Abstract
Epstein-Barr virus (EBV) causes hairy leukoplakia (HL), a benign lesion of oral epithelium that occurs primarily in the setting of human immunodeficiency virus (HIV)-associated immunodeficiency. However, the mechanisms of EBV infection of oral epithelium are poorly understood. Analysis of HL tissues shows a small number of EBV-positive intraepithelial macrophages and dendritic/Langerhans cells. To investigate a role for these cells in spreading EBV to epithelial cells, we used tongue and buccal explants infected ex vivo with EBV. We showed that EBV first infects submucosal CD14+ monocytes, which then migrate into the epithelium and spread virus to oral epithelial cells, initiating productive viral infection within the terminally differentiated spinosum and granulosum layers. Incubation of EBV-infected monocytes and oral explants with antibodies to CCR2 receptor and monocyte chemotactic protein 1 prevented entry of monocytes into the epithelium and inhibited EBV infection of keratinocytes. B lymphocytes played little part in the spread of EBV to keratinocytes in our explant model. However, cocultivation of EBV-infected B lymphocytes with uninfected monocytes in vitro showed that EBV may spread from B lymphocytes to monocytes. Circulating EBV-positive monocytes were detected in most HIV-infected individuals, consistent with a model in which EBV may be spread from B lymphocytes to monocytes, which then enter the epithelium and initiate productive viral infection of keratinocytes.
Epstein-Barr virus (EBV) is a human herpesvirus with oncogenic potential, contributing to the development of lymphoproliferative diseases of B lymphocytes and nasopharyngeal carcinoma (18). EBV infects about 90% of the human population, but in most immunocompetent individuals EBV persists in latent form and does not cause any significant disease. During human immunodeficiency virus (HIV)-associated immunosuppression, however, EBV may reactivate and may be associated with development of a benign lesion of oral mucosal epithelium known as hairy leukoplakia (HL) (12-14). The histopathology of HL includes acanthosis, irregular hyperparakeratosis, and balloon cell formation within the spinosum and granulosum layers of the epithelium, which may result from high-level EBV replication (14).
HL is a common lesion in HIV-positive patients with low CD4+ counts, suggesting that immunosuppression is an important factor in its development. The source of the EBV in HL is not known. Several lines of evidence support hematogenous spread from circulating white blood cells (WBC) (11, 29). The main reservoir of latent EBV infection in the body is memory B lymphocytes, but mechanisms of spread from cells in the blood compartment to the mucosal epithelium are not known. Furthermore, HL epithelium may support both latent and lytic replication of EBV (43), with lytic EBV replication and cell-to-cell spread of virions restricted exclusively to the terminally differentiated stratum spinosum and granulosum layers (28, 31, 44). Neither the mechanisms by which EBV enters and establishes productive infection in these cell layers nor the reasons for its absence in the basal and parabasal cell layers are understood.
In this work we investigated EBV infection and dissemination in HL biopsy specimens and in freshly isolated normal tongue and buccal explants infected ex vivo with EBV. Analysis of HL sections showed that intraepithelial macrophages and Langerhans cells (LC) were positive for EBV. Cocultivation of oral explants with EBV-infected monocytes led to migration of these monocytes/macrophages/LC into mucosal epithelium and spread of virus within the terminally differentiated oral keratinocytes. Consistent with this mechanism of EBV infection of oral epithelium, we confirmed the presence of circulating EBV-infected monocytes in HIV-positive individuals. We further showed that B lymphocytes can transmit EBV to monocytes in vitro, suggesting that these cells may be the ultimate source of EBV infection of monocytes. Our data show for the first time that EBV-infected monocytes/macrophages/LC may migrate into oral epithelium and may facilitate dissemination of EBV to oral keratinocytes.
MATERIALS AND METHODS
HL tissue biopsy samples.
Biopsy samples of HL tongue tissue containing epithelium and connective tissue were obtained using 4-mm-diameter biopsy punches from 19 HIV-positive individuals. These biopsy samples were collected between 1986 and 1997, and the tissues were frozen and stored in the tissue bank of the Oral AIDS Center Clinic of the Department of Orofacial Sciences, University of California, San Francisco. The biopsy tissues were sectioned in 7-μm-thick slices.
Establishment of an ex vivo oral tissue system for EBV infection.
Fresh biopsy samples of tongue and buccal mucosa containing epithelium and connective tissue were obtained using 4-mm-diameter biopsy punches from 25 healthy HIV-seronegative volunteers (age range, 30 to 41 years; 15 males and 10 females) who had no inflammation in the oral cavity. Some of these individuals donated their tissues more than once. Approval for this project was obtained from the Institutional Review Board at the University of California, San Francisco. Immediately after biopsy, the tissues were placed in a tube with 2 ml of RPMI medium (explant medium) containing 10% heat-inactivated fetal bovine serum, 20 mM HEPES, 100 mM glutamine, 20 μg/ml gentamicin, 200 U/ml penicillin, 200 μg/ml streptomycin, and 50 ng/ml amphotericin B. Tissues were washed three times with cold explant medium and placed in 24-well plates with 1 ml of explant medium.
Cell-free EBV infection of oral tissue explants.
To infect tissue explants, we used the B95-8 strain of EBV. Virus was propagated by inducing B95-8 cells with 30 ng/ml phorbol 12-myristate 13-acetate and 4 mM butyric acid (both from Sigma) for 10 days. Virus was then concentrated using a Centricon-100 concentrator (Millipore), and viral titer was determined by quantitative real-time PCR using BZLF-1-specific primers (forward, 5′-AAA TTT AAG AGA TCC TCG TGT AA ACA TC-3′; reverse, 5′-CGC CTC CTG TTG CCG CAG AT-3′). For some experiments we used concentrated EBV B95-8 virus from Advanced Biotechnologies Inc. (ABI). Tissue explants were infected with 108 cell-free B95-8 virions per explant and incubated for 1 h at 37°C on a shaker. Under these conditions, the virus had access to tissue from the epithelial and stromal surfaces. EBV-infected tissues were incubated at 37°C in 5% CO2, and one-third of the medium from each well was changed daily. The tissues were fixed with 4% paraformaldehyde, embedded with optimal cutting temperature compound (Fisher), and frozen in liquid nitrogen. The explants were sectioned in 7-μm-thick slices.
Cell-associated EBV infection of oral tissue explants.
To examine EBV infection of tissue explants by EBV-infected B lymphocytes or monocytes, peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood using a Ficoll-Paque Plus density gradient (Sigma). The B lymphocytes and monocytes were then isolated by positive selection using anti-CD19 and anti-CD14 Microbeads (Miltenyi Biotec), respectively, and separated in a MACS LS column (Miltenyi Biotec). The purity of monocytes and B lymphocytes was examined by fluorescence-activated cell sorting (FACS) assay using antibodies to CD14 and CD20 markers, respectively. B lymphocytes and monocytes of >95% purity were infected with cell-free EBV B95-8 virions at a multiplicity of 10 virions/cell for 3 days. Each tongue or buccal explant was independently cocultivated with 106 monocytes or B lymphocytes. One-third of the medium from each explant was changed daily.
Assay for inhibition of monocyte transmigration into oral epithelium.
To inhibit monocyte transmigration into oral epithelium, we treated the oral explants and monocytes with antibodies to monocyte chemotactic protein 1 (MCP-1) (Santa Cruz Biotechnology, Inc.) and CCR2 (Epitomics). Three tongue biopsy samples were collected, and each was cut in two pieces. The first piece was incubated with antibodies to MCP-1 (10 μg/ml) for 24 h. The second piece was not treated and served as a control. Freshly isolated monocytes were infected with EBV at a multiplicity of infection of 10 virions/cell, and at 3 days postinfection cells were divided between two tubes. Cells in the first tube were treated with antibodies to CCR2 (10 μg/ml) for 1 h on ice, and cells in the second tube were not treated. EBV-infected and anti-CCR2 antibody-treated monocytes were then cocultivated with MCP-1-treated tongue explants. As a negative control, the untreated monocytes were cocultivated with untreated tissue explants. Tissue explants were cultured for 7 days, and every other day one-third of the medium was replaced with fresh medium. Medium in experimental tissues was replaced with fresh medium containing antibodies to CCR2 and MCP-1. Tissues were fixed, sectioned, and immunostained for EBV viral capsid antigen (VCA) p18 and CD68.
Antibodies and immunofluorescence laser scanning confocal microscopy.
Immune serum from a nasopharyngeal carcinoma patient with a high titer for EBV VCA was used for immunostaining EBV proteins. To reduce background signals, the anti-EBV human serum was incubated with EBV-negative normal tongue sections for 1 h. The HL sections and tongue and buccal sections infected ex vivo with EBV were incubated overnight with blocking buffer containing 3% bovine serum albumin (BSA) in phosphate-buffered saline, pH 7.2. Before incubation with EBV-specific human serum, tissue sections were incubated with EBV-negative human serum (Blackhawk BioSystem, Inc.) and 10% affinity-purified Fab fragment of goat anti-human immunoglobulin G (Jackson Immunochemicals) for 1 h.
To detect individual EBV proteins, we used the following antibodies: mouse monoclonal antibodies (MAbs) to gp350/220 (ABI), BZLF-1 (Argene), and rat antiserum to VCA p18 (Virostat). For immunostaining of keratinocytes and junctional proteins we used rabbit antibodies to keratin 1 (Covance), guinea pig antibodies to cytokeratin (Sigma), and rabbit antibodies to ZO-1 (Zymed). For staining of immune cells we used mouse MAb (BD Biosciences Pharmingen) and rat antibody to CD1a (marker for LC) (Biosource); mouse MAb and rabbit antibody to CD14 (marker for monocytes/macrophages) (Cell Science Inc.); mouse MAb (Dako) and goat antibody (R&D Systems) to CD68 (marker for macrophages); mouse MAbs to CD20 and CD19 (B lymphocytes), CD3 (T cells), and CD86 (marker for activated LC and macrophages) (all from BD Biosciences Pharmingen). Mouse antibody to CD138 (marker for differentiated B lymphocytes) was purchased from US Biological. All immunostaining was performed using double or triple immunofluorescence assays with antibodies to EBV proteins and immune cell or keratinocyte markers. Secondary antibodies conjugated with fluorescein isothiocyanate, Texas red, or Cy5 were purchased from Jackson Immunochemicals. The specificity of each antibody was confirmed by negative staining with corresponding isotype control antibodies. Cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes) (blue). For quantitative analysis the sections of HL and EBV-infected oral explants were costained with anti-EBV human serum and antibodies to immune cell markers, and the EBV-positive cells expressing immune cell markers were counted. EBV-infected oral keratinocytes were detected by costaining with anti-EBV human serum and anti-keratin 1 antibody. The size of sections in all experiments was approximately 12 mm2. For each staining a minimum of five sections was used, and the number of EBV-infected immune and epithelial cells in mucosal epithelium was counted within the entire epithelium of the section. Immunostained tissue sections were analyzed with a Bio-Rad MRC1024 confocal microscope.
Western blot assay.
Gradient-purified EBV virions (ABI) were extracted with radioimmunoprecipitation assay lysis buffer containing 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate, Tris, pH 8.0, and a cocktail of protease inhibitors: phenylmethylsulfonyl fluoride (1 mM), aprotinin (10 μg/ml), leupeptin (10 μg/ml), and pepstatin A (10 μg/ml). The viral proteins were separated on a 4 to 20% gradient Tris-glycine gel (Invitrogen) and transferred to polyvinylidene difluoride membranes (Millipore, Eschborn, Germany). EBV proteins were analyzed by using EBV-positive serum from a nasopharyngeal carcinoma patient and normal serum from an EBV-seronegative individual (Blackhawk BioSystem, Inc.). Protein bands were visualized using the SuperSignal West Pico chemiluminescent substrate kit (Pierce, Rockford, IL) according to the manufacturer's protocols.
Quantitation of EBV copy numbers in B lymphocytes and monocytes using a QC-PCR assay.
We analyzed EBV copy numbers in peripheral blood B lymphocytes and monocytes from 50 HIV-negative (mean age, 36 years) and 28 HIV-positive (mean age, 47 years) individuals. CD14+ monocytes and CD19+ B lymphocytes were isolated from PBMC using anti-CD14 and anti-CD19 Microbeads (Miltenyi Biotec). The purity of monocytes and B lymphocytes was monitored by FACS assay using antibodies to CD14 and CD20, respectively. Total DNA was extracted using the QIAamp blood kit (QIAGEN Inc.). Twenty nanograms of EBV DNA was detected and quantitated using a quantitative comparative real-time PCR (QC-PCR) assay (30) with EBV BZLF-1 gene-specific primers (forward, 5′-AAA TTT AAG AGA TCC TCG TGT AA ACA TC-3′; reverse, 5′-CGC CTC CTG TTG CCG CAG AT-3′) and a fluorigenic probe [5′-(6-carboxyfluorescein)-ATA ATG GAG TCA ACA TCC AGG CTT GGG C-(6-carboxytetramethylrhodamine)-3′]. The beta-globin gene was used as a positive control [forward, 5′ TGG CCA ATC TAC TCC CAG GA-3′; reverse, 5′-CAT GGT GTC TGT TTG AGG TTG C-3′; fluorigenic probe, 5′-(6-carboxyfluorescein)-CAG GGC TGG GCA TAA AAG TCA GGG C-(6-carboxytetramethylrhodamine)-3′]. QC-PCR analysis was performed on an ABI Prism 7900 detection system (Applied Biosystems). Thermocycling conditions were 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min for 40 cycles. Each reaction mixture contained 1× TaqMan Universal Master Mix with final concentrations of 5.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 20 pmol each of forward and reverse primers, 10 pmol of TaqMan probe, and 0.5 U of Hotstart AmpliTaq Gold (Applied Biosystems) in a 20-μl volume in a 384-well plate. Experimental samples were run in triplicate, and the mean viral load was calculated. Each run had at least two “no-template” controls to check for amplicon contamination. DNA from the Namalwa cell line (American Type Culture Collection), which contains two copies of EBV per cell, was used to generate standard curves (30).
Electron microscopy.
HL biopsy specimens were fixed in 1.5% Karnovsky fixative, postfixed in 1% Palade buffer, and then dehydrated and embedded. Sections were stained in uranyl acetate and lead citrate, and were examined with a JEM-1200EX electron microscope.
Fluorescence in situ hybridization (FISH) assay.
Cryosections of infected and uninfected tongue explants and HL sections were hybridized with a 555-bp EBV BMRF-2 biotinylated probe. Before hybridization samples were treated with 0.1% pepsin in 0.2% HCl for 10 min at 37°C. Cells on slides were denatured in 70% formamide-2× SSC (l× SSC is 0.15 M NaCl plus 0.015 M sodium citrate, pH 7) at 70°C for 2 to 3 min and dehydrated in ethanol. Ten microliters of hybridization mixture, consisting of a total of 40 ng of labeled probe in 50% formamide-50% hybridization buffer, was denatured for 5 min at 95°C. Hybridization was performed overnight at 37°C under a coverslip in a moist chamber. For fluorescent probe detection, the slides were washed three times in 50% formamide-2× SSC at 60°C, blocked in 1% BSA-2× SSC for 5 min, and stained with Cy3-labeled streptavidin at a 1:100 dilution (Vector Laboratories) in 1% BSA-2× SSC. Next, the slides were washed in 2× SSC three times, and in the final step cell nuclei were counterstained with TO-PRO-3 iodide (Molecular Probes). Then cells were analyzed by confocal microscopy.
Statistical analysis.
To compare differences in EBV copy numbers in monocytes and B lymphocytes between HIV-positive and HIV-negative individuals, QC-PCR data were analyzed using the Wilcoxon rank sum test; P values of <0.05 were considered to be significant.
RESULTS
Mucosal epithelium of HL contains EBV-infected LC and macrophages.
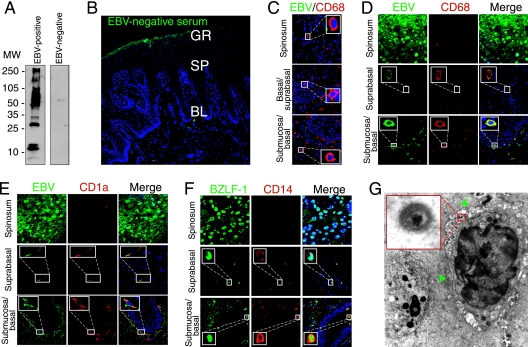
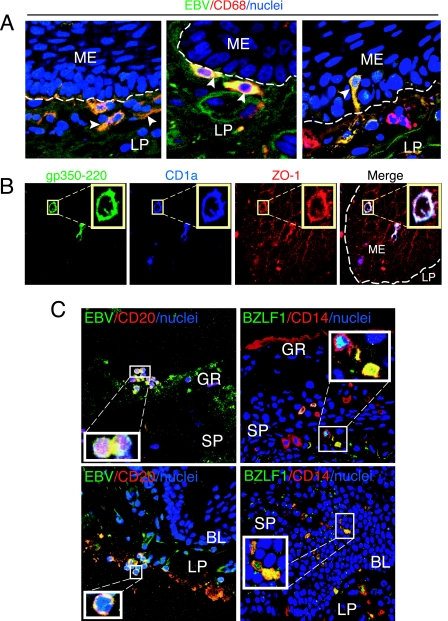
To analyze the presence of LC, macrophages, and EBV infection in HL and normal oral epithelium, we immunostained cryosections of HL lesions and normal oral epithelium with antibodies to CD1a and CD68, which are expressed in LC and macrophages, respectively. LC were also identified morphologically by the presence of dendrocytes. A comparison of the numbers of LC and macrophages in 19 HL tissues and eight normal tongue/buccal explants showed that both LC and macrophages in HL mucosal epithelium were reduced in number compared with those in normal epithelium (Table 1). To examine the presence of EBV in HL epithelium, cryosections of all 19 HL biopsy samples were immunostained for EBV using serum containing high titers of anti-EBV VCA from a patient with nasopharyngeal carcinoma. As a negative control we used human serum that was EBV negative, as confirmed by Western blot assay with total EBV proteins (Fig. 1A) and by immunostaining of HL sections (Fig. 1B). Coimmunostaining of normal tongue sections with EBV-positive serum and markers for CD68 (Fig. 1C) and CD1a or CD14 (data not shown) did not show EBV-infected immune or epithelial cells. Confocal immunofluorescence analysis showed that the epithelium of all HL tissues was infected with EBV. Six HL tissues contained EBV-positive macrophages and LC (Fig. 1D and E; Table 1). These cells were detected both in the lamina propria and within keratinocytes (one to five cells/section). Costaining of EBV BZLF-1 protein with CD14 showed that in two of the six tissues the submucosal and intraepithelial CD14+ cells expressed BZLF-1 (Fig. 1F), indicating that these cells may have lytic EBV infection. Electron microscopy analysis of four HL lesions showed that three had suprabasal intraepithelial WBC (Table 1; Fig. 1G, green arrowheads) containing herpesvirus-like virions consistent with EBV (Fig. 1G, inset). EBV-positive intraepithelial B lymphocytes were not detected in any of the HL samples. Thus, analysis of HL sections showed a small number of EBV-infected LC and macrophages in the lamina propria and mucosal epithelium of HL, despite overall LC depletion compared with normal tissue.
TABLE 1.
Presence of EBV-positive LC and macrophages in HL mucosal epithelium
| Tissue type and designation | Total no. of cells per section positive for marker (no. of EBV-positive cells)c:
|
|
|---|---|---|
| CD1a | CD68 | |
| HL | ||
| 045 | 26.3 ± 2.05 (2.6 ± 0.4) | 33.6 ± 2.05 (3 ± 0.8) |
| 143 | 35.6 ± 2.4 (1.3 ± 0.4) | 60.6 ± 4.2 (4.3 ± 0.9) |
| 052 | 22.3 ± 2.4 (2.3 ± 0.4) | 73.3 ± 3.8 (2 ± 0.8) |
| 090a | 36.3 ± 4.1 (2.6 ± 0.4) | 65.3 ± 4.4 (3.6 ± 0.4) |
| 034a | 33.3 ± 3.2 (5.3 ± 0.4) | 70.5 ± 4.9 (4.3 ± 0.4) |
| 059a | 55.3 ± 4.4 (2 ± 0.8) | 74.5 ± 4.1 (2.3 ± 0.9) |
| 065 | 6.3 ± 0.4 (0) | 19.3 ± 2.05 (0) |
| 122 | 4.6 ± 0.4 (0) | 13.3 ± 3.09 (0) |
| 064 | 0 (0) | 5.6 ± 1.6 (0) |
| 033 | 0 (0) | 7.6 ± 1.2 (0) |
| 385 | 3.6 ± 0.4 (0) | 7.3 ± 0.9 (0) |
| 080 | 5.6 ± 0.4 (0) | 9 ± 3.2 (0) |
| 068 | 0 (0) | 4.3 ± 0.4 (0) |
| 024 | 0 (0) | 5.6 ± 1.2 (0) |
| 044 | 4.3 ± 0.4 (0) | 8.7 ± 2.4 (0) |
| 109 | 0 (0) | 7.3 ± 2.05 (0) |
| 123 | 3 (0) | 11.6 ± 1.6 (0) |
| 611 | 0 (0) | 6.3 ± 1.5 (0) |
| 612 | 0 (0) | 12.6 ± 2.1 (0) |
| Normal oralb | ||
| 730B | 95.6 ± 4.2 | 104.3 ± 12.7 |
| 467T | 248.6 ± 13.2 | 368.3 ± 30.7 |
| 240B | 235.5 ± 18.6 | 366.3 ± 51.3 |
| 240T | 153.4 ± 14.3 | 227.6 ± 14.7 |
| 294T | 227.6 ± 16.6 | 231.4 ± 19.4 |
| 311B | 147.3 ± 19.6 | 380.6 ± 19.8 |
| 310T | 137.6 ± 17.1 | 256.6 ± 13.7 |
| 276B | 189.6 ± 14.6 | 68.3 ± 13.8 |
EBV virions were detected in intraepithelial WBC by electron microscopy.
T, tongue; B, buccal.
Values are means ± standard deviations.
FIG. 1.
Analysis of EBV-infected immune cells in HL lesions. (A) Total proteins of purified EBV virions were analyzed using a Western blot assay with EBV-positive and -negative human sera. MW, molecular weight (in thousands). (B) HL sections were immunostained with EBV-negative human serum. GR, granulosum; SP, spinosum; BL, basal lamina. (C) Normal tongue sections were coimmunostained with EBV-positive human serum (green) and MAbs to CD68 (red). Only merged panels are shown. (D and E) HL sections were costained with EBV-specific immune serum (green) and MAbs to CD68 or CD1a markers (red). (F) HL section costained with MAb to BZLF-1 (green) and rabbit antibodies to CD14 (red). (D to F) Yellow in merged panels shows colocalization of EBV proteins with immune cell markers. (B to F) Cell nuclei were stained in blue. (G) Detection of EBV-infected WBC by electron microscopy in the suprabasal layer of HL tissue (green arrowheads). The inset shows a magnified EBV virion.
EBV-infected submucosal monocytes/macrophages migrate into mucosal epithelium.
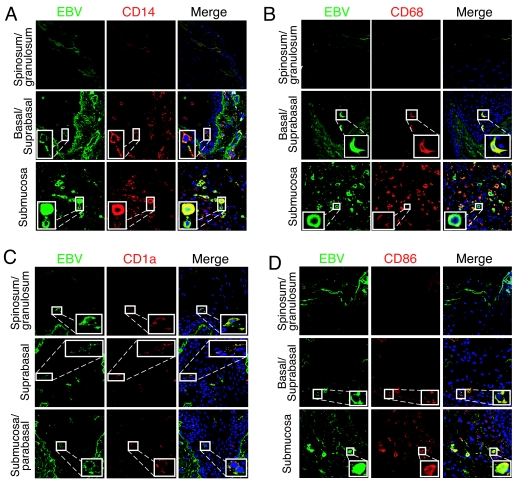
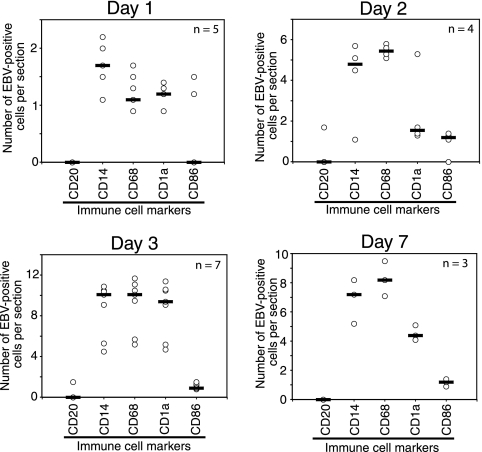
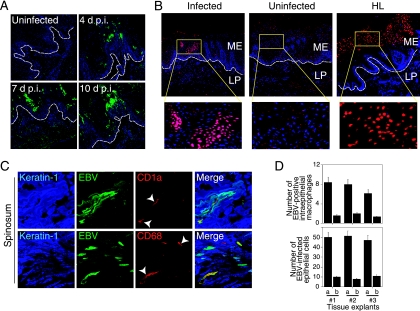
The presence of EBV-positive LC and macrophages within the epithelial compartment of HL suggested that these cells might migrate from the lamina propria and disseminate virus within the epithelium. To explore this hypothesis experimentally, we established normal tongue and buccal tissue explants from healthy individuals. Twenty oral explants (15 tongue and five buccal) were infected with cell-free EBV virions under floating conditions, and at 1, 2, 3, and 7 days postinfection tissues were fixed, sectioned, and coimmunostained for EBV proteins and immune cell markers. Confocal immunostaining analysis showed that in these explants the CD14+/CD68+ monocytes/macrophages (Fig. 2A and B), CD1a+ LC (Fig. 2C), and CD86+ activated LC/macrophages (Fig. 2D) were infected with EBV. EBV-infected immune cells were detected within the lamina propria and mucosal epithelium. To study the pattern of their distribution within the oral mucosal epithelium, the EBV-positive cells were quantitatively analyzed. At 1 day postinfection, the cells were detected predominantly in the lamina propria, and only a few (one to two cells/section) were detected within the mucosal epithelium (Fig. 3). At 2 and 3 days postinfection, the number of EBV-infected intraepithelial CD1a+ LC and CD68+ macrophages gradually increased to 6 to 12 cells/section (Fig. 3). At 7 days postinfection, the number of intraepithelial EBV-positive CD1a+ LC decreased by about twofold. EBV-infected intraepithelial CD86+-activated macrophages or LC were detected at 2, 3, and 7 days postinfection, and their numbers did not change significantly. EBV-positive cells expressing B-lymphocyte markers CD19 and CD20 were rare (approximately one to two cells/section) and were detected mostly in the lamina propria. Only 2 of 20 oral explants contained intraepithelial B lymphocytes, and they were rare (one to two cells/section) (Fig. 3). A small number of EBV-positive CD3+ cells (approximately one to two cells/section) were also detected in the mucosal epithelium in 3 of 20 tissues. The distribution pattern and migration efficiency of EBV-infected immune cells did not differ significantly between tongue and buccal explants.
FIG. 2.
EBV infection of ex vivo tongue explants. EBV-infected tongue explants were grown in vitro for 3 days and then sectioned and coimmunostained with EBV-specific human serum (green) and markers for CD14 (A), CD68 (B), CD1a (C), and CD86 (D) (all in red). Nuclei were stained in blue. Yellow in merged panels shows colocalization of EBV proteins with immune cell markers.
FIG. 3.
Ex vivo cell-free EBV infection of tongue and buccal tissue explants. Tongue and buccal tissue explants were infected with cell-free EBV for 1, 2, 3, and 7 days. Then the explants were fixed, sectioned, and coimmunostained with EBV-specific human serum and antibodies to B lymphocytes (CD20), monocytes (CD14), macrophages (CD68), LC (CD1a), and activated macrophages/LC (CD86). The intraepithelial EBV-positive immune cells were counted within the oral epithelium, and their average numbers from five sections are presented. Each data symbol indicates one sample. n, number of tissue explants. Horizontal lines represent median values.
To confirm the migration of EBV-infected monocytes but not B lymphocytes into epithelium, we purified CD14+ and CD19+ cells from the peripheral blood of healthy volunteers and infected the cells with EBV. At 3 days postinfection cells were examined for BZLF-1 expression by immunofluorescence assay, and these results showed that approximately 50% of B lymphocytes and 80% of monocytes were positive for BZLF-1 expression (data not shown). These EBV-infected monocytes and B lymphocytes were used for cocultivation of tongue and buccal explants.
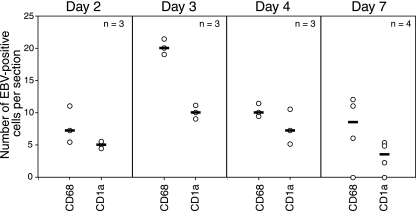
Thirteen (nine tongue and four buccal) oral explants were cocultivated with EBV-infected CD14+ cells for 2, 3, 4, and 7 days. Confocal immunostaining analysis of these explants showed intensive migration of EBV-infected macrophages and LC into oral epithelium. The highest number of intraepithelial macrophages and LC was detected at 3 days postcocultivation (20 cells/section), and numbers were reduced at 7 days postcocultivation (Fig. 4). There were more EBV-infected intraepithelial macrophages than EBV-infected intraepithelial LC. Significant differences between tongue and buccal epithelium in migration of EBV-infected macrophages and LC were not observed. EBV-infected CD14+/CD68+ cells were found close to the basement membrane (Fig. 5A, left), on the basement membrane (Fig. 5A, middle), and within the basal/parabasal epithelial cell layers (Fig. 5A, right). They had a shape consistent with motility, suggesting transmigration of submucosal macrophages into epithelium. EBV-infected intraepithelial CD1a+ (Fig. 5B) and CD68+ (data not shown) cells expressed the tight junction protein ZO-1, consistent with the ability of these cells to migrate by forming transient tight junctions with keratinocytes. Expression of ZO-1 and the migration pattern of EBV-infected macrophages/LC were not significantly different from those of uninfected macrophages/LC. About half of intraepithelial monocytes/macrophages were positive for BZLF-1, indicating that these cells had lytic EBV infection (Fig. 5C, right panels). Migration of CD14+ cells from the mucosal (apical) surface of the epithelium was not detected (Fig. 5C, upper right panel).
FIG. 4.
Cocultivation of tongue and buccal tissue explants with EBV-infected monocytes. Purified CD14+ monocytes were infected with EBV and 3 days later were cocultured with oral explants. At 2, 3, 4, and 7 days after cocultivation, cryosections of explants were coimmunostained with anti-EBV human serum and markers for macrophages (CD68) or LC (CD1a). EBV-positive intraepithelial macrophages and LC were counted, and the average numbers from five sections are presented. n, number of explants. Horizontal lines indicate median values.
FIG. 5.
Migration of EBV-infected submucosal monocytes into oral epithelium. (A) Tongue tissue sections at 24 h post-cocultivation with EBV-infected monocytes were coimmunostained with EBV-specific serum (green) and MAb to CD68 (red). Artificial white lines were added to the basement membrane to show translocation of EBV-infected submucosal cells to the epithelium. Yellow indicates colocalization of EBV proteins with CD68+ cells (arrowhead). (B) The same tongue tissue as in panel A was coimmunostained with MAb to EBV gp350/220 (green), rat antibody to CD1a (blue), and rabbit antibody to ZO-1 (red). White in the merged panel indicates colocalization of gp350/220, CD68, and ZO-1. (C) EBV-infected monocytes and B lymphocytes were cocultivated with tongue tissue for 2 days, and a section of this tissue was coimmunostained with EBV-specific human serum (green) and mouse MAb to CD20 (red) (left panels) and with mouse MAb to EBV BZLF-1 (green) and rabbit antiserum to CD14 (red) (right panels). Yellow in the merged panels shows colocalization of EBV proteins with CD20 and CD14. (A and C) Only merged panels are shown. Nuclei were stained in blue. ME, mucosal epithelium; GR, granulosum; SP, spinosum; BL, basal lamina; LP, lamina propria.
In parallel experiments six tongue explants were cocultured with EBV-infected B lymphocytes for 2 and 7 days. Immunostaining of these explants showed that the EBV-positive B lymphocytes had accumulated as a cluster on the mucosal surface of the tongue explants (Fig. 5C, upper left panel). Rare EBV-infected B lymphocytes (one to two cells/section) were detected in the lamina propria (Fig. 5C, lower left panel). Migration of EBV-infected B lymphocytes into the epithelium was not detected in any of the tissues cocultivated with EBV-infected CD19+ cells. Immunostaining of all six explants for CD138 showed that none had EBV-positive differentiated B lymphocytes. In these explants we detected a low number of EBV-infected CD68+ and CD1a+ cells in the lamina propria and mucosal epithelium (one to three cells/section).
EBV-infected intraepithelial macrophages and LC facilitate spread of EBV infection to keratinocytes of the oral epithelium.
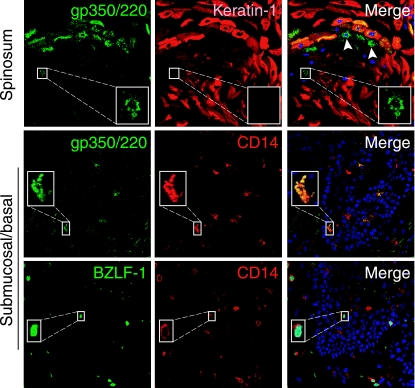
To determine whether virus could be transferred to oral mucosal keratinocytes by EBV-infected intraepithelial macrophages/LC, we coimmunostained all 20 tongue/buccal explants infected with cell-free EBV with antibodies to EBV proteins and keratin 1. Only 1 of 20 tongue tissues had a small cluster (9 to 10 cells) of EBV-infected keratinocytes within the spinosum layer (Fig. 6, top). Positive staining of these cells for EBV glycoprotein 350/220 (gp350/220) indicated lytic EBV infection. An EBV-infected, keratin-1-negative WBC was detected near the EBV-infected keratinocytes (Fig. 6, top, white arrows and inset). Detection of gp350/220- and BZLF-1-positive intraepithelial and submucosal CD14+ cells (Fig. 6, middle and bottom, respectively) in this tissue suggested that these monocytes/macrophages may migrate from the lamina propria into oral epithelium and infect the nearby oral keratinocytes.
FIG. 6.
Detection of EBV-infected monocytes and epithelial cells in tongue tissue explants. Tongue explant was infected with cell-free EBV and at 3 days postinfection was coimmunostained for EBV proteins and markers for CD14 or keratin 1. Upper panels show costaining with mouse MAb to gp350/220 (green) and rabbit antiserum to keratin 1 (red). Middle panels show costaining with mouse MAb to gp350/220 and rabbit antiserum to CD14 (red). Lower panels show costaining with mouse MAb to BZLF-1 (green) and rabbit antiserum to CD14 (red). White arrowheads and insets in upper panels show EBV-infected WBC in close proximity to EBV-infected keratinocytes. Nuclei were stained in blue.
Given the low rate of EBV infection of keratinocytes in explants exposed to cell-free EBV virions ex vivo, we next sought to determine whether oral mucosal keratinocytes could be infected by cocultivation of tissue explants with EBV-infected monocytes. We detected EBV-positive keratinocytes within the spinosum of 9 of 14 oral explants cocultivated with CD14+ cells. At 2 to 3 days of cocultivation a small number of EBV-infected keratinocytes were detected (≈5 to 10 cells/section), and their numbers substantially increased after 4 (≈10 to 20 cells/section), 7 (≈40 to 50 cells/section), and 10 (≈60 to 100 cells/section) days of cocultivation (Fig. 7A). EBV infection was detected within the spinosum layers of the oral epithelium but not in the basal and parabasal layers. EBV infection of oral epithelial cells was also confirmed by FISH assay using an EBV-specific DNA probe (Fig. 7B). FISH staining was detected in the nuclei of the small foci of keratinocytes in the spinosum layer of EBV-infected tongue explants (Fig. 7B, left). The EBV-specific DNA probe did not detect EBV signals in uninfected tissue explants (Fig. 7B, middle) but did detect EBV DNA in all keratinocytes of the spinosum and granulosum layers of HL epithelium (Fig. 7B, right). In oral explants cocultivated with EBV-infected CD14+ cells, the EBV-positive CD68+ and CD1a+ cells were detected in close proximity to the EBV-infected keratinocytes (Fig. 7C, white arrowhead).
FIG. 7.
EBV infection of oral keratinocytes by transmigrated EBV-infected monocytes. (A) Tongue explants were cocultivated with EBV-infected CD14+ monocytes for 4, 7, and 10 days, and cryosections of these explants were immunostained with EBV-positive human serum (green). Uninfected tongue tissue was immunostained with the same EBV-positive human serum and served as a control. White lines show basement membranes, and cell nuclei were stained in blue. d p.i., days postinfection. (B) A tongue explant that was cocultivated with EBV-infected CD14+ monocytes for 7 days was analyzed by FISH assay using EBV BMRF-2 probe (red). An uninfected tongue explant and HL tissue served as negative and positive controls, respectively. Cell nuclei were stained in blue, and only merged panels are shown. White lines indicate basement membranes. LP, lamina propria; ME, mucosal epithelium. (C) Tongue tissue was cocultivated with EBV-infected CD14+ cells for 7 days; sectioned; and immunostained for keratin 1 (blue), EBV-positive human serum (green), and CD1a or CD68 (red). Images were obtained from the spinosum layer. Yellow and light blue in the merged section indicate colocalization of EBV signals with CD1a or CD68 and keratin 1, respectively. (D) The tongue biopsy samples were cut in two pieces, one of which was treated with antibodies to MCP-1 and the other of which was untreated. Freshly isolated monocytes were infected with EBV, and at 3 days postinfection cells were divided between two tubes. Cells in one tube were treated with CCR2 antibodies, and those in the other tube were not. Then antibody-treated monocytes were cocultivated with antibody-treated explants (experimental explants) for 7 days. As a control, untreated monocytes were cocultured with untreated explants. Experimental and control explants were coimmunostained for CD68 and EBV VCA p18. The numbers of EBV-infected intraepithelial macrophages (top) and oral keratinocytes (bottom) were counted and are presented as number of infected cells per section. #1, #2, and #3, independently obtained tongue tissue biopsy samples from three individuals. a, cocultivation of untreated explants with untreated monocytes; b, cocultivation of treated explants with treated monocytes. Data are presented as the means ± standard errors.
Analysis of explants cocultured with EBV-infected B lymphocytes showed that a small number of EBV-infected keratinocytes (approximately five cells/section) was detected in three of six oral explants after 7 days of cocultivation (data not shown). However, in these explants EBV-positive intraepithelial B lymphocytes were not detected, in contrast to the detection of EBV-positive macrophages and LC (approximately one to two cells/section) in the mucosal epithelium.
Inhibition of monocyte migration into oral epithelium reduces EBV spread to keratinocytes within the oral epithelium.
Transmigration of monocytes from lamina propria into mucosal epithelium is mediated by the chemotactic function of MCP-1, which is secreted in mucosal epithelial cells and binds with the CCR2 receptor of monocytes in the lamina propria (16, 26). To inhibit monocyte transmigration into epithelium, we incubated EBV-infected monocytes with or without antibodies to CCR2 before cocultivating them with explants from three tongue biopsy samples. Each of these tongue tissue explants was divided into two pieces: one piece was incubated with antibodies to MCP-1, and the other piece was left untreated. The treated tissue explants were then cocultivated with the treated monocytes for the next 7 days. Explants cocultivated with untreated monocytes served as a control. Immunostaining analysis of these three explants showed that transmigration of CCR2-antibody-treated monocytes into epithelium in explants treated with antibodies to MCP-1 was three to fourfold lower than that in control explants (Fig. 7D, top). In explants treated with antibodies to MCP-1, the number of EBV-positive keratinocytes was about fivefold less (Fig. 7D, bottom) than that in control explants, indicating that transmigration of EBV-infected monocytes into epithelium mediates the spread of EBV to keratinocytes within the oral epithelium.
Peripheral blood monocytes from HIV-positive patients contain EBV.
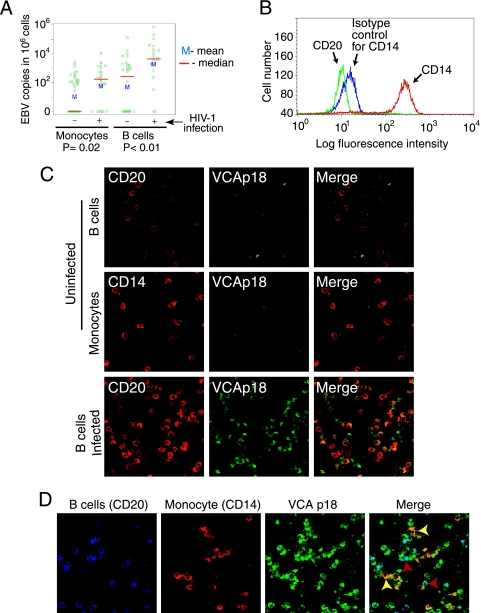
Our data show that EBV dissemination to keratinocytes within oral mucosal epithelium is mediated by EBV-infected macrophages and/or LC. The likeliest source of these cells in vivo is peripheral blood monocytes. To characterize EBV infection of peripheral blood monocytes, we isolated peripheral blood monocytes from 50 HIV-negative and 28 HIV-positive individuals (Fig. 8A). FACS analysis showed that the purity of monocyte populations from HIV-negative and -positive individuals was between 95 and 99% (Fig. 8B). The B lymphocytes were found at a frequency of 0.1 to 0.5% in monocyte populations. Analysis of EBV DNA by real-time PCR showed that 47% of HIV-negative and 69% of HIV-positive individuals had EBV-infected peripheral blood monocytes (Fig. 8A). The EBV copy number in monocytes from the HIV-negative individuals ranged from 50 to 5 × 103 EBV copies per 106 cells. In contrast, the copy number of EBV in monocytes of HIV-positive individuals was approximately twofold higher, ranging from 102 to 104 EBV copies per 106 cells. The EBV copy number in B lymphocytes of HIV-positive individuals was approximately 10-fold higher than that in HIV-negative individuals, ranging from 102 to 106 EBV copies per 106 cells.
FIG. 8.
Spread of EBV between B lymphocytes and monocytes in vivo and in vitro. (A) CD14+ monocytes and CD19+ B lymphocytes were isolated from PBMC of HIV-positive and -negative individuals. Detection and quantification of EBV DNA were performed using a QC-PCR assay. EBV DNA copy numbers are expressed on the logarithmic scale as numbers of copies per 1 million cells. Each data symbol indicates one sample. (B) Purity of monocytes was examined using antibodies to CD14 and CD20 and isotype control antibodies to CD14. (C) (Top and middle) Freshly isolated B lymphocytes and monocytes from HIV-negative individuals were coimmunostained with antibodies to EBV VCA p18 and CD20 or CD14 markers, respectively. (Bottom) B lymphocytes were infected with EBV at 10 virions/cell for 3 days, and cells were coimmunostained for CD20 and VCA p18. Yellow in the merged panel shows EBV-infected B lymphocytes. (D) EBV-infected B lymphocytes were cocultivated with monocytes from the same donor at a ratio of 1:1 for 3 days. Cocultured cells were triply coimmunostained for CD20, CD14, and VCA p18. The light blue signals (red arrowheads) in the merged section show EBV-infected B lymphocytes, and yellow signals (yellow arrowheads) show EBV-infected monocytes.
EBV may spread from B lymphocytes to monocytes.
It is well established that the main reservoir of latent EBV infection in vivo is the B-lymphocyte population. To test the hypothesis that EBV infection in monocytes may derive from B lymphocytes, we isolated primary CD19+ B lymphocytes and CD14+ monocytes from HIV-negative individuals, and both of these cell types were shown to be negative for VCA p18 (Fig. 8C, top and middle). The B lymphocytes were infected with EBV, and at 3 days postinfection immunostaining of VCA p18 showed that approximately 80% of B lymphocytes were positive for this protein (Fig. 8C, bottom). These cells were then cocultivated with circulating monocytes from the same donor at a 1:1 ratio. Three days later the mixed culture of B lymphocytes and monocytes was coimmunostained for CD20, CD14, and EBV VCA p18 (Fig. 8D). Confocal microscopy analysis showed that in this mixed culture, approximately 80% of monocytes were positive for EBV (Fig. 8D, merged panel, yellow signals and arrowheads), indicating that EBV-infected B lymphocytes may transmit virus to the monocytes.
DISCUSSION
Accumulating evidence indicates that infection of circulating monocytes by herpesviruses, including human cytomegalovirus, Kaposi's sarcoma-associated herpesvirus, and herpesvirus 6, may play a critical role in establishing a reservoir for latent infection in the blood compartment (2, 20, 22, 24, 37-39). EBV infection of monocytes, macrophages, and dendritic cells has been observed in vitro and in vivo (15, 19, 25, 32, 33, 36). Detection of EBV-infected macrophages in healthy asymptomatic individuals (36) suggests that, like B lymphocytes, circulating monocytes and tissue macrophages may serve as a reservoir for EBV infection. We also found EBV-positive circulating monocytes in both HIV-negative and HIV-positive individuals. The presence of EBV DNA in circulating monocytes has been shown recently by Schlitt et al. (33). Since circulating monocytes can migrate to tissue sites (17), including oral mucosa (5, 10, 34), EBV-infected monocytes may serve as a vehicle for virus transmission between the blood compartment and oral epithelium. Detection of similar EBV strains (29) in WBC and HL and the absence of reinfection of epithelium by WBC of EBV-eradicated bone marrow transplants (11) suggest that EBV infection of oral epithelium may occur from the blood compartment.
Consistent with this hypothesis, our data from oral tissue explants infected ex vivo with cell-free and cell-associated EBV showed that EBV-infected macrophage and dendritic cell precursors from the lamina propria migrate into the mucosal epithelium and infect oral keratinocytes within the spinosum layer. Expression of tight junction proteins in EBV-infected intraepithelial macrophages/LC indicated their migrating status (6, 40). Reduction of EBV infection of oral keratinocytes by inhibiting migration of EBV-infected monocytes to the mucosal epithelium using CCR2 and MCP-1 antibodies indicated that EBV-infected monocytes may play a key role in EBV dissemination within the oral epithelium. Also consistent with infection of epithelium from the blood compartment was our observation that the mucosal surface of oral epithelium was resistant to EBV infection, as well as our earlier findings that EBV did not enter from the apical surface of polarized oral keratinocytes (42). This mechanism of spread of EBV to epithelium may also be occurring in cutaneous T-cell lymphoma, in which both keratinocytes (8) and intraepithelial LC of skin T-cell lymphoma were shown to be positive for EBV (19).
In comparison with EBV-positive monocytes, migration of EBV-infected B lymphocytes into mucosal epithelium was rare and inefficient. However, we cannot completely exclude a role for EBV-infected B lymphocytes in virus spread within the oral epithelium. Rare EBV-infected intraepithelial B and T lymphocytes have been described previously in nasopharyngeal mucosa (41). EBV replication in vivo occurs in terminally differentiated plasma B lymphocytes (21), and these cells may migrate into mucosal epithelium and play a role in virus dissemination within the mucosal epithelium. We did not detect EBV-infected submucosal or intraepithelial CD138+ plasma B lymphocytes in any of the tissue explants that were cocultivated with EBV-infected B lymphocytes. This could reflect the lack of essential factors for terminal differentiation of B lymphocytes in our ex vivo explant system, and if so, our system may not be sufficient to fully evaluate the role of B lymphocytes in EBV spread within the oral epithelium. However, the lack of EBV-infected CD19+ cells in HL strongly suggests that the role of B lymphocytes in EBV spreading to oral epithelium is limited at best. Furthermore, it has been shown that EBV infection in B lymphocytes inhibits CXCR4 expression on the B-lymphocyte surface (9, 27), leading to reduced migration of EBV-infected B lymphocytes toward stromal cell-derived factor 1 in vitro (9). Stromal cell-derived factor 1 expression has been detected in tonsil epithelium (4), and we also detected its expression in tongue and buccal mucosal epithelium (data not shown). Thus, the absence or low level of EBV-infected B-lymphocyte migration into oral mucosal epithelium may be due to inhibition of CXCR4 expression, consistent with a lack of a significant role for B lymphocytes in EBV dissemination within the oral epithelium.
Detection of EBV infection in keratinocytes in only 1 of 20 tongue explants infected with cell-free EBV indicated that cell-free EBV infection of keratinocytes was very inefficient. In contrast, EBV infection of keratinocytes of tongue explants cocultivated with EBV-infected monocytes was highly efficient, indicating that dissemination of EBV to keratinocytes depends on the manner of EBV presentation to these cells, the number of EBV-infected monocytes, and the duration of their exposure to the explants. A small number of EBV-infected keratinocytes (approximately five cells/section) was detected in three oral explants cocultivated with EBV-infected B lymphocytes. In these explants EBV-infected intraepithelial B lymphocytes were not found, indicating that these B lymphocytes were not able to spread virus within the oral epithelium. In contrast, EBV-infected submucosal and intraepithelial macrophages/LC were detected in these explants. While it has been shown elsewhere that B lymphocytes may transfer membrane-bound EBV virions into epithelial cells (35), our data show that EBV may spread from B lymphocytes and into monocytes. The presence of EBV-infected macrophages/LC in these explants suggests that a likelier scenario is that EBV-infected B lymphocytes in the lamina propria transferred EBV to macrophages/LC, and these EBV-infected macrophages/LC then migrated into the epithelium and spread virus the keratinocytes (Fig. 9). However, we cannot exclude the possibility that the monocytes could be infected with EBV in the circulating blood compartment from B lymphocytes. If this were to occur, these EBV-infected circulating monocytes subsequently might migrate into mucosal epithelium, differentiate into macrophages and LC, and disseminate EBV within the oral epithelium (Fig. 9).
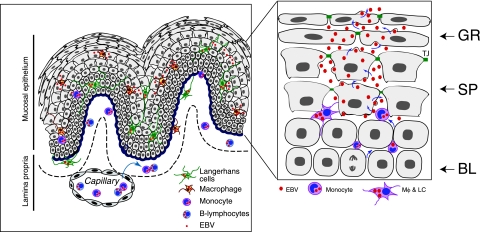
FIG. 9.
Model of EBV infection in stratified oral mucosal epithelium. In immunocompromised individuals, particularly those with HIV-mediated immunodeficiency, EBV replication may reactivate in latently infected B lymphocytes, and infectious progeny virions from B lymphocytes may infect monocytes in peripheral blood or in the lamina propria of oral mucosal epithelium. Subsequently these monocytes will differentiate into EBV-infected macrophages and LC. EBV-infected macrophages/LC may migrate into mucosal epithelium and transmit virus to terminally differentiated epithelial cells in the spinosum layer. The epithelial cells of the spinosum layer are highly susceptible to lytic EBV infection and therefore may produce a large number of infectious progeny virions. Productive EBV infection in the spinosum layer may lead to spread of virus to upper granulosum layers of oral epithelium and cause development of the HL. Mφ, macrophage; GR, stratum granulosum; SP, stratum spinosum; BL, stratum basale; TJ, tight junction.
Like the explants infected with EBV ex vivo, analysis of HL tissues also showed the presence of EBV-infected submucosal and intraepithelial macrophages and LC. Individuals with HIV infection have both higher numbers of circulating EBV-infected monocytes and higher EBV copy numbers per cell than HIV-negative individuals. While we cannot exclude possible detection of EBV in the monocyte populations due to B-lymphocyte contamination, the level of B-lymphocyte contamination of the monocyte population was low at 0.1 to 0.5%, and this small number of cells could not account for the high EBV copy numbers in the monocyte population. EBV infection of circulating monocytes could be due to EBV reactivation in B lymphocytes (23) in the setting of HIV-associated immunosuppression. Migration of EBV-infected monocytes into oral mucosal epithelium may then spread EBV infection to keratinocytes within the epithelium and initiate development of HL.
It has been shown elsewhere that EBV replication is activated in the tongue in the early stages of HIV infection, well before HL becomes clinically apparent (3). The depletion of EBV-positive intraepithelial macrophages and LC in HL lesions (7) also suggests that intensive migration of EBV-infected monocytes into oral epithelium might occur at an early stage of HL development. Reduction of EBV-infected intraepithelial macrophages/LC at 7 days post-infection of oral explants suggested that EBV infection might cause the death of these cells. Alternatively, detection of CD86 in EBV-infected intraepithelial macrophages/LC suggested that these activated immune cells may migrate in the opposite direction, i.e., from the epithelium toward the lamina propria (1). Thus, EBV infection of oral mucosal macrophages/LC may be involved in depletion of macrophages/LC in HL epithelium.
In summary, our findings show that intraepithelial EBV-infected monocytes/macrophages/LC migrate from the submucosa into the epithelium and disseminate EBV infection within the spinosum/granulosum layers of oral epithelium. These data suggest that EBV infection of oral epithelium via intraepithelial monocytes/macrophages/LC may play a key role in development of the HL lesion during immunosuppression, particularly at the early stages of lesion development before macrophages/LC cells become depleted. This may also be the major mechanism by which low-level EBV infection of oral epithelium occurs in an ongoing basis in healthy individuals.
Acknowledgments
We thank Vibeke Petersen for electron microscopy, Su-Chun Cheng for statistical analysis, Deborah Airo for editorial assistance, Piamkamon Vacharotayangul and Herve Sroussi for isolation of biopsy samples, and Nusi Dekker for sectioning of biopsy samples.
This project was supported by National Institutes of Health grants R01 DE14894 and R21 DE016009 (to S.T.) and P01 DEO7946 (to J.M.P.).
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 2.Blasig, C., C. Zietz, B. Haar, F. Neipel, S. Esser, N. H. Brockmeyer, E. Tschachler, S. Colombini, B. Ensoli, and M. Sturzl. 1997. Monocytes in Kaposi's sarcoma lesions are productively infected by human herpesvirus 8. J. Virol. 71:7963-7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulter, A. W., N. Soltanpoor, A. V. Swan, W. Birnbaum, N. W. Johnson, and C. G. Teo. 1996. Risk factors associated with Epstein-Barr virus replication in oral epithelial cells of HIV-infected individuals. AIDS 10:935-940. [DOI] [PubMed] [Google Scholar]
- 4.Casamayor-Palleja, M., P. Mondiere, A. Amara, C. Bella, M. C. Dieu-Nosjean, C. Caux, and T. Defrance. 2001. Expression of macrophage inflammatory protein-3alpha, stromal cell-derived factor-1, and B-cell-attracting chemokine-1 identifies the tonsil crypt as an attractive site for B cells. Blood 97:3992-3994. [DOI] [PubMed] [Google Scholar]
- 5.Challacombe, S. J., and S. P. Sweet. 2002. Oral mucosal immunity and HIV infection: current status. Oral Dis. 8(Suppl. 2):55-62. [DOI] [PubMed] [Google Scholar]
- 6.Collins, J. E. 2002. Adhesion between dendritic cells and epithelial cells maintains the gut barrier during bacterial sampling. Gut 50:449-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels, T. E., D. Greenspan, J. S. Greenspan, E. Lennette, M. Schiodt, V. Petersen, and Y. de Souza. 1987. Absence of Langerhans cells in oral hairy leukoplakia, an AIDS-associated lesion. J. Investig. Dermatol. 89:178-182. [DOI] [PubMed] [Google Scholar]
- 8.Dreno, B., P. Celerier, M. Fleischmann, B. Bureau, and P. Litoux. 1994. Presence of Epstein-Barr virus in cutaneous lesions of mycosis fungoides and Sezary syndrome. Acta Derm. Venereol. 74:355-357. [DOI] [PubMed] [Google Scholar]
- 9.Ehlin-Henriksson, B., F. Mowafi, G. Klein, and A. Nilsson. 2006. Epstein-Barr virus infection negatively impacts the CXCR4-dependent migration of tonsillar B cells. Immunology 117:379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gemmell, E., C. L. Carter, D. N. Hart, K. E. Drysdale, and G. J. Seymour. 2002. Antigen-presenting cells in human periodontal disease tissues. Oral Microbiol. Immunol. 17:388-393. [DOI] [PubMed] [Google Scholar]
- 11.Gratama, J. W., M. A. Oosterveer, F. E. Zwaan, J. Lepoutre, G. Klein, and I. Ernberg. 1988. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc. Natl. Acad. Sci. USA 85:8693-8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenspan, D., and J. S. Greenspan. 1997. Oral manifestations of HIV infection. AIDS Clin. Care 9:29-33. [PubMed] [Google Scholar]
- 13.Greenspan, D., J. S. Greenspan, N. G. Hearst, L. Z. Pan, M. A. Conant, D. I. Abrams, H. Hollander, and J. A. Levy. 1987. Relation of oral hairy leukoplakia to infection with the human immunodeficiency virus and the risk of developing AIDS. J. Infect. Dis. 155:475-481. [DOI] [PubMed] [Google Scholar]
- 14.Greenspan, J. S., D. Greenspan, E. T. Lennette, D. I. Abrams, M. A. Conant, V. Petersen, and U. K. Freese. 1985. Replication of Epstein-Barr virus within the epithelial cells of oral “hairy” leukoplakia, an AIDS-associated lesion. N. Engl. J. Med. 313:1564-1571. [DOI] [PubMed] [Google Scholar]
- 15.Guerreiro-Cacais, A. O., L. Li, D. Donati, M. T. Bejarano, A. Morgan, M. G. Masucci, L. Hutt-Fletcher, and V. Levitsky. 2004. Capacity of Epstein-Barr virus to infect monocytes and inhibit their development into dendritic cells is affected by the cell type supporting virus replication. J. Gen. Virol. 85:2767-2778. [DOI] [PubMed] [Google Scholar]
- 16.Han, K. H., R. K. Tangirala, S. R. Green, and O. Quehenberger. 1998. Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler. Thromb. Vasc. Biol. 18:1983-1991. [DOI] [PubMed] [Google Scholar]
- 17.Imhof, B. A., and M. Aurrand-Lions. 2004. Adhesion mechanisms regulating the migration of monocytes. Nat. Rev. Immunol. 4:432-444. [DOI] [PubMed] [Google Scholar]
- 18.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2575-2627. In B. N. Fields and D. M. Knipe (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 19.Knol, A. C., G. Quereux, M. C. Pandolfino, A. Khammari, and B. Dreno. 2005. Presence of Epstein-Barr virus in Langerhans cells of CTCL lesions. J. Investig. Dermatol. 124:280-282. [DOI] [PubMed] [Google Scholar]
- 20.Kondo, K., T. Kondo, T. Okuno, M. Takahashi, and K. Yamanishi. 1991. Latent human herpesvirus 6 infection of human monocytes/macrophages. J. Gen. Virol. 72:1401-1408. [DOI] [PubMed] [Google Scholar]
- 21.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson, S., C. Soderberg-Naucler, F. Z. Wang, and E. Moller. 1998. Cytomegalovirus DNA can be detected in peripheral blood mononuclear cells from all seropositive and most seronegative healthy blood donors over time. Transfusion 38:271-278. [DOI] [PubMed] [Google Scholar]
- 23.Legoff, J., C. Amiel, O. Calisonni, D. Fromentin, B. Rajoely, N. Abuaf, E. Tartour, W. Rozenbaum, L. Belec, and J. C. Nicolas. 2004. Early impairment of CD8+ T cells immune response against Epstein-Barr virus (EBV) antigens associated with high level of circulating mononuclear EBV DNA load in HIV infection. J. Clin. Immunol. 24:125-134. [DOI] [PubMed] [Google Scholar]
- 24.Maciejewski, J. P., E. E. Bruening, R. E. Donahue, S. E. Sellers, C. Carter, N. S. Young, and S. St. Jeor. 1993. Infection of mononucleated phagocytes with human cytomegalovirus. Virology 195:327-336. [DOI] [PubMed] [Google Scholar]
- 25.Masy, E., E. Adriaenssens, C. Montpellier, P. Crepieux, A. Mougel, B. Quatannens, G. Goormachtigh, N. Faumont, F. Meggetto, C. Auriault, H. Groux, and J. Coll. 2002. Human monocytic cell lines transformed in vitro by Epstein-Barr virus display a type II latency and LMP-1-dependent proliferation. J. Virol. 76:6460-6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monteclaro, F. S., and I. F. Charo. 1997. The amino-terminal domain of CCR2 is both necessary and sufficient for high affinity binding of monocyte chemoattractant protein 1. Receptor activation by a pseudo-tethered ligand. J. Biol. Chem. 272:23186-23190. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, T., R. Fujisawa, D. Izawa, K. Hieshima, K. Takada, and O. Yoshie. 2002. Human B cells immortalized with Epstein-Barr virus upregulate CCR6 and CCR10 and downregulate CXCR4 and CXCR5. J. Virol. 76:3072-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niedobitek, G., L. S. Young, R. Lau, L. Brooks, D. Greenspan, J. S. Greenspan, and A. B. Rickinson. 1991. Epstein-Barr virus infection in oral hairy leukoplakia: virus replication in the absence of a detectable latent phase. J. Gen. Virol. 72:3035-3046. [DOI] [PubMed] [Google Scholar]
- 29.Palefsky, J. M., J. Berline, D. Greenspan, and J. S. Greenspan. 2002. Evidence for trafficking of Epstein-Barr virus strains between hairy leukoplakia and peripheral blood lymphocytes. J. Gen. Virol. 83:317-321. [DOI] [PubMed] [Google Scholar]
- 30.Ryan, J. L., H. Fan, S. L. Glaser, S. A. Schichman, N. Raab-Traub, and M. L. Gulley. 2004. Epstein-Barr virus quantitation by real-time PCR targeting multiple gene segments: a novel approach to screen for the virus in paraffin-embedded tissue and plasma. J. Mol. Diagn. 6:378-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandvej, K., L. Krenacs, S. J. Hamilton-Dutoit, J. L. Rindum, J. J. Pindborg, and G. Pallesen. 1992. Epstein-Barr virus latent and replicative gene expression in oral hairy leukoplakia. Histopathology 20:387-395. [DOI] [PubMed] [Google Scholar]
- 32.Savard, M., C. Belanger, M. Tardif, P. Gourde, L. Flamand, and J. Gosselin. 2000. Infection of primary human monocytes by Epstein-Barr virus. J. Virol. 74:2612-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlitt, A., S. Blankenberg, K. Weise, B. C. Gartner, T. Mehrer, D. Peetz, J. Meyer, H. Darius, and H. J. Rupprecht. 2005. Herpesvirus DNA (Epstein-Barr virus, herpes simplex virus, cytomegalovirus) in circulating monocytes of patients with coronary artery disease. Acta Cardiol. 60:605-610. [DOI] [PubMed] [Google Scholar]
- 34.Seguier, S., G. Godeau, and N. Brousse. 2000. Immunohistological and morphometric analysis of intra-epithelial lymphocytes and Langerhans cells in healthy and diseased human gingival tissues. Arch. Oral Biol. 45:441-452. [DOI] [PubMed] [Google Scholar]
- 35.Shannon-Lowe, C. D., B. Neuhierl, G. Baldwin, A. B. Rickinson, and H. J. Delecluse. 2006. Resting B cells as a transfer vehicle for Epstein-Barr virus infection of epithelial cells. Proc. Natl. Acad. Sci. USA 103:7065-7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimakage, M., M. Kimura, S. Yanoma, M. Ibe, S. Yokota, G. Tsujino, T. Kozuka, T. Dezawa, S. Tamura, A. Ohshima, M. Yutsudo, and A. Hakura. 1999. Expression of latent and replicative-infection genes of Epstein-Barr virus in macrophage. Arch. Virol. 144:157-166. [DOI] [PubMed] [Google Scholar]
- 37.Smith, M. S., G. L. Bentz, J. S. Alexander, and A. D. Yurochko. 2004. Human cytomegalovirus induces monocyte differentiation and migration as a strategy for dissemination and persistence. J. Virol. 78:4444-4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soderberg-Naucler, C., K. N. Fish, and J. A. Nelson. 1998. Growth of human cytomegalovirus in primary macrophages. Methods 16:126-138. [DOI] [PubMed] [Google Scholar]
- 39.Soderberg-Naucler, C., D. N. Streblow, K. N. Fish, J. Allan-Yorke, P. P. Smith, and J. A. Nelson. 2001. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 75:7543-7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takano, K., T. Kojima, M. Go, M. Murata, S. Ichimiya, T. Himi, and N. Sawada. 2005. HLA-DR- and CD11c-positive dendritic cells penetrate beyond well-developed epithelial tight junctions in human nasal mucosa of allergic rhinitis. J. Histochem. Cytochem. 53:611-619. [DOI] [PubMed] [Google Scholar]
- 41.Tao, Q., G. Srivastava, A. C. Chan, L. P. Chung, S. L. Loke, and F. C. Ho. 1995. Evidence for lytic infection by Epstein-Barr virus in mucosal lymphocytes instead of nasopharyngeal epithelial cells in normal individuals. J. Med. Virol. 45:71-77. [DOI] [PubMed] [Google Scholar]
- 42.Tugizov, S. M., J. W. Berline, and J. M. Palefsky. 2003. Epstein-Barr virus infection of polarized tongue and nasopharyngeal epithelial cells. Nat. Med. 9:307-314. [DOI] [PubMed] [Google Scholar]
- 43.Webster-Cyriaque, J., J. Middeldorp, and N. Raab-Traub. 2000. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. J. Virol. 74:7610-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young, L. S., R. Lau, M. Rowe, G. Niedobitek, G. Packham, F. Shanahan, D. T. Rowe, D. Greenspan, J. S. Greenspan, A. B. Rickinson, and P. J. Farrell. 1991. Differentiation-associated expression of the Epstein-Barr virus BZLF1 transactivator protein in oral hairy leukoplakia. J. Virol. 65:2868-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]