Abstract
α-Dystroglycan (DG) is an important cellular receptor for extracellular matrix (ECM) proteins and also serves as the receptor for Old World arenaviruses Lassa fever virus (LFV) and lymphocytic choriomeningitis virus (LCMV) and clade C New World arenaviruses. In the host cell, α-DG is subject to a remarkably complex pattern of O glycosylation that is crucial for its interactions with ECM proteins. Two of these unusual sugar modifications, protein O mannosylation and glycan modifications involving the putative glycosyltransferase LARGE, have recently been implicated in arenavirus binding. Considering the complexity of α-DG O glycosylation, our present study was aimed at the identification of the specific O-linked glycans on α-DG that are recognized by arenaviruses. As previously shown for LCMV, we found that protein O mannosylation of α-DG is crucial for the binding of arenaviruses of distinct phylogenetic origins, including LFV, Mobala virus, and clade C New World arenaviruses. In contrast to the highly conserved requirement for O mannosylation, more generic O glycans present on α-DG are dispensable for arenavirus binding. Despite the critical role of O-mannosyl glycans for arenavirus binding under normal conditions, the overexpression of LARGE in cells deficient in O mannosylation resulted in highly glycosylated α-DG that was functional as a receptor for arenaviruses. Thus, modifications by LARGE but not O-mannosyl glycans themselves are most likely the crucial structures recognized by arenaviruses. Together, the data demonstrate that arenaviruses recognize the same highly conserved O-glycan structures on α-DG involved in ECM protein binding, indicating a strikingly similar mechanism of receptor recognition by pathogen- and host-derived ligands.
Arenaviruses merit significant attention as being powerful experimental models for virus infections and important human pathogens (8). Infection of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV) in its natural host, the mouse, provided fundamental concepts for virology and immunology (38). LCMV is also a prevalent human pathogen (20, 25). Lassa fever virus (LFV) causes severe hemorrhagic fever in humans, with over 300,000 infections and several thousand deaths per year, and represents a major threat to human health (21, 33).
The genome of arenaviruses is negative stranded and bisegmented, consisting of two single-stranded RNA species, a larger segment encoding the virus polymerase (L) and a small zinc finger motif protein (Z), and a smaller segment encoding the virus nucleoprotein (NP) and glycoprotein (GP) precursor (GPC) (8). GPC is processed into GP1, which is implicated in receptor binding, and the transmembrane GP2, which is structurally similar to the fusion-active portions of the GPs of other enveloped viruses.
The cellular receptor for Old World arenaviruses and clade C New World arenaviruses is α-dystroglycan (DG), a cell surface receptor for proteins of the extracellular matrix (ECM) (11, 46). Encoded as a single protein, DG is cleaved into α-DG and membrane-anchored β-DG and provides a molecular link between the ECM and the actin-based cytoskeleton (3). The extracellular α-DG has a central, highly glycosylated mucin-type domain that connects the globular N- and C-terminal domains. DG is expressed in most developing and adult tissues, typically at high levels in cell types that adjoin basement membranes. At the extracellular site, α-DG undergoes high-affinity interactions with the ECM proteins laminin, agrin, perlecan, and neurexins. α-DG is noncovalently associated with β-DG, which binds intracellularly to the cytoskeletal adaptor proteins dystrophin and utrophin.
In mammals, α-DG is subject to a highly complex and specific pattern of O glycosylation that is crucial for its function as an ECM receptor (3, 12, 27). These functional modifications involve the known and putative glycosyltransferases protein O-mannosyltransferases POMT1 and POMT2, protein O- mannose β1,2-N-acetylglucosamine (GlcNAc) transferase 1 (POMGnT1), LARGE1, LARGE2, fukutin, and fukutin-related protein (FKRP). The genes of these enzymes are targeted in a number of congenital neuromuscular diseases in humans, and some of these genetic defects occur with remarkable frequency in the human population (3, 27). POMT1/2 and POMGnT1 are involved in the biosynthesis of the unusual O-mannosyl oligosaccharide SiaAα2-3Galβ1-4GlcNAcβ1-2Man, which is found in high abundance on α-DG. Protein O mannosylation of α-DG is catalyzed by a dimer of POMT1 and POMT2 and takes place in the endoplasmic reticulum (32). In the Golgi apparatus, the O-mannosyl sugars are extended by POMGnT1, which attaches a GlcNAc residue to the O-linked mannose, resulting in the O-linked disaccharide GlcNAcβ1-2Man (53), which is then extended to SiaAα2-3Galβ1-4GlcNAcβ1-2Man by more generic galactosyltransferases and sialyltransferases. Another crucial glycan modification of α-DG involves the putative glycosyltransferases LARGE and LARGE2 (3, 4, 26). The LARGE proteins localize in the Golgi apparatus and contain two catalytic domains with similarities to glucose transferases and N-acetylglucosamine transferases (3, 40). LARGE binds directly to the N-terminal globular domain of α-DG and modifies the N-terminal part of α-DG's central mucin-type domain (26). Studies in vivo and in vitro indicated that the differential expression of LARGE is a crucial determinant for the functional glycosylation of α-DG (3, 4). The exact glycosyltransferase activity of LARGE is currently unknown. However, several lines of evidence strongly indicate that LARGE is involved in the synthesis of sugar chains different from the O-mannosyl tetrasaccharide SiaAα2-3Galβ1-4GlcNAcβ1-2Man (3, 4, 26, 27). Enzymes that appear to be related to LARGE catalyze modifications unrelated to O mannosylation (40). Furthermore, the overexpression of LARGE generates highly glycosylated, functional α-DG even in cells with defects in the biosynthesis of the O-mannosyl sugar chains (4, 40).
Recent studies reported a critical role for protein O mannosylation in the infection of cells with different LCMV isolates (24). Independently, LARGE-dependent modification was also found to be crucial for arenavirus binding (29). Together, these findings suggest that arenaviruses likely recognize some of α-DG's O-glycan structures involved in ECM protein binding. Our present study therefore aimed at the identification of the specific glycan structures on α-DG that are recognized by arenaviruses. As for LCMV, we found an absolute requirement for protein O mannosylation of α-DG for the binding of LFV, Mobala virus, and clade C New World arenaviruses. In contrast, the more generic O-glycans of α-DG were dispensable for arenavirus binding. Despite the requirement for O mannosylation for arenavirus binding under normal conditions, the overexpression of LARGE completely restored α-DG's function as an arenavirus receptor in O-mannosylation-deficient cells. Thus, modifications by LARGE but not O-mannosyl glycans themselves are the crucial structures recognized by arenaviruses of distinct phylogenetic origin.
MATERIALS AND METHODS
Proteins and antibodies.
α-DG was either purified from rabbit skeletal muscle as described previously (18) or isolated from cell membrane extracts by wheat germ agglutinin (WGA) affinity purification (35). Mouse laminin-1 was obtained from GIBCO-BRL (Gaithersburg, MD). Monoclonal antibodies (mAbs) 83.6 (anti-LCMV GP2) and 113 (anti-LCMV NP) were described previously (9, 51), as were anti-α-DG mAb IIH6 (18) and GT20ADG polyclonal antibody (4). Polyclonal rabbit anti-laminin-1 and mouse immunoglobulin M (IgM) isotype control antibody were obtained from Sigma (St. Louis, MO). Fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated anti-mouse IgM were obtained from Jackson Immuno-Research (West Grove, PA). Horseradish peroxidase (HRP)-conjugated secondary antibodies and streptavidin-HRP were obtained from Pierce. The biotinylated lectins peanut agglutinin, Erythrina cristagalli agglutinin, and Griffonia simplicifolia lectin II were obtained from Vector laboratories (Burlingame, CA). The Steady-Glo and Bright-Glo luciferase assay systems were obtained from Promega (Madison WI). Heparan sulfate (HS) and heparin were purchased from Sigma.
Cell lines.
Vero-E6 cells, HEK293 cells, A549 cells (ATCC CCL-185), and human hepatoma Huh7 cells (36) were cultured in Dulbecco's modified Eagle's medium (DMEM), 10% (vol/vol) fetal bovine serum (FBS) supplemented with glutamine, and penicillin-streptomycin. Human umbilical cord vascular endothelial cells (HUVEC) purchased from Cambrex (Walkersville, MD) were cultured according to the company's recommendations. The human B-cell lines WIL-2 NS and Daudi, the human T-cell line Jurkat, and immortalized human T cells (kindly provided by Philippe Galley, Scripps Research Institute) were cultured as described previously (41). The O-mannosylation-deficient cell lines Lec15.2 and Lec35 were maintained as described previously (24), and the mutant line psgΑ-745 was maintained as outlined previously (41). DG+/− and DG−/− embryonic stem (ES) cells were maintained as described previously (22).
Virus strains, purification, and quantification.
Recombinant adenoviral vector (AdV)-Ad5-LARGE-enhanced green fluorescent protein (GFP) (EGFP) and Ad5-EGFP have been described previously (4), as have LCMV clone 13 (cl-13) and WE2.2 (1, 47). Purified LCMV stocks were produced and titers were determined as described previously (15). UV inactivation of LCMV was done as described previously (30). LFV (Josiah), Mobala virus, Oliveros virus, Parana virus (PAR), and Amapari virus (AMA) were grown in Vero-E6 cells in a biosafety level 4 (BSL-4) facility, polyethylene glycol precipitated, and γ-inactivated at the Center for Disease Control and Prevention in Atlanta, GA, according to a method described previously (16). Inactivation was verified by infection assay. Work with infectious viruses was done at BSL-3, with the exception of LFV, which was handled in the BSL-4 laboratories at the Special Pathogens Branch, and LCMV, which was handled at BSL-2 at The Scripps Research Institute.
Production of retroviral pseudotypes.
Recombinant Moloney murine leukemia virus (MLV) pseudotypes were produced as described previously (41). The package cell line GP2-293 (BD Biosciences) was transfected with the packable MLV genome pLZRs-Luc-gfp, which contains a luciferase reporter gene and a GFP reporter (52), provided by Gary Nabel. The GPs of AMA, LCMV cl-13, LFV, and VSV were provided in trans by cotransfection with expression plasmids containing their full-length cDNAs. Briefly, 1.2 × 107 GP2-293 cells were plated in poly-l-lysine-coated T175 tissue culture flasks. After 16 h, cells were cotransfected with 20 μg each of pLZRs-Luc-gfp and the GP expression plasmid using calcium phosphate. Forty hours after transfection, cell supernatants were harvested and cleared by centrifugation for 15 min at 3,000 rpm. Retroviral pseudotypes were then concentrated by ultracentrifugation at 25,000 rpm at 4°C for 2 h using an SW28 rotor. Supernatants were discarded after centrifugation, and pellets were resuspended for 16 h in DMEM-20 mM HEPES (pH 7.5) at 4°C as described above. For determinations of titers, monolayers of Vero-E6 cells were infected with serial dilutions of pseudotypes. After 48 h, cells were fixed for 10 min in 2% (wt/vol) paraformaldehyde-phosphate-buffered saline (PBS). GFP was detected by using a rabbit anti-GFP polyclonal antibody (3080; Chemicon) diluted 1:100 in PBS-1% (vol/vol) FBS-0.1% (wt/vol) saponin that was incubated overnight at 4°C. After several washes, bound primary antibody was detected with an anti-rabbit IgG-FITC (Fab)2 (1:50 in PBS-1% [vol/vol] FBS-0.1% [wt/vol] saponin) applied for 45 min in the dark. Specimens were examined under a fluorescence microscope, clusters of GFP-positive cells were counted, and titers were calculated.
Detection of arenavirus GP incorporated into retroviral pseudotypes.
For the detection of GP in the retroviral pseudotypes, three independent preparations of concentrated viruses were analyzed for each variant studied. Pseudotypes were produced, concentrated, and titered as described above and diluted to 2 × 106 infectious units (IU)/ml. Pseudotypes were coated in triplicate wells in 96-well EIA/RIA High-Bond microtiter plates (Corning) for 2 h at 6°C, and nonspecific binding was blocked with 1% (wt/vol) bovine serum albumin (BSA)-PBS. For the detection of GP, mAb 83.6 (anti-LCMV GP2) was applied at 20 μg/ml for 2 h at 6°C and detected with peroxidase-conjugated anti-mouse IgG (1:1,000) in a color reaction using ABTS [2,2′azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)] substrate. The optical density at 405 nm was measured with an enzyme-linked immunosorbent assay reader. For the determination of specific binding, background binding to BSA was subtracted.
Infection of cells with recombinant adenoviruses and retroviral pseudotypes.
The overexpression of LARGE and EGFP using recombinant AdV-Ad5-LARGE-EGFP and Ad5-EGFP was described previously (4, 29).
For infection with retroviral pseudotypes, cells were plated in 96-well plates at a density of 104 cells/well. After 24 h, retroviral pseudotypes were added at the indicated multiplicities of infection (MOIs) and incubated for 1 h at 37°C. The viral particles were removed, cells were washed twice with DMEM, and fresh medium was added. Luciferase activity was determined after 48 h by using Steady-Glo luciferase assay (Promega), except for murine ES cells, in which the more sensitive Bright-Glo assay (Promega) was used.
Blocking of pseudotype infection of cells with inactivated viruses.
For blocking, HEK293 cells cultured in 96-well plates (2 × 103 cells/well) were incubated with the indicated concentration of inactivated viruses in a total volume of 100 μl/well in 50% OPTIMEM-PBS for 2 h on ice. Next, 2 × 103 IU of retroviral pseudotypes were mixed with the same concentrations of inactivated viruses in a total volume of 100 μl OPTIMEM-PBS, and the was inoculum added to the cells. After 45 min, supernatants were removed, and cells were washed three times with medium and incubated for 48 h. Infection was quantified by luciferase assay as described above.
Enzymatic remodeling of oligosaccharides on immobilized α-DG.
Enzymatic remodeling of oligosaccharides on immobilized α-DG was performed as described previously (13) by using the following enzymes: O-glycosidase from Diplococcus pneumoniae (Roche), neuraminidase from Arthrobacter urefaciens (Calbiochem), and β(1-4)-galactosidase from Streptococcus pneumoniae (Calbiochem). Enzymes were removed by five wash steps with Tris-buffered saline-0.1% (wt/vol) Tween 20. Biotinylated lectins were applied at 10 μg/ml in lectin binding buffer (3% [wt/vol] BSA, 50 mM Tris-HCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 150 mM NaCl [pH 7.5]) for 1 h at room temperature. After extensive washing, bound lectins were detected with streptavidin-HRP (1:500) in lectin binding buffer, followed by enhanced chemiluminescence (ECL) detection.
Flow cytometry.
For cell surface staining of α-DG with mAb IIH6, cells were detached with enzyme-free cell dissociation solution (Sigma), resuspended in fluorescence-activated cell sorter (FACS) buffer (1% [vol/vol] FBS, 0.1% [wt/vol] sodium azide, PBS), and plated in conical 96-well trays. For cell surface staining of functionally glycosylated α-DG, cells were incubated with mAb IIH6 (1:145) or GT20ADG (1:50) in FACS buffer for 1 h on ice. Cells were then washed twice in FACS buffer and labeled with PE-conjugated secondary antibodies (1:100 in FACS buffer) for 45 min on ice in the dark. After two wash steps in 1% (vol/vol) FBS in PBS, cells were fixed with 4% (wt/vol) paraformaldehyde-PBS for 10 min at room temperature in the dark. The cells were washed twice with PBS and analyzed with a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) using Cell Quest software. Image analysis was done using FloJo software (Tree Star, Inc.). Intracellular staining for LCMV NP using mAb 113 was performed as described previously (28).
Immunoblotting, LOA, and VOPBA.
For immunoblotting, proteins were separated by gel electrophoresis and transferred onto nitrocellulose. After blocking in 5% (wt/vol) skim milk in PBS, membranes were incubated with mAb IIH6 at 10 μg/ml in 2% (wt/vol) skim milk-PBS for 12 h at 6°C. Secondary antibodies coupled to HRP were applied at 1:5,000 in PBS-0.1% (wt/vol) Tween for 1 h at room temperature. Blots were developed by ECL using Super Signal West Pico ECL substrate (Pierce). Laminin overlay assay (LOA) and virus overlay protein binding assay (VOPBA) with LCMV cl-13 and γ-inactivated LFV, Mobala virus, and Oliveros virus were performed as described previously (29). A solid-phase laminin binding assay was performed as described previously (29). For inhibition of laminin binding to α-DG by heparin, 1 μg/ml Engelbreth-Holm-Swarm sarcoma laminin-1 was preincubated with the indicated amounts of heparin in laminin overlay buffer containing 1 mM MgCl2 and 1 mM CaCl2 for 1 h on ice. Laminin was then added to purified α-DG from rabbit skeletal muscle immobilized in microtiter plates. After incubation for 2 h at room temperature, bound laminin was detected with a polyclonal rabbit anti-laminin-1 antibody and an HRP-conjugated secondary antibody in a color reaction using ABTS substrate as described previously (29).
RESULTS
Functional glycosylation of α-DG is critical for receptor binding and infection of human cells with LFV and LCMV.
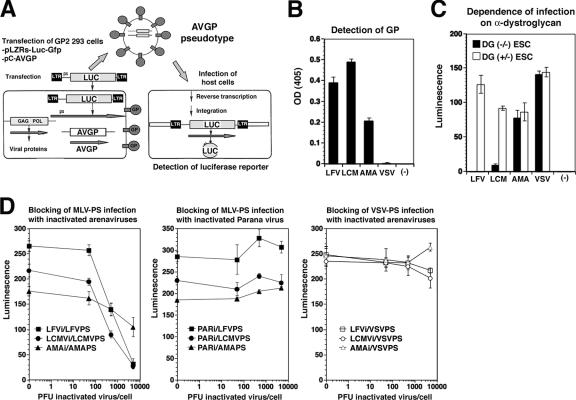
Glycosylation has recently been implicated in α-DG's function as an arenavirus receptor (24, 29), causing us to address the correlation between functional receptor glycosylation, virus binding, and cellular tropism for the highly human-pathogenic LFV and the prototypic immunosuppressive LCMV isolate cl-13 in human cells. Since LFV is a category A pathogen, studies with live virus are restricted to BSL-4 facilities. To study the interaction of LFV with its cellular receptor under BSL-2 conditions, we generated recombinant retroviruses containing the LFV GP in their envelope by pseudotyping. In addition, we produced retroviral pseudotypes of LCMV cl-13, the clade B New World arenavirus AMA, and vesicular stomatitis virus (VSV). In contrast to LFV and LCMV cl-13, AMA and VSV do not use α-DG as a receptor (11, 46). Using the strategy outlined in Fig. 1A, we inserted the GPs of LFV (strain Josiah), LCMV cl-13, AMA, and VSV into virions of recombinant MLV, which contain luciferase and a GFP reporter gene. Titers of the pseudotypes were determined by the infection of Vero cell monolayers using the GFP reporter and are given in Table 1. The arenavirus pseudotypes were specifically recognized by mAb 83.6, which binds to a highly conserved epitope in arenavirus GP2 (51) (Fig. 1B). Consistent with previous studies (11, 46), infection with pseudotypes of LCMV and LFV, but not AMA and VSV, was dependent on α-DG (Fig. 1C). Infection of cells by pseudotypes containing the GPs of LFV, LCMV, and AMA was specifically blocked by the corresponding inactivated viruses but not by inactivated PAR, which belongs to the clade A New World arenaviruses (Fig. 1D). Together, the data indicate that our retroviral pseudotypes adopt the receptor binding specificities of the viruses from which their GPs were derived and represent powerful tools to study virus-receptor interactions and viral entry. Since receptor binding and entry of arenaviruses are mediated exclusively by the viral GP (8), retroviral pseudotypes allow the separation of effects on virus entry from subsequent steps of viral replication that may be subject to cell-type-specific restrictions.
FIG. 1.
Recombinant retroviral vectors pseudotyped with arenavirus GPs. (A) The packaging cell line GP2-293 (BD Biosciences) stably transfected with MLV Gag and Pol was cotransfected with a plasmid containing the packable MLV genome pLZRs-Luc-gfp (52) carrying a luciferase and a GFP reporter and an expression plasmid for the heterologous GP (pC-AVGP). Retroviral pseudotypes were released into the cell supernatant. LTR, long terminal repeat. (B) Binding of mAb 83.6 to pseudotypes. Equal amounts of concentrated pseudotypes containing the GPs of LFV, LCMV cl-13, AMA, and VSV were immobilized in microtiter plates and probed with mAb 83.6. Primary antibodies were detected with HRP-conjugated anti-mouse IgG in a color reaction using the substrate ABTS. The optical density (OD) at 405 nm was measured using an enzyme-linked immunosorbent assay reader (n = 3 ± standard deviation [SD]). (C) Infection of cells with LFV and LCMV pseudotypes is dependent on α-DG. DG-deficient (DG−/−) and DG+/− mouse ES cells (ESC) cultured in 96-well plates were infected with retroviral pseudotypes of LFV, LCMV cl-13, AMA, or VSV or pseudotypes containing no GP (−) at an MOI of 10. Infection was assessed by Bright-Glo luciferase assay after 48 h (n = 3 ± SD). (D) Blocking of arenavirus pseudotype (PS) infection by inactivated viruses. HEK293 cells cultured in 96-well plates were blocked with inactivated LFV, LCMV, AMA, or PAR at the indicated ratios of virus particles per cell. After blocking for 2 h at 4°C, cells were infected with the indicated pseudotypes at an MOI of 1, and infection was assessed after 24 h by Steady-Glo luciferase assay (n = 3 ± SD).
TABLE 1.
Representative titers of retroviral pseudotypes in this studya
| GP | Mean pseudotype titer (IU/ml) ± SD | % Recovery after concn (±SD) |
|---|---|---|
| LCMV | (5.2 ± 0.6) × 105 | 81 (±15) |
| LFV | (1.4 ± 0.4) × 106 | 95 (±21) |
| AMA | (2.8 ± 0.6) × 105 | 77 (±11) |
| VSV | (3.1 ± 1.2) × 106 | 83 (±14) |
| No GP | <10 | ND |
Recombinant retroviruses were pseudotyped with the GPs of LCMV cl-13, LFV, AMA, and VSV using the strategy described in the legend of Fig. 1A. In the negative controls (no GP), the empty vector pcAGGS was transfected. For the determination of titers, serial dilutions of the pseudotypes were added to monolayers of Vero-E6 cells, and GFP-positive cells were detected by immunofluorescence staining. Clusters of GFP-positive cells were counted, and titers were calculated as IU. ND, not determined.
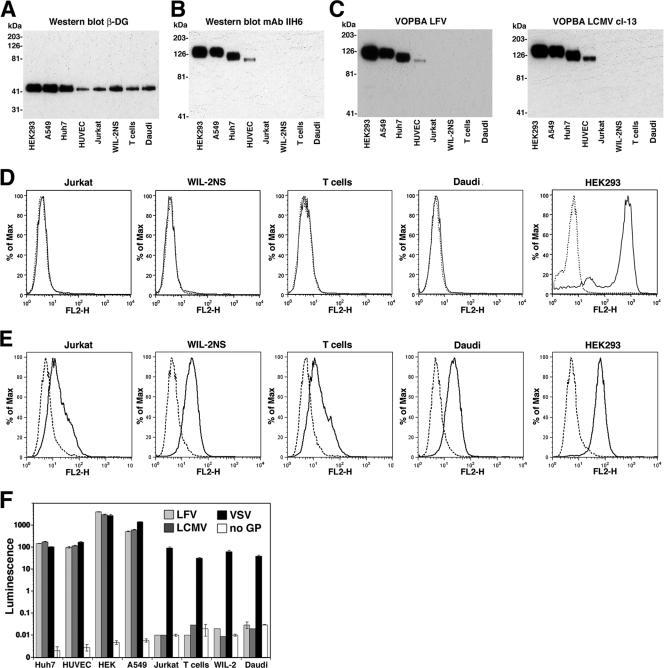
While the core protein of DG is ubiquitously expressed, the functional glycosylation of α-DG is regulated in a cell-type-specific manner (3). Human cell lines derived from fibroblasts, epithelial cells, hepatocytes, vascular endothelial cells, T cells, and B cells expressed significant levels of DG core protein, as detected by Western blot analysis for β-DG (Fig. 2A). However, examination of membrane protein fractions with the glycosylation-sensitive anti-α-DG mAb IIH6 (35) in Western blots revealed significant differences in the expression of glycosylated α-DG. High levels of functionally glycosylated α-DG were found in established cultures of fibroblasts, epithelial cells, and hepatocytes; lower but significantly detectable levels were found in vascular endothelial cells; and undetectable levels were found in both T cells and Β cells (Fig. 2B). The levels of IIH6-reactive α-DG in membrane protein fractions of different cell types correlated well with the binding to LFV and LCMV cl-13 in VOPBA (Fig. 2C). The absence of functionally glycosylated α-DG on established T-cell and B-cell lines was verified independently by cell surface staining on live cells using mAb IIH6 and flow cytometry (Fig. 2D). Despite the absence of IIH6 staining at the surface of the B- and T-cell lines examined, we were able to detect significant levels of α-DG core protein using polyclonal antibody GT20ADG, which recognizes the core protein of α-DG independent of functional glycosylation (4, 35) (Fig. 2E). Although the cell surface levels of α-DG core protein in B- and T-cell lines were significantly lower than that in HEK293 cells (Fig. 2E), the larger differences in IIH6 surface staining between the lymphocyte lines and HEK293 cells (Fig. 2D) suggest that α-DG on B and T cells indeed has a reduced level of functional glycosylation.
FIG. 2.
Receptor binding and infection of human cells with LFV and LCMV depend on the functional glycosylation of α-DG. (A) Detection of β-DG in human cell lines. Equal amounts of total cell protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), blotted onto nitrocellulose, and probed with anti-β-DG mAb 8D5 in Western blots using an HRP-conjugated secondary antibody and ECL for detection. Molecular masses are indicated. (B) Detection of glycosylated α-DG. Equal amounts of total membrane proteins were separated and probed in Western blots with mAb IIH6 using an HRP-conjugated secondary antibody and ECL. (C) VOPBA. Membrane proteins from B were probed with 107 PFU/ml LCMV cl-13 and inactivated LFV. Bound virus was detected with anti-arenavirus GP2 mAb 83.6 using an HRP-conjugated secondary antibody and ECL. (D) Detection of functionally glycosylated α-DG at the cell surface of human cell lines by flow cytometry. Live, nonpermeabilized cells were stained with mAb IIH6, combined with a PE-labeled secondary antibody, and analyzed by flow cytometry using a FACSCalibur flow cytometer. Data were acquired and analyzed using Cell Quest and FloJo software packages. In histograms, the y axis represents cell numbers, and the x axis represents PE fluorescence intensity. The solid line indicates primary and secondary antibodies, and the broken line indicates secondary antibody only. (E) Detection of α-DG core protein at the cell surface of T- and B-cell lines. Cells were subjected to cell surface staining with the polyclonal antibody GT20ADG, which recognizes the core protein of α-DG independent of functional O glycosylation. Bound primary antibody was detected with a PE-conjugated anti-goat IgG secondary antibody and flow cytometry as in D. The solid line indicates primary and secondary antibodies, and the broken line indicates secondary antibody only. (F) Infection of cells with retroviral pseudotypes. Cells cultured in 96-well plates were infected with LFV, LCMV, and VSV pseudotypes and with pseudotypes without GP (no GP) at MOIs of 1 (HEK293, A549, Huh7, and HUVEC) and 10 (T- and B-cell lines). After 48 h, infection was quantified by luciferase assay, and luminescence was expressed as the increase (n-fold) over uninfected cells (n = 3 ± SD).
To assess the susceptibilities of the different human cell lines to infection with LFV and LCMV cl-13, cells were infected with retroviral pseudotypes of LFV, LCMV cl-13, and VSV, and infection was detected after 48 h by luciferase assays. Consistent with the VOPBA data, pseudotypes of LFV and LCMV cl-13 infected fibroblasts, epithelial cells, endothelial cells, and hepatocytes but not T and B cells (Fig. 2F). Since the T- and B-cell lines were efficiently infected by VSV pseudotypes, the resistance to LFV and LCMV pseudotypes is likely not due to the lack of retroviral integration or reporter gene expression. As both arenaviruses and VSV enter cells by endocytosis involving pH-dependent fusion between viral and endosomal cell membranes (6, 42), it is unlikely that the resistance of T and B cells to arenavirus pseudotypes is caused by a blockade in endocytosis or pH-dependent membrane fusion. The lack of infection of T and B cells is therefore likely to be due to the absence of a functional receptor.
Protein O mannosylation is crucial for α-DG binding to Old World arenaviruses and clade C New World viruses.
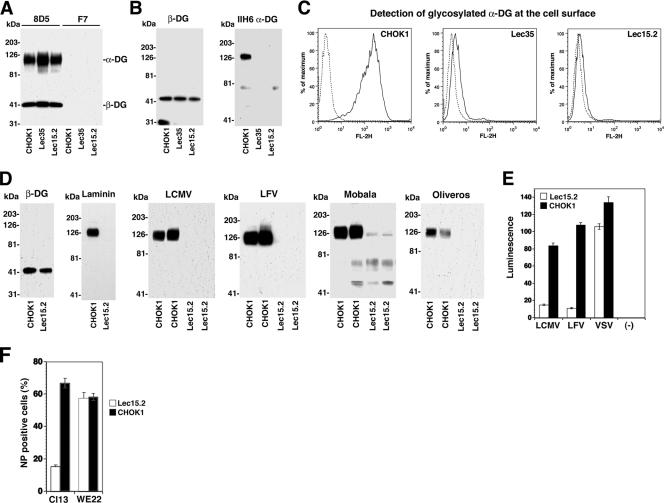
Since protein O mannosylation has been implicated in α-DG's function as a receptor for LCMV (24), we studied the role of this modification in receptor binding of arenaviruses derived from phylogenetically distinct groups represented by LFV, the related African arenavirus Mobala virus, and the clade C New World arenavirus Oliveros virus. To address this issue, we chose the CHO cell mutants Lec15.2 and Lec35.1 that are deficient in the biosynthesis of dolichol-P-mannose (10, 31), the donor substrate for the protein O-mannosyltransferases POMT1 and POMT2, which are involved in the attachment of O-linked mannose to α-DG (32). The cells also lack glycosylphosphatidylinositol-anchored proteins, do not have N-glycans of the high-mannose type (Man6-9GlcNAc2), and are unable to synthesize C-mannosylated proteins. However, as DG is not glycosylphosphatidylinositol anchored and the other glycan modifications have so far not been implicated in the biosynthesis of functional DG, we do not expect these biochemical defects to affect the virus-α-DG interaction. The expression of α-DG core protein at the surface of Lec15.2 and Lec35.1 mutants and wild-type CHOK1 cells was verified by using a method combining cell surface biotinylation with immunoprecipitation (IP) of β-DG (44). As shown in Fig. 3A, we detected at least similar, if not higher, levels of the α-DG core protein in cells impaired in O mannosylation. However, the probing of total membrane proteins with mAb IIH6 in Western blot and flow cytometry analyses revealed reduced functional glycosylation of α-DG in Lec35.1 and Lec15.2 cells as reported previously (24) (Fig. 3B and C). For our experiments, we chose the mutant Lec15.2, which was less leaky than Lec35.1. First, α-DG was prepared from Lec15.2 and CHOK1 cells by WGA purification as described previously (35). The presence of comparable amounts of DG core protein in WGA fractions was confirmed by the detection of β-DG in Western blots (Fig. 3D). Compared to the wild type, α-DG from Lec15.2 cells showed undetectable binding to laminin-1, LFV, LCMV cl-13, Mobala virus, and Oliveros virus (Fig. 3D). Since the arenavirus binding site on α-DG is subject to O glycosylation, but not N glycosylation, and previous studies excluded a significant role for N-glycans in arenavirus receptor binding (5), the lack of virus binding to α-DG from Lec15.2 cells is most likely due to the absence of O-mannosyl glycans.
FIG. 3.
Protein O mannosylation is critical for α-DG's function as a receptor for Old World and clade C New World arenaviruses. (A) Detection of α-DG core protein. CHOK1, Lec35.1, and Lec15.2 cells were subjected to cell surface biotinylation using the reagent N-hydroxysuccinimide-X-biotin. After the quenching of the reaction, cells were lysed, and cleared lysates were subjected to IP with anti-β-DG mAb 8D5 or an isotype control. IPs were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with streptavidin-HRP using ECL detection. The positions of α-DG and β-DG are indicated. (B) Detection of functionally glycosylated α-DG. Membrane lysates of CHOK1, Lec35.1, and Lec15.2 cells were subjected to Western blot analysis using mAb 8D5 (β-DG) and mAb IIH6. (C) Detection of functionally glycosylated α-DG on CHOK1, Lec35.1, and Lec15.2 cells by flow cytometry. Live, nonpermeabilized cells were stained with mAb IIH6, combined with a PE-labeled secondary antibody, and analyzed by flow cytometry as described in the legend of Fig. 2D. In the histograms, the y axis represents cell numbers, and the x axis represents PE fluorescence intensity. The solid line indicates primary and secondary antibodies, and the broken line indicates secondary antibody only. (D) Laminin and virus binding to α-DG from CHOK1 and Lec15.2 cells. α-DG was isolated from CHOK1 and Lec15.2 cells by WGA affinity purification, and eluted proteins were probed for the presence of β-DG in Western blots. Binding to laminin was assessed by LOA using 10 μg/ml mouse laminin-1, a polyclonal anti-laminin antibody, and ECL for detection. For VOPBA, duplicate samples were probed with 107 PFU/ml of LCMV cl-13 and inactivated LFV, Mobala virus, and Oliveros virus as described in the legend of Fig. 1C. (E) Infection of Lec15.2 and wild-type CHOK1 cells with retroviral pseudotypes of LFV, LCMV, and VSV. CHOK1 and LEC15.2 cells cultured in 96-well plates were infected with the indicated pseudotypes (MOI of 1), and infection was quantified by a Steady-Glo luciferase assay after 48 h. Luminescence was expressed as the increase (n-fold) over uninfected cells (n = 3 ± SD). (F) Infection of Lec15.2 cells and wild-type CHOK1 cells with LCMV cl-13 and LCMV WE22. CHOK1 and LEC15.2 cells cultured in 24 well plates were infected with LCMV cl-13 and WE22 (MOI of 1). After 16 h, cells were detached, and infection was quantified by intracellular staining with anti-LCMV NP mAb 113 combined with an FITC-labeled secondary antibody and analyzed by flow cytometry as in C (n = 3 ± SD).
Next, we infected Lec15.2 cells and wild-type controls with retroviral pseudotypes of LFV, LCMV, and VSV. Determinations of pseudotype infection by luciferase assay revealed a large reduction in infection with LFV and LCMV pseudotypes but not VSV pseudotypes (Fig. 3E). To directly correlate the pseudotype data with the previously reported effect on infection with live virus (24), Lec15.2 and wild-type CHO cells were infected with LCMV cl-13 virus, and infection was assessed after 16 h by detection of LCMV NP using mAb 113 and flow cytometry. To confirm the role of α-DG in the observed reduction of LCMV cl-13 infection, we included LCMV isolate WE22, which infects cells independently of α-DG (30, 43, 45). The similar infection levels of LCMV WE22 in Lec15.2 and wild-type cells demonstrate that the reduced infection with LCMV cl-13 indeed involves α-DG and not another step in LCMV replication and/or gene expression (Fig. 3F). In sum, our data show that the requirement for O mannosylation for α-DG's receptor function is highly conserved among arenaviruses.
More generic O-glycans on α-DG are dispensable for arenavirus binding.
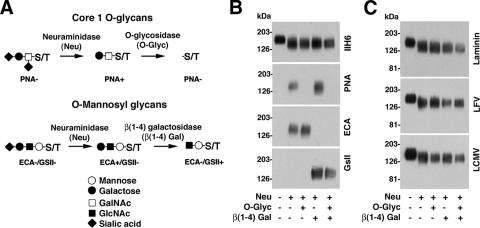
Apart from O-mannosyl glycans implicated in α-DG's function as a cellular receptor for ECM proteins and arenaviruses, α-DG is also modified by sialylated core 1-type O-glycans (mucin-type O-glycans) with the structure SiaAα(2-3)Galβ(1-3)GalNAc-O-S/T. These more generic O-glycans are not involved in the binding of α-DG to ECM proteins (18) but were recently found to be important for the ability of α-DG to mediate the clustering of acetylcholine receptors during muscle differentiation (34). This function of α-DG's core 1 O-glycans in a molecular recognition process raises the question of whether arenaviruses have also evolved to recognize these glycan structures. To address the role of these sugars in virus binding, mature, fully glycosylated α-DG was purified from rabbit muscle membranes and subjected to enzymatic remodeling by O-glycosidase, which hydrolyzes O-linked Galβ(1-3)GalNAc moieties. Since the activity of O-glycosidase is blocked by sialic acid linked to the Galβ(1-3)GalNAc core, we used the enzyme in combination with neuraminidase from Arthrobacter urefaciens that cleaves α(2-3,6,8)-linked sialic acid (Fig. 4A). The removal of specific sugar moieties was verified by probing α-DG with lectins recognizing the corresponding glycan structures (Fig. 4B). In addition, we enzymatically removed the more generic distal SiaAα2-3Galβ1-4 residues from the O-linked SiaAα2-3Galβ1-4GlcNAcβ1-2Man glycans to address their function in virus binding. As shown in Fig. 4C, removal of mucin-type core 1 O-glycans and the SiaAα2-3Galβ1-4 parts of α-DG's O-mannosyl glycans did not affect the binding of laminin, LFV, or LCMV, indicating that these sugars are dispensable for the binding of both laminin and arenaviruses.
FIG. 4.
Generic O-glycans of α-DG are dispensable for virus binding. (A) Enzymatic removal of the sialylated core 1 O-glycans (Galβ1-3GalNAc-S/T) and the SiaAα2-3Galβ1-4 parts of α-DG's O-mannosyl glycans (SiaAα2-3Galβ1-4GlcNAcβ1-2Man). (B) Verification of glycan removal. Purified α-DG was treated with glycosidases as described above (A), blotted onto nitrocellulose, and probed with either mAb IIH6 or the indicated lectins. Peanut agglutinin (PNA) is specific for Galβ(1-3)GalNAc, Erythrina cristagalli agglutinin (ECA) recognizes Galβ(1-4)GlcNAc, and Griffonia simplicifolia lectin II (GsII) detects terminal α- or β-linked GlcNAc and α- or β-linked GalNAc. (C) Binding of laminin, LFV, and LCMV. Nitrocellulose transfers of glycosidase-treated α-DG were subjected to LOA and VOPBA as described in the legend of Fig. 3D.
Modifications by LARGE but not O-mannosyl glycans themselves are crucial structures for arenavirus binding.
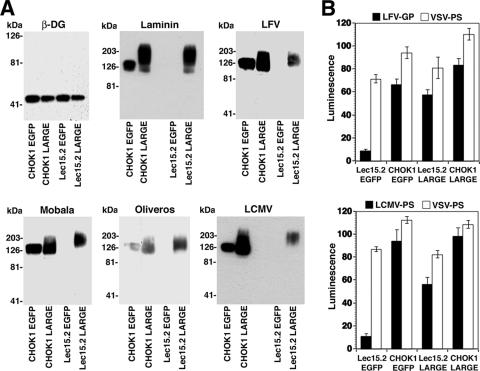
Since our previous studies demonstrated that arenavirus binding to α-DG critically depended on modifications by the glycosyltransferase LARGE (29), we addressed the relative contribution of α-DG's O-mannosyl glycans and the LARGE-derived sugars for arenavirus binding. A striking property of LARGE is its ability to functionally bypass defects in other enzymes implicated in the functional glycosylation of α-DG (4), suggesting that the sugars attached by LARGE are crucial for the binding of ECM proteins. To address the ability of LARGE to restore the arenavirus receptor function of α-DG in the absence of O mannosylation, we overexpressed LARGE in Lec15.2 cells deficient in O mannosylation. Briefly, Lec15.2 and wild-type CHOK1 cells were infected with AdVs expressing either LARGE or EGFP. After 48 h, cells were lysed, and α-DG was prepared by WGA affinity purification and subjected to Western blot analysis with anti-β-DG antibody, LOA, and VOPBA (Fig. 5A). The overexpression of LARGE did not change the amounts of β-DG detected in the WGA fractions, indicating no significant effect on DG core protein biosynthesis. Consistent with previous studies (40), the overexpression of LARGE in both Lec15.2 and wild-type CHO cells resulted in the appearance of a hyperglycosylated form of α-DG that bound laminin. This hyperglycosylated form of α-DG also showed high-affinity binding to LFV, LCMV, Mobala virus, and Oliveros virus (Fig. 5A). The stronger binding of laminin to the hyperglycosylated α-DG derived from LARGE-overexpressing cells was consistently observed and may be due to the fact that laminin-1, but not the viruses, can self-aggregate under the experimental conditions used. Next, we tried to restore arenavirus receptor function in O-mannosylation-deficient cells by LARGE overexpression. Lec15.2 and wild-type CHO cells were infected with AdVs expressing LARGE and EGFP. Forty-eight hours later, cells were infected with retroviral pseudotypes of LFV, LCMV, and VSV. As shown in Fig. 5B, the overexpression of LARGE but not the EGFP control efficiently restored infection with LFV and LCMV pseudotypes. These data demonstrate that similar to ECM proteins like laminin, the LARGE-dependent modification, but not the O-mannosyl glycans per se, is the crucial glycan structure recognized by arenaviruses.
FIG. 5.
LARGE-derived glycan modifications but not O-mannosyl glycans are the crucial structures recognized by arenaviruses. (A) Binding of laminin and viruses to α-DG derived from Lec15.2 cells and wild-type CHO cells with or without overexpression of LARGE. O-mannosylation-deficient Lec15.2 and wild-type CHO cells were transfected with recombinant AdVs expressing LARGE or EGFP. After 48 h, membrane lysates were subjected to WGA affinity purification, and eluted proteins were separated by SDS-PAGE and probed with mAb 8D5 to β-DG in Western blots as described in the legend of Fig. 2A. α-DG was tested for the binding of laminin, LFV, Mobala virus, Oliveros virus, and LCMV as described in the legend of Fig. 3D. (B) Rescue of LFV and LCMV infection in Lec15.2 cells. Lec15.2 and wild-type CHO cells were plated in 96-well plates and infected with AdV-LARGE or AdV-EGFP. After 24 h, cells were infected with pseudotypes of LFV, LCMV, and VSV (MOI of 1), and infection was assessed after 48 h as described in the legend of Fig. 2F.
LFV and LCMV do not recognize other laminin-binding GAGs.
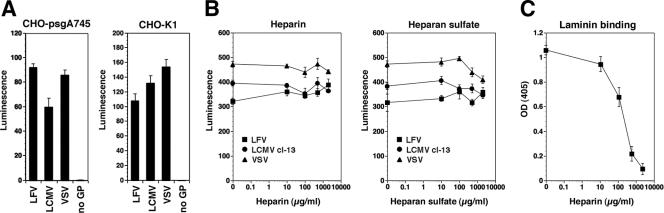
Our data show a striking similarity in the recognition of α-DG-derived sugars between arenaviruses and laminin. Interestingly, on laminin-1, the binding sites for the α-DG-derived glycans and for heparan sulfate both map to the LG4 module present at the C terminus of the laminin α1 chain and involve overlapping amino acid residues (2, 23). As a consequence, low concentrations of heparin block the binding of laminin-1 to α-DG (18). To address a possible interaction of arenavirus GPs with HS and other glycosaminoglycans (GAGs), we studied arenavirus pseudotype infection in a GAG-deficient cell line. For this purpose, we used the mutant CHO cell line pgsΑ-745, deficient in UDP-d-xylose:serine-1,3-d-xylosyltransferase, that lacks all GAGs (19). As shown in Fig. 6A, pseudotypes of LFV, LCMV, and VSV infected both the mutant psgΑ-745 line and wild-type CHO cells with similar efficiencies. In a complementary approach, we attempted to block pseudotype infection with soluble HS and heparin. Consistent with our findings with GAG-deficient cells, no significant blocking of infection was observed with either HS or heparin at concentrations of up to 2 mg/ml (Fig. 6B). In line with previously reported data (18), the same concentrations of heparin significantly reduced the binding of laminin-1 to immobilized α-DG (Fig. 6C). Together, our results exclude a significant role for GAGs in Old World arenavirus infection and indicate important differences in cell surface glycan recognition between arenavirus GPs and laminin laminin globular domains.
FIG. 6.
Infection of LFV and LCMV is not dependent on GAGs. (A) GAG-deficient psgΑ-745 cells and wild-type CHOK1 cells were infected with LFV, LCMV, and VSV pseudotypes at an MOI of 1 or with pseudotypes without GP (no GP). Infection was quantified after 48 h by luciferase assay as described in the legend of Fig. 2F (n = 3 ± SD). (B) Blocking of LFV infection with HS and heparin. Pseudotypes were preincubated with the indicated concentrations of HS and heparin for 1 h on ice and then added to monolayers of Vero cells (MOI of 1). Infection was quantified as described above (A) (n = 3 ± SD). (C) Heparin blocks binding of laminin-1 to α-DG. One microgram of mouse Engelbreth-Holm-Swarm sarcoma laminin-1 per milliliter was preincubated with the indicated concentrations of heparin and then added to purified rabbit skeletal muscle α-DG immobilized in microtiter plates. Bound laminin was detected with a polyclonal anti-laminin-1 antibody, followed by an HRP-conjugated secondary antibody and a color reaction using the substrate ABTS (n = 3 ± SD).
DISCUSSION
The present study's focus was the identification of the α-DG-derived glycan structures involved in the binding of arenaviruses. We show that under normal physiological conditions, the binding of Old World and clade C New World arenaviruses critically depends on protein O mannosylation of α-DG but not on more generic O-linked glycans present on the receptor. However, the overexpression of LARGE can restore arenavirus receptor function of α-DG in cells deficient in O mannosylation, indicating that the LARGE-dependent glycan modifications are the structures that are actually recognized by the viruses. Our results reveal a remarkable similarity in α-DG glycan binding between arenaviruses and ECM proteins and provide a striking example of how a pathogen mimics receptor recognition by host-derived ligands.
Carbohydrate parts of cell surface GPs, glycolipids, and proteoglycans function as receptors for a wide variety of enveloped and nonenveloped viruses (37, 49). Most cell-surface-associated glycans that serve as viral receptors are negatively charged carbohydrates, like, e.g., sialic acid and sulfated GAGs, that can be associated with a variety of cell surface proteins and/or lipids (39). In contrast, the functional O glycosylation of the arenavirus receptor α-DG, which is implicated in arenavirus binding (24, 29), is of remarkable specificity, with α-DG being currently the only known substrate (3, 26).
Considering the tissue-specific nature of the functional O glycosylation of α-DG, we addressed the correlation between receptor glycosylation, virus binding, and infection in a variety of human cell types for the highly pathogenic LFV and a prototypic immunosuppressive LCMV isolate. The use of well-characterized retroviral pseudotypes for LFV and LCMV allowed us to separate virus-receptor binding and entry from subsequent steps of viral replication that may be subject to cell-type-specific restrictions. Using a combination of virus binding assays and retroviral pseudotypes, we found that across the board, the functional glycosylation of α-DG was strictly required for virus binding and susceptibility to infection. This direct correlation between α-DG binding and infection pinpoints α-DG as being the main receptor used by both viruses. Cell types that adjoin ECM structures, like epithelial cells, hepatocytes, endothelial cells, and fibroblasts, express significant levels of functionally glycosylated α-DG and are highly susceptible to infection. In contrast, the established human B- and T-cell lines tested express a form of α-DG that lacks a detectable functional modification, rendering these cell types highly resistant. There is little evidence for the infection of T and B cells in humans infected with LFV (33, 50). However, several studies reported a detectable infection of T cells and B cells in acute and chronic LCMV infection in mouse (7, 14, 48). LCMV infection of T and B lymphocytes in mouse in vivo suggests that either the functional glycosylation of α-DG or the expression of an alternative receptor distinct from α-DG may occur during certain stages of lymphocyte differentiation and/or activation. Both possibilities are currently under investigation in our laboratory.
Based on the crucial role of α-DG's glycans for arenavirus binding and infection, we identified the specific sugar structures on the receptor that are directly involved in virus binding. Previous studies implicated O-mannosyl glycans in α-DG's function as a receptor for different isolates of LCMV (24). While a common glycan modification of proteins in plants and fungi, protein O mannosylation is rare in mammalian GPs (17). Since this modification of α-DG is highly conserved from invertebrates to humans, we were interested in the dependence on α-DG O mannosylation among arenaviruses belonging to phylogenetically distant groups. Using well-characterized cell lines deficient in the biosynthesis of O-mannosyl glycans (10, 31), we found that α-DG O mannosylation is also crucial for binding to LFV, the Old World virus Mobala virus, and the genetically more distant clade C New World arenaviruses. In contrast, the more generic core 1 O-glycan SiaAα(2-3)Galβ(1-3)GalNAc and SiaAα2-3Galβ1-4 moieties of α-DG's O-linked SiaAα2-3Galβ1-4GlcNAcβ1-2Man glycans were dispensable for virus binding. Together, our data suggest that the unusual and conserved O-linked GlcNAcβ1-2Man structure is, directly or indirectly, involved in α-DG's ability to serve as a high-affinity receptor for the envelope GPs of Old World and clade C New World arenaviruses.
Another crucial step in the biosynthesis of functional α-DG is modification by the putative glycosyltransferase LARGE, which is involved in the attachment of anionic sugar polymers of unknown structure to the N-terminal part of α-DG's mucin-type domain (3, 26). Although the glycosyltransferase activity of LARGE has not yet been identified, several previous reports strongly indicated that the LARGE-derived glycans are different from the O-mannosyl oligosaccharides found on α-DG (3, 4, 26, 27). The catalytic domains of LARGE are structurally unrelated to known protein O-mannosyltransferases and resemble enzymes with other activities (40). Moreover, the overexpression of LARGE results in highly glycosylated functional α-DG in cells bearing defects in POMT1 and POMGnT1, which are involved in different steps of the synthesis of the O-mannosyl sugar chains (4, 40).
Since our previous studies demonstrated a critical role for LARGE-dependent modification of α-DG in arenavirus binding (29), we addressed the relative contribution of α-DG's O-mannosyl oligosaccharides and the LARGE-derived glycans to arenavirus binding. In the present study, we found that the overexpression of LARGE restored α-DG's arenavirus receptor function in cells lacking the dolichol-P-mannose donor substrate for POMT1/2 (31, 32). Since LARGE is most likely involved in the synthesis of sugar chains different from O-mannosyl glycans, it appears unlikely that the overexpression of LARGE can lead to the O mannosylation of the receptor in Lec15.2 cells by a route that does not require the dolichol-P-mannose donor substrate. Our data therefore pinpoint the structurally unknown LARGE-derived glycans and not the O-mannosyl oligosaccharides to be directly involved in virus binding.
The similarity in the recognition of α-DG-derived glycans between laminin and arenaviruses raised the question of whether the viral GP might interact with other laminin-binding cell surface carbohydrates like HS. This was based on the fact that on laminin-1, binding to α-DG's O-glycans and HS involves overlapping amino acid residues on the LG4 modules located in the C-terminal G domain of the α1 chain (2, 23). Furthermore, GAGs like HS serve as cellular receptors for a variety of viruses (39). Using a cell line deficient in GAG biosynthesis and neutralization assays with soluble HS and heparin, we could exclude a significant role for GAGs in LFV and LCMV infection. This indicates important differences in the specificities of glycan recognition between arenavirus GPs and laminin laminin globular domains.
The recognition of a highly conserved functional glycan structure on α-DG by phylogenetically distant arenaviruses revealed by our present study is striking. We speculate that the ability of the virus to recognize this essential modification may have been advantageous during virus-host coevolution and is likely one reason for the broad host range and tissue tropism of arenaviruses. The remarkable similarity in glycan recognition between Old World arenaviruses and clade C New World arenaviruses further suggests that the use of α-DG as a receptor may have arisen prior to the evolutionary separation of New World from Old World viruses. While clade C viruses maintained the ability to recognize the ancient glycan structures on α-DG, clade A and clade B New World viruses evolved to recognize other cell surface receptors distinct from α-DG (41, 46). Our data also pinpoint LARGE-derived polymeric sugars to be the crucial binding motif for the human-pathogenic arenavirus LFV. The elucidation of this unique carbohydrate structure will likely provide a powerful basis for the development of antiviral drugs that can attack LFV before it can gain control over the host cell machinery for replication.
Acknowledgments
This is publication 18464 from the Molecular and Integrative Neurosciences Department of The Scripps Research Institute (TSRI).
We thank Michael B. A. Oldstone (TSRI) for his generous support and insightful discussions. We further acknowledge Michael Buchmeier (TSRI) for materials, Mark Lehrman (UT Southwestern Medical Center, Dallas, TX) for the Lec15.2 and Lec35 cells, and James Paulson (TSRI) for his advice. The retroviral construct pLZRs-Luc-gfp was kindly provided by Gary Nabel. The hepatoma cell line Huh7 was provided by the laboratory of Francis Chisari (TSRI).
This research was supported by U.S. Public Health grant AI55540 (to M. B. A. Oldstone and S. Kunz) and grant 1U54 AI065359 of the Pacific Southwest Regional Center of Excellence for Biodefense and Emerging Infectious Disease (S. Kunz and J. M. Rojek). K.P.C. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andac, Z., T. Sasaki, K. Mann, A. Brancaccio, R. Deutzmann, and R. Timpl. 1999. Analysis of heparin, alpha-dystroglycan and sulfatide binding to the G domain of the laminin alpha1 chain by site-directed mutagenesis. J. Mol. Biol. 287:253-264. [DOI] [PubMed] [Google Scholar]
- 3.Barresi, R., and K. P. Campbell. 2006. Dystroglycan: from biosynthesis to pathogenesis of human disease. J. Cell Sci. 119:199-207. [DOI] [PubMed] [Google Scholar]
- 4.Barresi, R., D. E. Michele, M. Kanagawa, H. A. Harper, S. A. Dovicio, J. S. Satz, S. A. Moore, W. Zhang, H. Schachter, J. P. Dumanski, and K. P. Campbell. 2004. LARGE can functionally bypass alpha-dystroglycan glycosylation defects in distinct congenital muscular dystrophy. Nat. Med. 7:696-703. [DOI] [PubMed] [Google Scholar]
- 5.Borrow, P., and M. B. A. Oldstone. 1992. Characterization of lymphocytic choriomeningitis virus-binding protein(s): a candidate cellular receptor for the virus. J. Virol. 66:7270-7281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., and M. B. Oldstone. 1994. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 198:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., A. Tishon, and M. B. Oldstone. 1991. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J. Exp. Med. 174:203-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, p. 1635-1668. In B. N. Fields, D. L. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 9.Buchmeier, M. J., H. A. Lewicki, O. Tomori, and M. B. Oldstone. 1981. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology 113:73-85. [DOI] [PubMed] [Google Scholar]
- 10.Camp, L. A., P. Chauhan, J. D. Farrar, and M. A. Lehrman. 1993. Defective mannosylation of glycosylphosphatidylinositol in Lec35 Chinese hamster ovary cells. J. Biol. Chem. 268:6721-6728. [PubMed] [Google Scholar]
- 11.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 12.Cohn, R. D. 2005. Dystroglycan: important player in skeletal muscle and beyond. Neuromuscul. Disord. 15:207-217. [DOI] [PubMed] [Google Scholar]
- 13.Combs, A. C., and J. M. Ervasti. 2005. Enhanced laminin binding by alpha-dystroglycan after enzymatic deglycosylation. Biochem. J. 390:303-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doyle, M. V., and M. B. Oldstone. 1978. Interactions between viruses and lymphocytes. I. In vivo replication of lymphocytic choriomeningitis virus in mononuclear cells during both chronic and acute viral infections. J. Immunol. 121:1262-1269. [PubMed] [Google Scholar]
- 15.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689-1698. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, L. H., J. B. McCormick, and K. M. Johnson. 1982. Inactivation of Lassa, Marburg, and Ebola viruses by gamma irradiation. J. Clin. Microbiol. 16:704-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endo, T. 2004. Structure, function and pathology of O-mannosyl glycans. Glycoconj. J. 21:3-7. [DOI] [PubMed] [Google Scholar]
- 18.Ervasti, J. M., and K. P. Campbell. 1993. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J. Cell Biol. 122:809-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer, S. A., M. B. Graham, M. J. Kuehnert, C. N. Kotton, A. Srinivasan, F. M. Marty, J. A. Comer, J. Guarner, C. D. Paddock, D. L. DeMeo, et al. 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 354:2235-2249. [DOI] [PubMed] [Google Scholar]
- 21.Geisbert, T. W., and P. B. Jahrling. 2004. Exotic emerging viral diseases: progress and challenges. Nat. Med. 10:S110-S121. [DOI] [PubMed] [Google Scholar]
- 22.Henry, M. D., J. S. Satz, C. Brakebusch, M. Costell, E. Gustafsson, R. Fassler, and K. P. Campbell. 2001. Distinct roles for dystroglycan, beta1 integrin and perlecan in cell surface laminin organization. J. Cell Sci. 114:1137-1144. [DOI] [PubMed] [Google Scholar]
- 23.Hohenester, E., D. Tisi, J. F. Talts, and R. Timpl. 1999. The crystal structure of a laminin G-like module reveals the molecular basis of alpha-dystroglycan binding to laminins, perlecan, and agrin. Mol. Cell 4:783-792. [DOI] [PubMed] [Google Scholar]
- 24.Imperiali, M., C. Thoma, E. Pavoni, A. Brancaccio, N. Callewaert, and A. Oxenius. 2005. O Mannosylation of α-dystroglycan is essential for lymphocytic choriomeningitis virus receptor function. J. Virol. 79:14297-14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamieson, D. J., A. P. Kourtis, M. Bell, and S. A. Rasmussen. 2006. Lymphocytic choriomeningitis virus: an emerging obstetric pathogen? Am. J. Obstet. Gynecol. 194:1532-1536. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 26.Kanagawa, M., F. Saito, S. Kunz, T. Yoshida-Moriguchi, R. Barresi, Y. M. Kobayashi, J. Muschler, J. P. Dumanski, D. E. Michele, M. B. Oldstone, and K. P. Campbell. 2004. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell 117:953-964. [DOI] [PubMed] [Google Scholar]
- 27.Kanagawa, M., and T. Toda. 2006. The genetic and molecular basis of muscular dystrophy: roles of cell-matrix linkage in the pathogenesis. J. Hum. Genet. 13:13. [DOI] [PubMed] [Google Scholar]
- 28.Kunz, S., K. H. Edelmann, J.-C. de la Torre, R. Gorney, and M. B. A. Oldstone. 2003. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology 314:168-178. [DOI] [PubMed] [Google Scholar]
- 29.Kunz, S., J. Rojek, C. Spiropoulou, R. Barresi, K. P. Campbell, and M. B. A. Oldstone. 2005. Posttranslational modification of α-dystroglycan, the cellular receptor for arenaviruses, by the glycosyltransferase LARGE is critical for virus binding. J. Virol. 79:14282-14296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kunz, S., N. Sevilla, J. M. Rojek, and M. B. Oldstone. 2004. Use of alternative receptors different than alpha-dystroglycan by selected isolates of lymphocytic choriomeningitis virus. Virology 325:432-445. [DOI] [PubMed] [Google Scholar]
- 31.Maeda, Y., S. Tanaka, J. Hino, K. Kangawa, and T. Kinoshita. 2000. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 19:2475-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manya, H., A. Chiba, A. Yoshida, X. Wang, Y. Chiba, Y. Jigami, R. U. Margolis, and T. Endo. 2004. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. USA 101:500-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCormick, J. B., and S. P. Fisher-Hoch. 2002. Lassa fever. Curr. Top. Microbiol. Immunol. 262:75-109. [DOI] [PubMed] [Google Scholar]
- 34.McDearmon, E. L., A. C. Combs, and J. M. Ervasti. 2003. Core 1 glycans on alpha-dystroglycan mediate laminin-induced acetylcholine receptor clustering but not laminin binding. J. Biol. Chem. 278:44868-44873. [DOI] [PubMed] [Google Scholar]
- 35.Michele, D. E., R. Barresi, M. Kanagawa, F. Saito, R. D. Cohn, J. S. Satz, J. Dollar, I. Nishino, R. I. Kelley, H. Somer, V. Straub, K. D. Mathews, S. A. Moore, and K. P. Campbell. 2002. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418:417-422. [DOI] [PubMed] [Google Scholar]
- 36.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 37.Ohtsubo, K., and J. D. Marth. 2006. Glycosylation in cellular mechanisms of health and disease. Cell 126:855-867. [DOI] [PubMed] [Google Scholar]
- 38.Oldstone, M. B. 2002. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Curr. Top. Microbiol. Immunol. 263:83-117. [DOI] [PubMed] [Google Scholar]
- 39.Olofsson, S., and T. Bergstrom. 2005. Glycoconjugate glycans as viral receptors. Ann. Med. 37:154-172. [DOI] [PubMed] [Google Scholar]
- 40.Patnaik, S. K., and P. Stanley. 2005. Mouse large can modify complex N- and mucin O-glycans on alpha-dystroglycan to induce laminin binding. J. Biol. Chem. 280:20851-20859. [DOI] [PubMed] [Google Scholar]
- 41.Rojek, J. M., C. F. Spiropoulou, and S. Kunz. 2006. Characterization of the cellular receptors for the South American hemorrhagic fever viruses Junin, Guanarito, and Machupo. Virology 349:476-491. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 42.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In B. N. Fields, D. L. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 43.Sevilla, N., E. Domingo, and J. C. de la Torre. 2002. Contribution of LCMV towards deciphering biology of quasispecies in vivo. Curr. Top. Microbiol. Immunol. 263:197-220. [DOI] [PubMed] [Google Scholar]
- 44.Singh, J., Y. Itahana, S. Knight-Krajewski, M. Kanagawa, K. P. Campbell, M. J. Bissell, and J. Muschler. 2004. Proteolytic enzymes and altered glycosylation modulate dystroglycan function in carcinoma cells. Cancer Res. 64:6152-6159. [DOI] [PubMed] [Google Scholar]
- 45.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. A. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spiropoulou, C. F., S. Kunz, P. E. Rollin, K. P. Campbell, and M. B. A. Oldstone. 2002. New World arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J. Virol. 76:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng, M. N., P. Borrow, M. B. A. Oldstone, and J. C. de la Torre. 1996. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J. Virol. 70:8438-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tishon, A., P. J. Southern, and M. B. Oldstone. 1988. Virus-lymphocyte interactions. II. Expression of viral sequences during the course of persistent lymphocytic choriomeningitis virus infection and their localization to the L3T4 lymphocyte subset. J. Immunol. 140:1280-1284. [PubMed] [Google Scholar]
- 49.Varki, A. 2006. Nothing in glycobiology makes sense, except in the light of evolution. Cell 126:841-845. [DOI] [PubMed] [Google Scholar]
- 50.Walker, D. H., J. B. McCormick, K. M. Johnson, P. A. Webb, G. Komba-Kono, L. H. Elliott, and J. J. Gardner. 1982. Pathologic and virologic study of fatal Lassa fever in man. Am. J. Pathol. 107:349-356. [PMC free article] [PubMed] [Google Scholar]
- 51.Weber, E. L., and M. J. Buchmeier. 1988. Fine mapping of a peptide sequence containing an antigenic site conserved among arenaviruses. Virology 164:30-38. [DOI] [PubMed] [Google Scholar]
- 52.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, A., K. Kobayashi, H. Manya, K. Taniguchi, H. Kano, M. Mizuno, T. Inazu, H. Mitsuhashi, S. Takahashi, M. Takeuchi, R. Herrmann, V. Straub, B. Talim, T. Voit, H. Topaloglu, T. Toda, and T. Endo. 2001. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev. Cell 1:717-724. [DOI] [PubMed] [Google Scholar]