly in proportion to the fraction of the total cliff swallow population contained there. This method allowed us to determine that most cliff swallows occupied the same cluster of colonies between years; among those birds banded as nestlings and whose breeding colonies were known in one or more subsequent years (Fig. 1; n = 2,581 birds), only 4.8% moved to a nonnatal cluster in at least 1 year as a breeder. This finding supported our assumption that a cluster in which a bird settled in a given year represented the set of colony sizes from which it chose that season.
Figure 1.
Midparent–offspring regression of breeding-colony size rank in cliff swallows for all offspring (a) and those that occupied only nonnatal colony sites (b). Means (±SEM) are shown; the numbers by dots (sample sizes) indicate number of offspring recaptured from each midparent rank.
We used linear parent–offspring regression (34, 35) to estimate heritability of colony-size decisions. When identities of both parent and offspring from a nest were known, we calculated both single-parent and midparent regressions; heritability for single-parent regression was taken to be twice the regression coefficient, and heritability for midparent regression was taken to equal the regression coefficient. Colony-size ranks for each bird (parent or offspring) were averaged over all seasons for which we knew the bird's breeding-colony size, and these average values per individual were used in analyses. When specific identities of parents were unknown, we considered the colony size that year to represent the midparent value for each offspring. We used Falconer's (34) method to weight families of unequal size. Sample sizes for the single-parent analyses that required known parents were smaller than for those that used natal-colony rank as the midparental value, because we had fewer nests with both mother and father identified and from which nestlings were encountered subsequently as breeders. Because different parents had been followed for different numbers of years and thus had potentially different average parental-colony ranks, we would not necessarily expect single-parent heritabilities using known parents to be identical for each sex or to match those computed by using only natal-colony rank as the midparent value.
Results
For nonexperimental birds banded as nestlings and encountered as breeders in 1 or more subsequent years (Fig. 1; n = 2581), a regression of their breeding-colony rank on that of their parents yielded midparent heritability estimates that differed significantly from zero for both males and females and for birds that always used their natal site as breeders and those that used only nonnatal sites (Table 1). There was close agreement between heritabilities for birds that were philopatric to the natal site and those that dispersed to nonnatal sites. For this reason, we combined both philopatric and dispersing birds for the single-parent heritability analyses (for which we had smaller sample sizes). Single-parent heritabilities tended to be larger than midparent heritabilities, with offspring–father heritabilities greater than offspring–mother heritabilities for each sex (Table 1). Thus, among nonmanipulated birds, offspring tended to select colony sizes as breeders similar to those of their parents regardless of whether they settled at their natal site (Fig. 1).
Table 1.
Heritability estimates (h2) for choice of breeding-colony size in cliff swallows
| Category of birds | h2 | SEM | P |
|---|---|---|---|
| Midparent, using offspring's natal-colony size as parental value | |||
| Male (n = 1,390)* | 0.346 | 0.027 | <0.0001 |
| Female (n = 1,078) | 0.377 | 0.032 | <0.0001 |
| Birds always using natal site (n = 1,654) | 0.369 | 0.020 | <0.0001 |
| Birds using only nonnatal sites (n = 927) | 0.326 | 0.036 | <0.0001 |
| Midparent, using parents' all known breeding-colony sizes | |||
| Male (n = 92) | 0.279 | 0.235 | 0.24 |
| Female (n = 58) | 0.553 | 0.216 | 0.013 |
| Single-parent, using parents' all known breeding-colony sizes | |||
| Male–father (n = 92) | 0.714 | 0.472 | 0.13 |
| Male–mother (n = 58) | 0.312 | 0.407 | 0.44 |
| Female–father (n = 92) | 1.131 | 0.349 | 0.002 |
| Female–mother (n = 58) | 0.642 | 0.474 | 0.018 |
| Midparent, 1997 cross-fostering, using offspring's natal-colony size as parental value | |||
| Males, not cross fostered, against birth colony (n = 143) | 0.470 | 0.107 | <0.0001 |
| Males, cross fostered, against birth colony (n = 130) | 0.536 | 0.102 | <0.0001 |
| Males, cross fostered, against rearing colony (n = 130) | −0.549 | 0.103 | <0.0001 |
| Females, not cross fostered, against birth colony (n = 114) | 0.345 | 0.116 | 0.003 |
| Females, cross fostered, against birth colony (n = 116) | 0.295 | 0.122 | 0.017 |
| Females, cross fostered, against rearing colony (n = 116) | −0.306 | 0.124 | 0.015 |
| Midparent, 1998 cross-fostering, using offspring's natal-colony size as parental value (sexes combined) | |||
| Not cross fostered, against birth colony (n = 87) | 0.375 | 0.061 | <0.0001 |
| Cross fostered, against birth colony (n = 88) | 0.179 | 0.072 | 0.015 |
| Cross fostered, against rearing colony (n = 88) | −0.117 | 0.068 | 0.086 |
Sample sizes differ in “Midparent, using offspring's natal-colony size as parental value,” because sex was unknown for some birds.
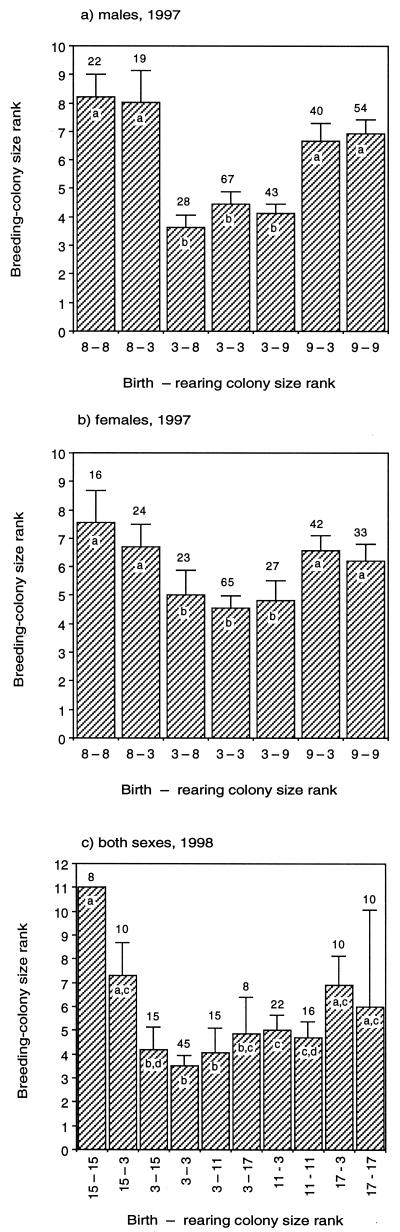
In a cross-fostering experiment performed from 1997 to 1998, we transferred nestlings between two large colonies (950 and 1,005 nests, each rank 3) and five small colonies (220, 193, 154, 98, and 55 nests, ranks 11, 8, 9, 15, and 17, respectively, in the different years). All transfers were made between colonies within the same cluster containing 25 total colony sites. In 1997, the two small colonies (193 and 154 nests) were 1.8 km apart, and they were 3.4 and 3.9 km from the large colony (950 nests), respectively. In 1998, the three small colonies were situated linearly along a railroad track, with 1.0 and 4.9 km separating them. They were 3.3, 3.4, and 5.6 km, from the large colony. We recaught 721 (36.6%) of the nestlings in the cross-fostering experiment (n = 1,968) as breeders in 1 or more subsequent years. Mean breeding-colony rank varied significantly with colony of birth but not with colony of rearing for both males and females from 1997 (Fig. 2 a and b) and both sexes combined from 1998 (Fig. 2c). Cross-fostered cliff swallows chose breeding colonies of size more similar to those where they were born than where they were reared; breeding-colony ranks of cross-fostered nestlings in most cases differed significantly from the ranks of birds born and raised at their foster colony but did not differ significantly from those of birds born and raised at their birth colony (Fig. 2).
Figure 2.
Breeding-colony size rank (mean ±SEM) chosen by cross-fostered cliff swallows of each sex in 1997 (a and b) and 1998 (c; sexes combined) in relation to rank of colony in which a bird was born and reared. The same rank for birth and rearing colonies are for control individuals that were not cross fostered. The numbers above bars (sample sizes) indicate number of birds from each treatment recaptured as breeders. Within each year, bars with the same letter denote means that did not differ significantly (Wilcoxon test, P > 0.10); bars with different letters denote means that differed significantly (Wilcoxon test, P ≤ 0.05). A two-way ANOVA revealed that birth–rearing colony combination had a significant effect on breeding-colony rank (F = 10.85, P < 0.001), but neither sex alone (F = 0.05, P = 0.82) nor an interaction between sex and birth–rearing colony (F = 0.69, P = 0.72) was significant.
We also examined the spatial settlement patterns of birds in the cross-fostering experiment. Among individuals that dispersed to nonnatal sites for breeding, birds born and raised at the same site moved to colonies that were a mean (±SEM) 3.07 km (±0.28) from their natal colony (n = 196) with the longest movement we detected being 46.3 km. Cross-fostered nestlings moved to colonies that were a mean (±SEM) 3.38 km (±0.28) from their birth colony (n = 216) with the longest movement being 41.7 km. This difference was not significant (Wilcoxon test, P = 0.21). This result illustrates that both classes of birds tended to settle in the same general area; most settled in the same cluster of their birth, and thus, both classes had the same set of colony sizes to choose from.
We estimated midparent heritabilities for birds in the cross-fostering experiment by regressing breeding-colony rank on birth- and rearing-colony rank for nestlings that were cross fostered and for those born and reared at the same site (Table 1). For birds born and raised at the same site, heritabilities were similar to those estimated from our larger sample of nonmanipulated birds (Table 1). Heritabilities estimated by regressing cross-fostered birds' breeding-colony ranks against those of their true parents (birth colony) were significantly positive and similar in magnitude to heritabilities estimated from birds not cross fostered (Table 1). A comparable analysis, in which we regressed cross-fostered birds' breeding-colony ranks against those of their foster parents (rearing colony), yielded significantly negative heritabilities (Table 1). This finding indicated an inverse relationship between cross-fostered birds' breeding- and rearing-colony ranks and illustrates that these birds actively avoided breeding colonies of rank similar to those in which they were fostered. These analyses (Fig. 2; Table 1) show that regardless of the colony size in which they were reared, cliff swallows chose breeding colonies that ranked approximately the same in size as their colony of birth.
Discussion
To show that choice of group size in cliff swallows is genetically based, we must rule out common environmental effects, maternal effects, and philopatry as explanations for our results. The cross-fostering experiment removed environmental effects by placing some birds in a different environment (foster colony) and comparing their choice of colony size to that of their siblings raised in the birth colony. We found that fostered birds chose colony sizes in the same manner as their genetic relatives despite the different rearing environments. Although fostering freshly laid eggs might have been better than fostering newly hatched nestlings to remove any potential environmental effect completely, we were constrained by the need to permanently mark (i.e., band) individuals before they mixed with their foster nest mates. It seems unlikely that newly hatched nestlings would be influenced by environmental effects (e.g., ambient noise) before fostering, given the enclosed nature of the cliff swallow's nest and the fact that no imprinting on social system for newly hatched altricial birds is known.
We also found little evidence for maternal effects. Single-parent regressions for both sexes revealed higher heritability with fathers than with mothers (Table 1). The opposite would have been expected if maternal effects had been important. Although the observed difference could in part reflect different parental histories for male parents versus female parents among the individuals used in the single-parent analyses, there is certainly no evidence that mother–offspring heritabilities were inflated enough, relative to father–offspring heritabilities, for maternal effects to be important.
The most likely alternative explanation for our results is natal philopatry. An association between parental and offspring breeding-colony ranks might occur if both classes of birds simply returned to the same colony site in successive years and colony size at a given site remained roughly similar from year to year. Although actual colony sizes at a site were not significantly repeatable between years (rI = 0.113, P = 0.30; intraclass correlation), yearly ranks at a site were significantly correlated (rI = 0.838, P < 0.001). Therefore, the same site tended to rank roughly the same between years, even though actual colony size might have differed from year to year.
However, two kinds of evidence suggest that philopatry does not explain our results. First, we found a similar heritability in choice of colony-size rank when confining the analysis only to dispersing nonphilopatric birds that used nonnatal sites (Fig. 1; Table 1). The estimated heritability for dispersing birds suggests that colony rank often figures in their decision. Second, had philopatry alone accounted for these results, cross-fostered birds should have returned to the site of their foster colony. That their later colony-size choice matched instead that of their siblings raised at another site indicates that these birds were sensitive to differences in colony size per se and were not simply philopatric to their place of rearing.
The spatial distributions of birds in the cross-fostering experiment bolsters this conclusion. Both cross-fostered birds and their siblings raised in the birth colony returned to the same general vicinity and had the same set of colony sites to choose from as breeders. Thus, even if there had been a spatial autocorrelation of colony size among adjacent sites that might have caused dispersers to settle in colonies of size similar to their birth, this scenario could not explain the observed differences in preferences for group sizes in the cross-fostering experiment (Fig. 2), because the settlement distances indicated that both classes of birds were choosing from the same set of colonies. Furthermore, because most of these birds returned to the same cluster of colonies (which happened to be the largest, containing 25 colony sites), both classes of birds had a relatively large number of colonies from which to choose.
A heritable basis to choice of group size could be established through genetic correlations between cognitive decision processes and morphological, behavioral, or physiological attributes, the performance of which varies with social environment. For example, within colonies some cliff swallows seem to have greater infestations of blood-feeding ectoparasites and are more negatively affected by them than other birds (15, 36), possibly reflecting degrees of genetically based resistance and susceptibility (37–40). Selection will favor susceptible individuals who are sensitive to group size in making settlement decisions and choose smaller colonies that have lower average ectoparasite loads (15). Resistant individuals who choose large colonies (and can tolerate more ectoparasitism) would be favored over resistant birds who choose small colonies, because the former receive greater net benefits from the social advantages (foraging, predator avoidance) of large colonies. Similarly, birds that are prone to high levels of stress hormones (e.g., corticosterone) in response to stressful social environments of large (or small) colonies would be selected to choose group sizes that minimize their stress response, especially if that stress response impairs immune function or is otherwise disadvantageous (41). Because no single colony size is best for all birds, relatively high additive genetic variance for colony-size choice can be maintained.
Cliff swallows probably do not choose breeding colonies strictly on the basis of genetic predisposition to large or small groups. Colony choice is a complex process that may also include assessment of one's own breeding performance and that of conspecifics, attraction to conspecifics, and assessment of local resource availability (15, 27, 42). These factors interact with genetically based choices of group size and probably account in part for the cases where birds used colony sizes unlike those of their birth. However, the existence of moderate heritable variation for choice of group size in cliff swallows helps to maintain a range of colony sizes.
Across all analyses involving birds not cross fostered, heritability for choice of breeding-colony size averaged 0.480. This heritability is higher than the mean heritability reported for behavioral traits in a tabulation of 105 estimates (0.304; ref. 43) and in other studies of complex behavior in vertebrates such as foraging-patch choice, migratory tendency, and dispersal distance (44–46). To our knowledge, this study is the first in vertebrates to report a strong genetic basis to the type of social system chosen. Ecological explanations for group-size variation, based on local resource availability or habitat characteristics, should perhaps consider that some animals may be inherently predisposed to occupy groups of particular sizes.
Acknowledgments
We thank our 48 research assistants from 1982 to 1999, J. Hoogland, D. Pilson, W. Wagner, and two anonymous reviewers for discussion and/or comments on the manuscript, the School of Biological Sciences at the University of Nebraska-Lincoln for use of the facilities at the Cedar Point Biological Station, and the National Science Foundation (BSR-8407329, BSR-8600608, BSR-9001294, BSR-9015734, DEB-9224949, DEB-9496113, and DEB-9613638), the Erna and Victor Hasselblad Foundation, the National Geographic Society (3244-85, 3996-89, 4545-91, and 5469-95), the American Philosophical Society, Princeton University, the University of Tulsa, Yale University, the Cedar Point Biological Station, the American Museum of Natural History, the National Academy of Sciences, Sigma Xi, and Alpha Chi for support.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bradbury J W, Emmons L H. Z Tierpsychol. 1974;36:137–183. doi: 10.1111/j.1439-0310.1974.tb02130.x. [DOI] [PubMed] [Google Scholar]
- 2.Jarman P J. Behaviour. 1974;48:215–267. [Google Scholar]
- 3.Brown J L. J Theor Biol. 1982;95:793–810. [Google Scholar]
- 4.Pulliam H R, Millikan G C. In: Avian Biology. Farner D S, King J R, Parkes K C, editors. Vol. 6. New York: Academic; 1982. pp. 169–197. [Google Scholar]
- 5.Caccamise D F, Lyon L A, Fischl J. Condor. 1983;85:474–481. [Google Scholar]
- 6.Terborgh J. Five New World Primates: A Study in Comparative Ecology. Princeton: Princeton Univ. Press; 1983. [Google Scholar]
- 7.Brown C R. Anim Behav. 1988;36:780–792. [Google Scholar]
- 8.Brown C R, Stutchbury B J, Walsh P D. Trends Ecol Evol. 1990;5:398–403. doi: 10.1016/0169-5347(90)90023-7. [DOI] [PubMed] [Google Scholar]
- 9.Hoglund J, Alatalo R V. Leks. Princeton: Princeton Univ. Press; 1995. [Google Scholar]
- 10.Hoogland J L, Sherman P W. Ecol Monogr. 1976;46:33–58. [Google Scholar]
- 11.Hoogland J L. Ecology. 1981;62:252–272. [Google Scholar]
- 12.Møller A P. Anim Behav. 1987;35:819–832. [Google Scholar]
- 13.Robinson J G. Behav Ecol Sociobiol. 1988;23:187–197. [Google Scholar]
- 14.Elgar M A. Biol Rev Camb Philos Soc. 1989;64:13–33. doi: 10.1111/j.1469-185x.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- 15.Brown C R, Brown M B. Coloniality in the Cliff Swallow: the Effect of Group Size on Social Behavior. Chicago: Univ. of Chicago Press; 1996. [Google Scholar]
- 16.Beauchamp G. Biol Rev Camb Philos Soc. 1998;73:449–472. [Google Scholar]
- 17.Sibly R M. Anim Behav. 1983;31:947–948. [Google Scholar]
- 18.Pulliam H R, Caraco T. In: Behavioural Ecology. Krebs J R, Davies N B, editors. Sunderlund, MA: Sinauer; 1984. pp. 122–147. [Google Scholar]
- 19.Giraldeau L-A, Gillis D. Anim Behav. 1985;33:666–667. [Google Scholar]
- 20.Higashi M, Yamamura N. Am Nat. 1993;142:553–563. [Google Scholar]
- 21.Brown C R, Brown M B. In: Proceedings of the 22nd International Ornithological Congress. Adams N J, Slotow R H, editors. Johannesburg: Birdlife South Africa; 1999. pp. 1293–1303. [Google Scholar]
- 22.Fretwell S D, Lucas H L., Jr Acta Biotheor. 1970;19:1–36. [Google Scholar]
- 23.Robinson S K. Anim Behav. 1986;34:113–122. [Google Scholar]
- 24.Burger J. Condor. 1988;90:575–582. [Google Scholar]
- 25.Stamps J A. Am Nat. 1988;131:329–347. [Google Scholar]
- 26.Ekman J. Ornis Scand. 1989;20:86–88. [Google Scholar]
- 27.Brown C R, Rannala B. Behav Ecol Sociobiol. 1995;36:221–228. [Google Scholar]
- 28.Rypstra A L. J Arachnol. 1985;13:71–78. [Google Scholar]
- 29.Smith D R R. J Arachnol. 1985;13:363–373. [Google Scholar]
- 30.Gibbs J P, Kinkel L K. Colonial Waterbirds. 1997;20:1–7. [Google Scholar]
- 31.Brown C R, Brown M B. In: Current Ornithology. Nolan V, Ketterson E, Thompson C F, editors. Vol. 16. New York: Plenum; 2001. , in press. [Google Scholar]
- 32.Ranta E, Lindstrom K. Ann Zool Fenn. 1990;27:67–75. [Google Scholar]
- 33.Widemo F, Owens I P F. Nature (London) 1995;373:148–151. [Google Scholar]
- 34.Falconer D S. Introduction to Quantitative Genetics. 2nd Ed. London: Longman; 1981. [Google Scholar]
- 35.Lynch M, Walsh B. Genetics and Analysis of Quantitative Traits. Sunderlund, MA: Sinauer; 1998. [Google Scholar]
- 36.Brown C R, Brown M B. Anim Behav. 1991;41:457–465. [Google Scholar]
- 37.Wakelin D. Adv Parasitol. 1978;16:219–308. doi: 10.1016/s0065-308x(08)60575-8. [DOI] [PubMed] [Google Scholar]
- 38.Møller A P. Evolution (Lawrence, Kans) 1990;44:771–784. [Google Scholar]
- 39.Boulinier T, Sorci G, Monnat J-Y, Danchin E. J Evol Biol. 1997;10:77–85. [Google Scholar]
- 40.Brinkhof M W G, Heeb P, Kolliker M, Richner H. Proc R Soc London Ser B. 1999;266:2315–2322. [Google Scholar]
- 41.Hillgarth N, Wingfield J C. In: Host-Parasite Evolution. Clayton D H, Moore J, editors. Oxford: Oxford Univ. Press; 1997. pp. 78–104. [Google Scholar]
- 42.Brown C R, Brown M B, Danchin E. J Anim Ecol. 2000;69:133–142. [Google Scholar]
- 43.Mousseau T A, Roff D A. Heredity. 1987;59:181–197. doi: 10.1038/hdy.1987.113. [DOI] [PubMed] [Google Scholar]
- 44.Waser P M, Jones W T. Anim Behav. 1989;37:987–991. [Google Scholar]
- 45.Lemon W C. Evol Ecol. 1993;7:421–428. [Google Scholar]
- 46.Berthold P, Pulido F. Proc R Soc London Ser B. 1994;257:311–315. [Google Scholar]