Abstract
Influenza A virus nonstructural protein 1 (NS1A protein) is a virulence factor which is targeted into the nucleus. It is a multifunctional protein that inhibits host cell pre-mRNA processing and counteracts host cell antiviral responses. We show that the NS1A protein can interact with all six human importin α isoforms, indicating that the nuclear translocation of NS1A protein is mediated by the classical importin α/β pathway. The NS1A protein of the H1N1 (WSN/33) virus has only one N-terminal arginine- or lysine-rich nuclear localization signal (NLS1), whereas the NS1A protein of the H3N2 subtype (Udorn/72) virus also has a second C-terminal NLS (NLS2). NLS1 is mapped to residues 35 to 41, which also function in the double-stranded RNA-binding activity of the NS1A protein. NLS2 was created by a 7-amino-acid C-terminal extension (residues 231 to 237) that became prevalent among human influenza A virus types isolated between the years 1950 to 1987. NLS2 includes basic amino acids at positions 219, 220, 224, 229, 231, and 232. Surprisingly, NLS2 also forms a functional nucleolar localization signal NoLS, a function that was retained in H3N2 type virus NS1A proteins even without the C-terminal extension. It is likely that the evolutionarily well-conserved nucleolar targeting function of NS1A protein plays a role in the pathogenesis of influenza A virus.
The influenza A virus genome consisting of eight separate RNA segments encodes 11 viral structural and nonstructural proteins. In addition to the viral hemagglutinin, nonstructural protein 1 (NS1A) is one of the major viral virulence factors. The evolution of NS1A genes appears to be species specific, and the evolution of the present human NS1A genes began in 1918 when H1N1 type viruses emerged and became pandemic (20).
The NS1A protein is a multifunctional protein that participates in both protein-RNA (7, 16, 28, 57) and protein-protein (23, 25, 38) interactions. The NS1A protein contains an N-terminal double-stranded RNA (dsRNA)-binding domain and a C-terminal effector domain (45). The three-dimensional structures of the dsRNA-binding and effector domains of NS1A have been determined (3, 6, 27). The NS1A protein exists as a dimer, and the structure of its RNA-binding domain differs markedly from all other known RNA-binding proteins. The effector domain binds two cellular proteins that are essential for the 3′ end processing of cellular pre-mRNAs (5, 26, 38). As a result, the processing of cellular pre-mRNAs, including beta interferon (IFN-β) pre-mRNA and the pre-mRNAs of other antiviral proteins, is inhibited, thereby suppressing the amount of mature IFN-β mRNA that is produced in infected cells (38, 39, 49, 55). The role of the dsRNA-binding activity is controversial and may be virus strain specific. The role of the dsRNA-binding activity of the NS1A protein of the human H3N2 influenza A/Udorn/72 virus was determined using a recombinant virus expressing a NS1 protein lacking dsRNA-binding activity. Analysis of the defect in virus replication demonstrated that the primary role of the NS1 dsRNA binding is to inhibit the activation of the IFN-induced 2′ to 5′ oligo(A) synthetase/RNase L pathway and showed that this dsRNA-binding activity has no role in inhibiting the production of IFN-β mRNA (34). In contrast, experiments with the mouse-adapted H1N1 influenza A/PR8/34 virus indicated that the RNA-binding domain participates in an NS1A protein-mediated inhibition of the activation of retinoic acid-inducible gene I, which is required for cytokine gene expression (19, 31, 50), leading to impaired synthesis of IFN during influenza A virus infection (33, 44).
Unlike most other RNA viruses, influenza viruses replicate in the nucleus of the host cells. The NS1A protein is efficiently targeted into the nucleus, and two nuclear localization signals (NLSs) have been identified in the H3N2 subtype influenza A/Alaska/6/77 virus NS1A protein (15). However, so far the molecular mechanisms mediating the nuclear import of NS1A proteins have not been determined.
Active nuclear import of proteins targeted to the nucleus is mediated by specific sequence elements, NLSs. A classical monopartite NLS is composed of a stretch of four to six arginines or lysines (18, 24), while in a bipartite NLS two stretches of basic amino acids are separated by a spacer 10 to 12 amino acids long (11). In the cytoplasm NLS-containing proteins are recognized by importin α, followed or preceded by binding of importin α to importin β. Cargo/importin α/importin β protein complexes are then translocated into the nucleus through the nuclear pore complex (NPC). Six human importin α isoforms have been identified: importin α1, importin α3, importin α4, importin α5, importin α6, and importin α7 (9, 10, 21, 22, 36, 48). Importin α isoforms show significant differences in their substrate specificity and binding mechanisms (12, 22, 32). The three-dimensional structure of the importin α NLS-binding domain has been determined (8, 14).
Eukaryotes have a specialized nuclear compartment, the nucleolus, which is a relatively large, dynamic, highly organized nonmembranous subcompartment of the nucleus. The nucleolus is the site for rRNA synthesis, processing, and maturation. Recently, it has become apparent that the nucleolus also has a role in regulating the cell cycle, tumor suppression and oncogenic activities, assembly of signal recognition particle, control of aging, and modulation of telomerase functions (41-43). Some of these functions are mediated through sequestration of transcription factors that control the cell cycle (4, 47). Nuclear proteins pass through the nucleolus randomly, and those with affinity to constitutive nucleolar components are retained. It has been suggested that nucleolar localization signals (NoLSs) act as retention signals rather than as classical targeting or transport signals (2, 4). Many NoLSs overlap with NLSs and contain basic amino acids (46, 54).
Here we show that influenza A virus NS1A proteins have a well-conserved N-terminal monopartite NLS (NLS1), where basic amino acids 35, 38, and 41 are critical for importin α binding and nuclear translocation. Remarkably, the NLS1 is coincident with the dsRNA-binding epitope of the NS1A protein. Viruses isolated between the years 1950 and 1987 also have a 7-amino-acid C-terminal extension (residues 231 to 237) in their NS1A protein that functions, together with other C-terminal basic residues, as an NoLS and as a second NLS (NLS2). Evolutionary analysis and fine mapping of the NoLS and NLS2 signals by site-directed mutagenesis indicated that the C-terminal basic amino acids constituted both of these signals. Even when the C-terminal extension (residues 231 to 237) of H3N2 virus NS1A proteins was deleted, the truncated NS1A protein (NS1AΔ231-237) was still localized in the nucleolus and contained a functional NLS2.
MATERIALS AND METHODS
Cells.
Human A549 lung carcinoma cell line (ATCC CCL 185) was maintained in continuous culture in minimum Eagle's medium-α (Invitrogen Corp., Carlsbad, CA) supplemented with 0.6 μg/ml penicillin, 60 μg/ml streptomycin, and 10% fetal calf serum (Integro, Zaandam, The Netherlands). Human hepatocellular carcinoma HuH7 (37) cells were maintained in minimum Eagle's medium-α with supplements as above. Spodoptera frugiperda (Sf9) cells were used for baculovirus expression and maintained in Grace's insect medium as described previously (52).
Viruses and infections.
A549 cells, grown on glass coverslips on 24-well plates, were infected with the following viruses: H3N2 viruses A/Udorn/72, A/Fin/001/73, A/Fin/008/85, A/Beijing/353/89, A/Fin/229/92, A/Fin/455/97, and A/Moscow/10/99; H1N1 viruses A/WSN/33, A/Fin/001/79, A/Fin/001/82, A/Fin/40/86, A/Fin/432/96, and A/New Caledonia/20/99. Virus stocks were cultivated in 8-day-old embryonated chicken eggs and stored at −70°C. The hemagglutination titers of the stock viruses ranged from 64 to 256, and the infectivity of the virus stocks in A549 cells was 1 × 107 to 4 × 107 PFU/ml. The multiplicity of infection used in the experiments varied from 0.5 to 5 PFU/cell.
Recombinant influenza A/Udorn/72 viruses were created as previously described (53). A mutant A/Udorn/72 virus with a deletion of amino acids 221 to 237 of NS1A (NS1AΔ221-237) was created by introducing a stop codon at position 221 of the NS1A gene sequence by using two rounds of PCR and specific oligonucleotide primers. The resulting DNA was sequenced and cloned into pHH21 vector. The virus encoding the mutant NS1A protein was generated by cotransfecting 293T cells with eight plasmids encoding the viral RNA segments and four plasmids expressing the PB1, PB2, PA, and NP proteins (53). Cell culture supernatants were collected, the virus was titered by plaque assay on MDCK cells, and individual plaques were amplified in 10-day-old embryonic chicken eggs.
Antibodies.
Rabbit anti-importin α1, α3, and α7 antibodies used in Western blot analysis were as previously described (22). Secondary horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:2,000; Daco, Glostrup, Denmark) were used as suggested by the manufacturer. For confocal laser microscopy, anti-influenza A NS1A protein antibodies were prepared in guinea pigs by immunizing the animals four times at 4-week intervals with an Escherichia coli-expressed, glutathione-Sepharose-purified (Amersham Biosciences, Buckinghamshire, United Kingdom) glutathione S-transferase (GST)-NS1A fusion protein (50 μg of protein/immunization/animal). Rabbit anti-human immunodeficiency virus type 1 (HIV-1) Rev antibodies have been described previously (30). Secondary antibodies used were rhodamine Red-X- or fluorescein isothiocyanate-labeled goat anti-guinea pig or anti-rabbit immunoglobulins, respectively (1:100; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA).
Plasmids and DNA manipulations.
GST-importin α1, α3, α5, α7, and β E. coli expression constructs have been described previously (32). The coding region of the cDNA for human importin α6 (O15131) was modified by PCR to create N- and C-terminal NcoI and XhoI sites, respectively, for further cloning into an E. coli GST fusion vector (pETM-30; kindly provided by G. Stier, EMBL Heidelberg, Germany). The importin α1, α3, α4, and α7 gene constructs in baculovirus GST expression vectors have been described previously (13). The importin α5 gene (NM_002264) was PCR modified and cloned into the BamHI site of a GST-pAc/YMI baculovirus expression vector, and GST-importin α5 was produced as described previously (13).
The wild-type (wt) A/Udorn/72 (H3N2 virus) NS1 gene (V01102) was expressed in E. coli GST (pGEX-3X; Amersham Biosciences) and eukaryotic pcDNA3.1(+) (Invitrogen) expression vectors. The wt A/WSN/33 (H1N1 virus) NS1 gene (M12597) was modified by PCR to create N- and C-terminal BglII sites for further cloning into the BamHI site of a pcDNA3.1(+) expression vector (Invitrogen). To create point mutations to A/Udorn/72 and A/WSN/33 NS1 cDNAs, a QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used.
To create green fluorescent protein (GFP)-NS1A fusion constructs, the C-terminal cDNAs for wt A/Udorn/72 (amino acids 203 to 237) and A/WSN/33 (amino acids 203 to 230) NS1 genes were modified by PCR to create N- and C-terminal SalI and NheI sites, respectively, for further cloning into the SalI and NheI cloning site of the pCMX-SAH/Y145F expression vector (40). Mutations to GFP-NS1A chimeric gene constructs were done using the QuikChange Site-Directed Mutagenesis Kit. All oligonucleotides used to modify the genes in the study will be provided upon request.
The HIV-1 Rev cDNA in the pBC12/CMV expression vector has been described previously (30). All DNA manipulations were performed according to standard protocols, and the newly created gene constructs were partially sequenced.
Importin binding assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting.
Human GST-importin α1, α3, α4, α5, α6, α7, and β or influenza A virus GST-NS1A fusion proteins were expressed in E. coli BL21 cells or by baculovirus in Sf9 cells, and GST-fusion proteins were purified as described previously (12, 32).
In vitro translated NS1A wt or NLS mutant proteins (TnT Coupled Reticulocyte Lysate Systems; Promega, Madison, WI) were 35S labeled (PRO-MIX; Amersham Biosciences) and allowed to bind to Sepharose-immobilized GST or GST-importin fusion proteins on ice for 60 min followed by washing. GST-importin-bound 35S-labeled proteins were separated by 12% SDS-PAGE. The gels were fixed and treated with Amplify reagent (Amersham Biosciences) as specified by the manufacturer and autoradiographed. GST pull-down experiments from A549 cell extracts were carried out as described previously (13).
Transfections, indirect immunofluorescence, and confocal laser microscopy.
For indirect immunofluorescence and confocal laser microscopy, HuH7 cells grown on glass coverslips for 24 h were transfected with wt and mutant NS1A, GST-NS1A fusion, and HIV-1 rev gene constructs using FuGENE6 transfection reagent (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were fixed with 3% paraformaldehyde at room temperature for 20 min, permeabilized with 0.1% Triton X-100 for 5 min, and processed for immunofluorescence microscopy. The cells positive for NS1A or HIV-1 Rev proteins were visualized and photographed on a Leica TCS NT confocal microscope.
RNA-binding assay.
The GST portion of GST fusion proteins in RNA-binding assays was cleaved using either the protease factor Xa (GST-NS1A A/Udorn/72; E. coli expression construct) or thrombin (GST-importin α1; E. coli expression construct). The RNA-binding experiment was performed as described previously (57).
RESULTS
All human importin α isoforms bind the influenza virus NS1A protein.
Although the influenza A virus NS1A protein has been shown to contain NLSs that target it into the host cell nucleus (15), the mechanism of its nuclear import has not be determined. To determine whether the NS1A protein can interact with different importin α isoforms, GST-importin α fusion proteins were expressed in E. coli and in Sf9 insect cells, and pull-down experiments with 35S-labeled the influenza A/Udorn/72 NS1A protein generated by in vitro translation were carried out. E. coli-expressed GST-importin α1 and α6 isoforms, but not those of importin α3, α5, or α7, were able to bind the influenza A/Udorn/72 NS1A protein (Fig. 1A). In contrast, all the Sf9-expressed GST-importin α isoforms (α1, α3, α4, α5, and α7) bound the NS1A protein (Fig. 1B). The same results were obtained using the influenza virus A/WSN/33 NS1A protein (Fig. 1C and D). These results show that the influenza NS1A protein is capable of binding to all human importin α isoforms. The differences in the binding patterns of E. coli- and Sf9-expressed importin α isoforms suggest that posttranslational modifications may be needed for the binding of the NS1A protein to certain importin α isoforms.
FIG. 1.
Influenza A virus NS1 binds to all importin α isoforms. (A) 35S-labeled and in vitro translated NS1A protein from A/Udorn/72 virus strain (H3N2) was allowed to bind to E. coli-expressed and Sepharose-immobilized GST-importin β, α1, α3, α5, α6, or α7 at +4°C for 1 h. After being washed twice with binding buffer, importin-bound NS1A was dissolved in Laemmli sample buffer, separated by 12% SDS-PAGE, and autoradiographed. A similar gel was also stained with Coomassie blue to visualize the amount of Sepharose-immobilized GST-importin β and α isoforms. C, control; M, Sepharose matrix-bound in vitro translated NS1A. (B) 35S-labeled and in vitro translated NS1A protein (A/Udorn/72) was allowed to bind to Sf9-expressed and Sepharose-immobilized GST and GST-importin α1, α3, α4, α5, or α7 as described above. 35S-labeled and in vitro translated NS1A protein from A/WSN/33 virus strain (H1N1) was allowed to bind to E. coli-expressed (C) or Sf9-expressed (D) and Sepharose-immobilized GST-importins as described above. C, the control of in vitro translated NS1. (E) Cell extracts of cultured A549 cells were prepared, and the proteins in cell extracts were allowed to bind to E. coli-expressed and Sepharose-immobilized GST and GST-NS1A (A/Udorn/72) at +4°C for 1 h. Sepharose-bound proteins were dissolved in Laemmli sample buffer followed by 8% SDS-PAGE and Western blotting with anti-importin α1, α3, and α7 antibodies. As a control, cell extracts of A549 cells containing 20 μg of protein were stained in lane C. A similar gel was also stained with Coomassie blue to visualize the amount of Sepharose-immobilized GST and GST-NS1A A/Udorn/72.
To determine whether the NS1A protein specifically binds to importin α molecules in human cells, extracts from A549 human lung carcinoma cells were allowed to bind to the GST-NS1A protein, followed by immunoblotting of the bound proteins with antibodies specific for anti-importin α1, α3, or α6 (α7 cross-reactive). Importins α1 and α6/7 and, to a lesser extent, importin α3 were found to bind to immobilized GST-NS1A (Fig. 1E). Since the recombinant and natural importins α1 and α6 were found to interact with the NS1A protein, E. coli-expressed GST-importins α1 and α6 were used in subsequent binding experiments.
The NLS1 of the NS1A protein is highly conserved among influenza A virus strains.
It has been shown previously that the NS1A protein of the H3N2 type influenza virus A/Alaska/6/77 has two NLSs. NLS1 was suggested to comprise amino acids 34 to 38 (DRLRR) (15). This sequence is located in helix 2/2′ of the dimeric RNA-binding domain (6, 27). We aligned 172 human, 650 avian, 103 swine, and 24 equine influenza A virus NS1A protein sequences found in the GenBank (29) to determine whether the amino acid sequence of NLS1 is conserved among influenza A viruses isolated from different species and at different times (Fig. 2A). Only 1 out of the 172 human strains and 12 out of the 650 avian strains differed from the consensus sequence. It should be noted that almost all of these differences are characterized by the substitution of a similar amino acid (I substituted for L or K substituted for R). In the avian virus A/pintai/Alberta/119/79, there is an alanine in place of an arginine at position 38. No differences from the consensus sequence were detected in swine or equine virus strains. Several amino acids C-terminal to the DRLRR sequence were also well conserved. Amino acid 41 was K or R in all 990 strains analyzed (Fig. 2A). Amino acid 44 was almost as well conserved, since only 5 out of the 990 strains analyzed had another amino acid apart from K or R at this position. Amino acid K20 was also highly conserved (data not shown). For site-directed mutagenesis of the NLS1, we selected conserved basic amino acids K20, R35, R37, R38, K41, and R44 as targets.
FIG. 2.
Variation of the amino acid sequence around NLS1 and NLS2 of influenza A virus NS1A protein. (A) Variation of NLS1 (amino acids 34 to 44). The number of human, avian, swine, and equine NS1A protein sequences from different virus strains is shown at right. The previously identified putative NLS1 (amino acids 34 to 38) (14) is underlined, and critical arginines (R) at positions 35 and 38 and lysine (K) at position 41, which regulate importin α binding, are shaded. (B) Variation of putative C-terminal NLS2 in selected influenza A virus NS1A proteins. Critical amino acids 219, 220, 224, 227, 229, 231, and 232 that regulate NS1A protein interaction with importin α (this study) are shaded.
A putative NLS2 is found in the NS1A proteins of a subset of influenza A virus strains.
The NLS2 in the NS1A protein of influenza virus A/Alaska/6/77 was mapped to amino acids 203 to 237 at the C terminus (15), but the specific amino acids comprising the NLS2 were not identified. The sequence in this NS1A protein from amino acids 219 to 237, KRKMARTARSKVRRDKMAD (Fig. 2B), resembles a classical bipartite NLS, in which two short basic amino acid sequences (underlined) are separated by a nonspecific 9-amino-acid spacer. To determine which amino acids in the putative NLS2 of the A/Udorn/72 NS1A protein are required for NLS function, we carried out site-directed mutagenesis of basic amino acids 219, 220, 224, 227, 229, 231, and 232. A similar, or identical, putative NLS2 is found in all human influenza A viruses isolated between 1950 and 1989, including influenza A/Udorn/72, the strain used in the present study. These virus strains, which are comprised of all human HN types during this period (H1N1, H2N2, and H3N2), encode an NS1A protein that contains 237 amino acids (Fig. 2B). In contrast, the NS1A proteins of all other human influenza A strains are shorter. The vast majority have a stop codon at position 230, thereby disrupting the putative NLS2. The NS1A protein of influenza A/WSN/33 is in this category. In avian virus strains, the length of the NS1A protein is between 217 and 230 amino acids (Fig. 2B).
Importin α binds both NLS1 and NLS2 of the NS1A protein of influenza virus A/Udorn/72.
To determine whether both the NLS1 and the putative NLS2 of A/Udorn/72 NS1A protein mediate the binding to importin α, we carried out pull-down experiments with E. coli-expressed GST-importin α1 and [35S]methionine-labeled A/Udorn/72 NS1A proteins containing specific mutations in the NLS1 and/or the putative NLS2 sequences (Fig. 3A). Mutations in NLS1 of R37A and R38A yielding NLS1(R37A R38A) or in the putative NLS2 yielding the individual mutants NLS2(K219A R220A) or NLS2(R231A R232A) or a two-step mutation of first NLS2(K219A R220A) and then NLS2(R231A R232A) did not eliminate the binding of the A/Udorn/72 NS1A protein to importin α1. In contrast, when both NLS1 and NLS2 sequences were simultaneously mutated [NLS1(R37A R38A) plus NLS2(K219A R220A) or NLS1(R37A R38A) plus NLS2(R231A R232A)], A/Udorn/72 NS1A protein binding to the importin α1 was eliminated. The same result was obtained with E. coli-expressed GST-importin α6 (data not shown). These results show that both NLS1 and NLS2 mediate importin α binding, and that NLS2 likely functions as a classical bipartite NLS consisting of a proximal part, K219 and R220, and a distal part, R231 and R232.
FIG. 3.
Influenza virus A/Udorn/72 NS1A protein has two importin α binding sites, and A/WSN/33 NS1A protein has one importin α binding site. (A) Influenza virus A/Udorn/72 NS1A protein binds to importin α protein via two separate NLSs. 35S-labeled and in vitro translated A/Udorn/72 NS1A wt and point mutant proteins were allowed to bind to E. coli-expressed and Sepharose-immobilized GST, GST-importin β, and GST-importin α1 proteins as indicated in the figure. Importin-bound NS1A was dissolved in Laemmli sample buffer, separated by 12% SDS-PAGE, and autoradiographed. A similar gel was also stained with Coomassie blue to visualize the amount of Sepharose-immobilized GST and GST-importin β and α1 proteins. For the translation control 1 μl of 35S-labeled and in vitro translated NS1 wt and six point mutants were separated by 12% SDS-PAGE and autoradiographed. (B) 35S-labeled and in vitro translated A/Udorn/72 NS1A wt and seven point mutant proteins covering putative NLS1 and NLS2 as indicated in the figure. The binding experiment was carried out as described for panel A. (C) 35S-labeled and in vitro translated A/WSN/33 NS1A wt and four point mutant proteins were allowed to bind to E. coli-expressed and Sepharose-immobilized GST and GST-importin α1 proteins as indicated in the figure. The binding experiment was as described for panel A.
To identify the amino acids in the NLS1 of the A/Udorn/72 NS1A protein that are required for importin α binding, single mutations were made in this sequence on top of the NLS2 mutant construct [NLS2(K219A R220A) followed by NLS2(R231A R232A)]. In this context, single point mutations, either R35A, R38A, or K41A, completely eliminated importin α1 binding, whereas K20A, R37A, or R44A single mutations did not significantly reduce binding (Fig. 3B). These results were fully confirmed with binding to E. coli-expressed GST-importin α6 or Sf9-expressed GST-importins α5 and α7 (data not shown). It should be noted that R38, which is required for importin α binding, is also the amino acid that is absolutely required for dsRNA binding (57).
The binding of the influenza virus A/WSN/33 NS1A protein to importin α is mediated solely by the NLS1 in the dsRNA-binding domain.
Since the NS1A protein of influenza A/WSN/33 has a stop codon at position 230, it lacks the putative NLS2. Consequently, it would be expected that mutations in the NLS1 of this NS1A protein would eliminate importin α binding. To determine whether this is the case, we carried out pull-down experiments with E. coli-expressed GST-importin α1 and [35S]methionine-labeled A/WSN/33 NS1A protein with point mutations in the NLS1 sequence (Fig. 3C). Mutations of R35A, R38A, or K41A completely eliminated the binding of the WSN NS1A protein to importin α1. These results were confirmed with binding to importin α6 (data not shown). These are the same amino acids that are required for the binding of NLS1 of A/Udorn/72 to importin α. These results also show that the A/WSN/33 NS1A protein lacks a C-terminal NLS2 that binds importin α. The two conserved basic amino acids, K219 and R220, that are part of NLS2 in the A/Udorn/72 NS1 protein are not able to mediate importin α binding without amino acids R231 and R232. Alignment of human influenza A virus NS1A protein C termini revealed that many virus strains lack the C-terminal NLS2 (Fig. 2B).
Importin α1 does not compete with dsRNA for binding the NS1A protein.
Based on previous site-directed mutagenesis experiments, R38 and K41 in the NS1A protein are the critical amino acids for dsRNA-binding (57). As R38 and K41 are also essential in the binding of NS1A to importin α1 (Fig. 3), we studied by direct binding experiments using highly purified proteins whether dsRNA would compete with importin α1 for binding to the NS1A protein. Our results clearly show that importin α1 does not compete with dsRNA for binding to the NS1A protein (Fig. 4). This was the case regardless of the order of addition of dsRNA and importin α. Since both dsRNA and importin α can bind to NS1A simultaneously, we can infer that the binding sites of these molecules are actually not totally overlapping.
FIG. 4.
Importin α1 does not compete with dsRNA for binding to the NS1A protein. (A) Gel shift assay of complexes formed between radiolabeled 55-bp dsRNA and C-terminally deleted A/Udorn/72 NS1A(1-215) protein in the absence and presence of importin α1. The indicated polypeptides [40 or 400 nM of NS1A(1-215) and 4 to 1,600 nM of importin α1] were incubated with the 55-bp dsRNA (10,000 cpm; 1 nM), and the polypeptide-RNA complexes were separated from free RNA by nondenaturing gel electrophoresis (6% acrylamide; bis:acrylamide 1:100). (B) Gel shift assay of complexes formed between radiolabeled 55-bp dsRNA and importin α1. The last lane shows the complex formed between this dsRNA and the NS1A(1-215) protein.
NS1A proteins containing mutated NLS sequence(s) fail to accumulate in the nucleus.
To determine the intracellular location of wt and NLS mutant A/Udorn/72 NS1A proteins, HuH7 hepatoma cells were transiently transfected with plasmids encoding these NS1A proteins, followed by analysis with laser scanning confocal microscopy (Fig. 5A). wt A/Udorn/72 NS1A proteins, as well as the NLS1 or NLS2 mutant proteins, were found in the cell nucleus (Fig. 5A). However, when both the NLS1 protein carried the mutation NLS1(R35A R38A) and/or NLS1(K41A) and the NLS2 protein carried the mutations NLS2(K219A R220A) plus NLS2(R231A R232A), the A/Udorn/72 NS1A protein was distributed throughout the cytoplasm, and only weak nuclear accumulation was seen. In contrast, the A/Udorn/72 NS1A protein was localized in the nucleus when the NLS2 mutations were combined with the single point mutations K20A, R37A, or R44A. In the case of the NS1A protein of A/WSN/33, mutations within the NLS1, specifically, the single point mutations R35A, R38A, and K41A and the double mutation R38A K41A, were sufficient to render NS1A protein predominantly cytoplasmic with only weak nuclear staining (Fig. 5B). The intracellular distribution of all NS1A mutant proteins fully correlated with their ability to bind to importin α (compare Fig. 3 and 5). However, it was unexpected that some NLS mutant NS1A proteins that lacked importin α binding still weakly entered the nucleus. Since NS1A protein is a relatively small protein, it is possible that some of it can passively diffuse into the nucleus.
FIG. 5.
Point mutations to NLSs regulate nuclear/cytoplasmic distribution of the influenza A virus NS1A protein. (A) HuH7 cells were transiently transfected with wt or NLS mutant (mt) influenza A/Udorn/72 NS1A gene constructs as indicated in the figure. NLS1, NLS1(R37A R38A); NLS2, NLS2(K219A R220A) plus NLS2(R231A R232A). (B) HuH7 cells were transiently transfected with wt or NLS mutant (mt) influenza A/WSN/33 NS1A gene constructs as indicated in the figure. The cells were stained with rabbit anti-NS1A protein and secondary rhodamine-labeled anti-rabbit antibodies. Bar, 5 μm.
Surprisingly, the NLS2 of the NS1A protein of influenza A/Udorn/72 is retained after deletion of amino acids 231 to 237.
Based on the above results, it may be predicted that deletion of the amino acid extension (residues 231 to 237) of the NS1A protein of the influenza A/Udorn/72 virus would disrupt the bipartite NLS2 and thereby eliminate the C-terminal sequence of the NS1A protein from binding to importin α. To test this prediction, we carried out GST-importin pull-down experiments with the NS1AΔ231-237 protein that also carried the mutant NLS1(R37A R38A) (Fig. 6A). Unexpectedly, this mutant NS1A protein still efficiently bound to GST-importin α6. In addition, this mutant NS1A protein was localized in the nucleus (Fig. 6B). This result indicated that the truncated C-terminal region retained NLS2 function, presumably as a monopartite NLS. To identify specific basic amino acids that mediate importin α6 binding, we changed both K219 and R220 to A residues in the NS1AΔ231-237(K37A K38A) mutant protein. These additional mutations eliminated both importin α binding and nuclear localization. Consequently, the retained NLS2 in the Udorn NS1A protein required K219 and R220. However, additional basic amino acids are needed because the WSN NS1A protein, which lacks the C-terminal extension, contains K219 and R220 but lacks NLS2 function. Two other basic amino acids (R224 and K229) are also required for NLS2 function (data not shown). WSN NS1A protein has a G rather than an R at position 224. In addition, other H1N1 type viruses have a negatively charged E rather than a positively charged K at position 229 (see Fig. 2B). In particular, a negatively charged amino acid within an NLS/NoLS seems to eliminate all binding capacity.
FIG. 6.
C-terminal deletion mutant influenza A/Udorn/72 NS1AΔ231-237 protein binds to importin α6. (A) 35S-labeled and in vitro translated A/Udorn/72 NS1A wt and three mutant proteins were allowed to bind to E. coli-expressed and Sepharose-immobilized GST and GST-importin α6 proteins as indicated in the figure. Importin-bound NS1A was dissolved in Laemmli sample buffer, separated by 12% SDS-PAGE, and autoradiographed. A similar gel was also stained with Coomassie blue to visualize the amount of Sepharose-immobilized GST and GST-importin (GST-imp) α6 proteins. For the translation control, 1 μl of 35S-labeled and in vitro translated NS1 wt and three mutants were separated by 12% SDS-PAGE and autoradiographed. (B) HuH7 cells were transiently transfected with wt or C-terminal deletion mutant influenza A/Udorn/72 NS1A gene constructs as indicated in the figure. NLS1, NLS1(R37A R38A); NLS2, NLS2(K219A R220A). Bar, 5 μm.
Localization of the NS1A protein in influenza A virus-infected cells: identification of a C-terminal NoLS.
Analysis of the intranuclear localization of the NS1A protein in virus-infected A549 cells revealed that in A/Udorn/72-infected cells, the NS1A protein was found in not only the nucleus but also the nucleolus at 6 to 12 h after infection (Fig. 7A). In contrast, in A/WSN/33-infected cells the NS1A protein localized in the nucleus but failed to accumulate significantly in the nucleolus. These results suggested that the C-terminal end of the Udorn NS1A protein containing the NLS2 was responsible for the nucleolar localization of its encoded NS1A protein. To test this possibility, we generated a recombinant A/Udorn/72 virus that encoded an NS1A protein with a deletion in its C-terminal end (NS1AΔ221-237). This virus was not attenuated, and it replicated with the same kinetics as wt Udorn virus. However, the mutant NS1AΔ221-237 protein completely failed to accumulate in the nucleolus (Fig. 7A), demonstrating that the C-terminal 17 amino acids of the A/Udorn/72 virus NS1A protein contains a functional NoLS.
FIG. 7.
Intracellular localization of NS1A protein during influenza A virus infection. (A) A549 cells grown directly on coverslips were infected with wt influenza A/Udorn/72, recombinant A/Udorn/72 NS1AΔ221-237 with a stop codon at position 221 of the NS1A protein gene, or wt A/WSN/33 viruses for 4 to 24 h as indicated in the figure. After fixation, the cells were stained with rabbit anti-NS1A and fluorescein isothiocyanate-labeled anti-rabbit antibodies, followed by analysis with confocal laser microscopy. Bar, 5 μm. (B) A549 cells were infected with different wt H3N2 or H1N1 influenza A virus strains as indicated in the figure. Nuclear and nucleolar localization of NS1A protein was detected at 6 and 12 h after infection by indirect immunofluorescence microscopy. For each virus the percentage of cells expressing NS1A protein in the nucleolus was calculated from 300 NS1A protein-expressing cells.
Because the C-terminal extension (residues 231 to 237) is not required for the NLS2 function of the Udorn NS1A protein, it was possible that this extension is also not required for NoLS function. To test this possibility, we utilized three H3N2-type viruses (A/Beijing/353/89, A/Finland/229/92, and A/Finland/455/97), each of which encodes an NS1A protein that lacks the C-terminal extension but has an amino acid sequence from position 219 to 230 that is identical to that of A/Udorn/72 (Fig. 2B). In A549 cells infected with these H3N2 viruses, nucleolar localization of the NS1A protein was observed in 78 to 91% of the cells (Fig. 7B). This is similar to the results with A/Udorn/72, whose NS1A protein contains the C-terminal extension. These results indicate that the shared sequence from 219 to 230 in the NS1A proteins of H3N2 viruses is sufficient for NoLS function, as is the case for NLS2 function (see above). This was not the case for influenza A/WSN/33 (Fig. 6A), as well as for two other H1N1 viruses that lack the C-terminal extension (A/Finland/432/96 and A/New Caledonia/20/99) (Fig. 2B and 7B). In A549 cells infected with these H1N1 viruses, only weak nucleolar localization of the NS1A protein was observed in approximately 7 to 15% of the cells. Consequently, the NS1A sequence from residue 219 to 230 of these H1N1 viruses, which lack basic amino acids at positions 224 and 229 (Fig. 2B), does not exhibit an NoLS function. In fact, even with the H1N1 viruses whose NS1A proteins contain the C-terminal extension (residues 231 to 237), for example, A/Finland/001/79 and A/Finland/40/86, nucleolar localization is weaker than that observed with H3N2 viruses: nucleolar localization was observed in only approximately 25 to 41% of the infected cells. These results indicate that the basic residues at positions 224 and 229 in the NS1A protein of H3N2 viruses function in the C-terminal NoLS of the NS1A protein.
C-terminal arginines and lysines form the functional NoLS of the NS1A protein.
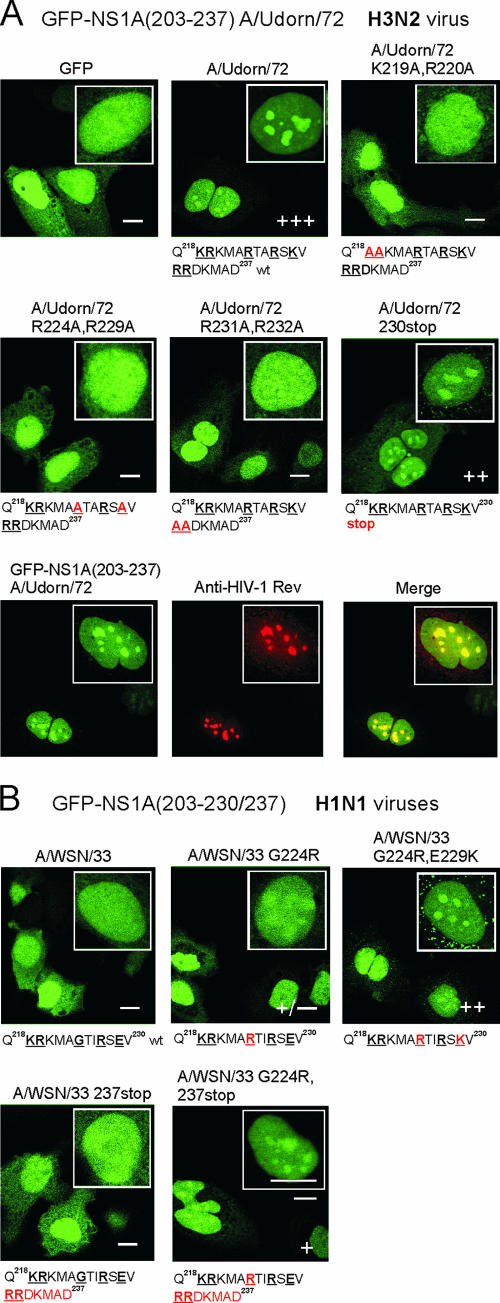
To verify the identity of the amino acids that constitute the NoLS of the NS1A protein of A/Udorn/72, we constructed plasmids expressing GFP fusion proteins containing the C-terminal amino acids 203 to 237 of this virus [GFP-NS1A(203-237)]. This plasmid was transiently transfected into HuH7 cells, and the subcellular localization of the GFP-containing proteins was analyzed by fluorescence microscopy (Fig. 8A). GFP expressed alone was distributed throughout the cells. In contrast, GFP-NS1A(203-237) accumulated predominantly in the nucleoli. Coexpression of this protein and the HIV-1 Rev protein, which has previously been localized into the nucleolus (29), showed an excellent colocalization, establishing that GFP-NS1A(203-237) is indeed localized in the nucleoli (Fig. 8A, lower panels). Double mutations, K219A R220A, R224A K229A, or R231A R232A, in the NS1A(203-237) sequence of the GFP fusion protein destroyed the NoLS, since these mutant proteins failed to accumulate into the nucleoli (Fig. 8A). Interestingly, the GFP-NS1A(203-230) protein, which lacks residues 231 to 237 (RRDKMAD) of the C-terminal extension, maintained its nucleolar localization, although a bit weakened, indicating that the presence of the C-terminal extension is not absolutely required for nucleolar localization.
FIG. 8.
C-terminal end of NS1A protein in H3N2 type influenza A viruses encode a functional NoLS. C-terminal fragments of NS1A genes encoding amino acids 203 to 237 in A/Udorn/72 and amino acids 203 to 230 in A/WSN/33 were inserted into GFP expression vector pCMX-SAH/Y145F to express GFP-NS1A fusion proteins [GFP-NS1A(9203-230/237)]. (A) HuH7 cells were transiently transfected with GFP-wt, mutant, or deletion A/Udorn/72 NS1A gene constructs for 48 h as indicated in the figure. The intensity of nucleolar localization was scored by immunofluorescence microscopy as no nucleolar staining (−) or weak (+), moderate (++), or strong (+++) nucleolar staining. Critical basic amino acids involved in nuclear/nucleolar targeting are marked in boldface and underlined. Mutated amino acids are marked in red. To verify nucleolar localization, HuH7 cells were transiently transfected with GFP-wt NS1A(203-237) A/Udorn/72 and HIV-1 Rev gene constructs for 48 h as indicated in the figure. After fixation the cells were stained with anti-HIV-1 Rev antibodies, and colocalization with GFP-NS1A(203-237) protein was detected with confocal microscopy. (B) HuH7 cells were transiently transfected with GFP-wt and mutant A/WSN/33 NS1A gene constructs for 48 h as indicated in the figure. Critical and mutated amino acids are marked as above. Bars, 5 μm.
As indicated by the results shown in Fig. 7, the NS1A protein of the H1N1 A/WSN/33 virus, which lacks a C-terminal extension, possess only a weak functional NoLS. Consistent with these results, the GFP-NS1A(203-230) A/WSN/33 fusion protein completely failed to accumulate in the nucleoli (Fig. 8B). Next, we determined whether NoLS function could be activated by the introduction of basic residues at 224 and/or at both 224 and 229. Mutation of G224 to R led to partial nucleolar localization, whereas mutation of both G224 and E229 to basic residues (R and K, respectively) resulted in full nucleolar localization. Consequently, basic residues at both of these positions were sufficient for a functional NoLS.
Next we concentrated on analyzing the role of the C-terminal extension in nucleolar targeting. The C-terminal extension (residues 231 to 237) by itself did not render the GFP-NS1A(203-230) A/WSN/33 fusion protein nucleolar (Fig. 8B). However, if G224 was mutated to R, this C-terminal extension rendered the protein faintly nucleolar. These results, coupled with those described above, demonstrate that the NoLS of the NS1A protein is comprised of basic residues at positions 219, 220, 224, and 229, and when the NS1A protein has a C-terminal extension, basic residues at positions 231 and 232 are also part of the NoLS and will strengthen its function (Fig. 8B and 9A).
FIG. 9.
(A) Schematic representation of NS1A intracellular targeting signals. Both WSN and Udorn virus NS1A proteins have an NLS constituting basic residues 35, 38, and 41 (NLS1). The Udorn NS1A protein also has a 7-amino-acid C-terminal extension (residues 231 to 237) and a second NLS (NLS2) at the end of the molecule, constituting arginines or lysines at positions 219, 229, 224, 229, 231, and 323. The same residues form a functional NoLS. NLS and NoLS signals are shown in bold and underlined. (B) Mechanisms of nuclear import of Udorn virus NS1A protein. Newly synthesized dimeric Udorn NS1A protein interacts via its NLS1 or NLS2 with different cytoplasmic importin (Imp) α (all 6 isotypes)/importin β complex, followed by nuclear translocation of the complex via the NPC. Most cytoplasmic NS1A is likely bound to importin α interfering with dsRNA binding to NS1A in the cytoplasm. In the nucleus NS1A protein is released from the transport complex, and importin α and importin β are transported back into the cytoplasm through the NPC. After the release of importin α, the C-terminal NoLS of the NS1A protein is exposed, and the NS1A protein is targeted into the nucleolus. Simultaneously, the dsRNA-binding domain of the NS1A protein is also exposed, and the protein becomes competent to bind dsRNA. This leads to sequestration of dsRNA and lack of activation of the oligoadenylate synthetase/RNase L antiviral pathway. RBD, dsRNA-binding domain.
DISCUSSION
In the present study we show that influenza A virus NS1A protein has a strong arginine- or lysine-rich NLS (NLS1) in the N-terminal part of the molecule, which is very well conserved among all known avian, human, and other mammalian NS1A proteins. NLS1 was found to mediate an interaction of the NS1A protein with all six human importin α isoforms, indicating that the nuclear import of the NS1A protein takes place via the classical importin α/β nuclear import pathway. This is likely to happen in all types of cells, since different importin α isoforms, although not always all of them, are expressed in all cells (21). The critical amino acids regulating the functionality of NLS1 were identified to be R35, R38, and K41. It is remarkable that the amino acids which form the NLS1 of the NS1A protein are also the same ones that participate in dsRNA-binding activity of the protein (16, 28, 57, 59). Previous site-directed mutagenesis experiments have shown that R38 and K41 are the most essential ones for dsRNA-binding (57).
Because of these mutagenesis results, it was surprising that the NS1A protein was able to bind to dsRNA and importin α simultaneously. This finding suggests that although the most important amino acids comprising the NLS1 (R35, R38, and K41) and dsRNA binding (R38 and K41) sites of the NS1A protein are practically the same, the binding sites of dsRNA and importin α are not totally overlapping, enabling both molecules to bind to the NS1A protein at the same time. Hopefully, future structural analyses will reveal the exact binding sites of dsRNA and importin α on the NS1A protein. It is noteworthy that relatively high concentrations of these molecules are required before an interaction between these molecules can be demonstrated, suggesting a relatively low binding affinity for both dsRNA and importin α binding to the NS1A protein. In fact, a low affinity of the NS1A RNA-binding domain for dsRNA has previously been demonstrated (7). Nonetheless, it is likely that the RNA-binding domain of newly synthesized NS1A protein molecules is efficiently bound to importin α molecules that mediate nuclear import, because at most times of infection the vast majority of the NS1A protein is found in the nucleus (Fig. 8). Based on our competition experiments, these NS1A proteins would be capable of importing dsRNA molecules into the nucleus.
In addition to NLS1 which overlaps with the dsRNA-binding domain, the C terminus of the NS1A proteins of H3N2 and H2N2 viruses contains an NLS2, which also functions as an NoLS, thereby localizing these NS1A proteins in the nucleolus (data summarized in Fig. 9). To further verify that the C-terminal NLS2 also functioned as an NoLS during virus infection, we generated a recombinant H3N2 Udorn virus encoding an NS1A protein that lacks the last 17 C-terminal amino acids. The NS1A protein expressed by the wt Udorn virus localized in the nucleolus, whereas the NS1A protein expressed by the mutant virus failed to do so. From an evolutionary point of view, it was of interest that the C-terminal extension of 7 amino acids (amino acids 231 to 237) was found in viruses isolated between the years 1950 and 1989. This extension existed in not only the H3N2 NS1A proteins but also H1N1 and H2N2 viruses as well (Fig. 2). A functional NLS2/NoLS of the Udorn NS1A protein required the basic arginine or lysine residues at positions 219, 220, 231, and 232. Evolutionary and mutational analyses (Fig. 2 and 8) revealed that R at position 224 and K at position 229 also played a role in the formation of a functional NLS2/NoLS. When the residues 224 and 229 were R and K, respectively, the NLS2/NoLS function was not lost, even if the C-terminal extension was eliminated by a stop codon at position 231 in the NS1A reading frame of the H3N2 viruses, as was the case in viruses that were isolated after the year 1989. An additional interesting observation was that, when the arginines at positions 231 and 232 were mutated to alanines, the NLS2/NoLS function was lost even if residues 224 and 229 were intact. This may indicate that the mutated C-terminal extension (residues 231 to 237) of NS1A protein may fold in such a way that it interferes with the interaction of basic residues 224 and 229 with importin α. Consequently, our data indicate that the C-terminal extension with its basic residues at positions 231 and 232 was able to create a functional second NLS and NoLS for the NS1A protein. However, this extension was no longer needed for NLS2/NoLS function, if the C terminus contained an arginine and a lysine at positions 224 and 229, respectively, as was the case in the evolutionary lineage of H3N2 type viruses (Fig. 2).
In contrast, the H1N1 NS1A proteins, which contain the C-terminal extension, show only weak nucleolar localization, which is lost when the C-terminal extension was eliminated in H1N1 viruses isolated after the year 1989. The absence of an NoLS was thus traced to the absence of basic residues at positions 224 and 229. In addition, the C-terminal end of the H5N1 (avian virus) NS1A protein resembles the sequences of the present H1N1 type viruses, and is thus likely to lack the C-terminal NLS2/NoLS.
The most likely interpretation of our results is that the important function of the C-terminal region of the NS1A proteins of the H3N2 and H2N2 viruses is to target these NS1A proteins to the nucleolus. Nucleolar localization of the NS1A protein is not unique, since many other viruses apart from influenza A virus encode proteins that are targeted into the nucleolus (17). The nucleolar localization of dengue, Kunjin, and Japanese encephalitis virus core proteins apparently plays a critical role in virus replication and pathogenesis in mammalian cells (35, 56, 58). It is possible that viral proteins localized in the nucleolus interfere with normal cell cycle regulation and/or associate with or reorganize nucleolar proteins like nucleolin, B23, and fibrillarin (17). Nucleolar sequestration of regulatory proteins may be another mechanism by which viral proteins regulate cellular gene expression, cell proliferation, and apoptosis (1, 4, 51). It has been shown previously that localization of the influenza virus NS1A protein in the nucleolus inhibits rRNA synthesis (23). Our working hypothesis is that the primary roles of the nucleolar localization of H3N2 and H2N2 NS1A proteins are on host functions and thus on pathogenesis, particularly because the H3N2 Udorn virus that expresses an NS1A protein lacking its 17 C-terminal amino acids does not exhibit any apparent defect during replication in tissue culture experiments (unpublished data). Consequently, an important future question will be to determine whether nucleolar localization of the NS1A protein plays a role in the pathogenesis of H3N2 and H2N2 influenza A virus infections.
Acknowledgments
We thank Reijo Pyhälä for the viruses and valuable discussions. We also thank Sinikka Sopanen and Raija Tyni for providing us with the cells, Anja Villberg and Riitta Santanen for growing different influenza viruses, and Hanna Valtonen and Johanna Lahtinen for their excellent technical assistance.
This study was supported by the Medical Research Council of the Academy of Finland and the Sigrid Juselius Foundation (I.J.) and by grant AI11772 from the National Institutes of Health of the U.S. Department of Health and Human Services (R.M.K.).
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Belmont, A. 2003. Dynamics of chromatin, proteins, and bodies within the cell nucleus. Curr. Opin. Cell Biol. 15:304-310. [DOI] [PubMed] [Google Scholar]
- 2.Birbach, A., S. T. Bailey, S. Ghosh, and J. A. Schmid. 2004. Cytosolic, nuclear and nucleolar localization signals determine subcellular distribution and activity of the NF-κB inducing kinase NIK. J. Cell Sci. 117:3615-3624. [DOI] [PubMed] [Google Scholar]
- 3.Bornholdt, Z. A., and B. V. Prasad. 2006. X-ray structure of influenza virus NS1 effector domain. Nat. Struct. Mol. Biol. 13:559-560. [DOI] [PubMed] [Google Scholar]
- 4.Carmo-Fonseca, M. 2002. The contribution of nuclear compartmentalization to gene regulation. Cell 108:513-521. [DOI] [PubMed] [Google Scholar]
- 5.Chen, Z., Y. Li, and R. M. Krug. 1999. Influenza A virus NS1 protein targets poly(A)-binding protein II of the cellular 3′-end processing machinery. EMBO J. 15:2273-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chien, C. Y., R. Tejero, Y. Huang, D. E. Zimmerman, C. B. Rios, R. M. Krug, and G. T. Montelione. 1997. A novel RNA-binding motif in influenza A virus non-structural protein 1. Nat. Struct. Biol. 4:891-895. [DOI] [PubMed] [Google Scholar]
- 7.Chien, C. Y., Y. Xu, R. Xiao, J. M. Aramini, P. V. Sahasrabudhe, R. M. Krug, and G. T. Montelione. 2004. Biophysical characterization of the complex between double-stranded RNA and the N-terminal domain of the NS1 protein from influenza A virus: evidence for a novel RNA-binding mode. Biochemistry 43:1950-1962. [DOI] [PubMed] [Google Scholar]
- 8.Conti, E., M. Uy, L. Leighton, G. Blobel, and J. Kuriyan. 1998. Crystallographic analysis of the recognition of a nuclear localization signal by the nuclear import factor karyopherin alpha. Cell 94:193-204. [DOI] [PubMed] [Google Scholar]
- 9.Cortes, P., Z. S. Ye, and D. Baltimore. 1994. RAG-1 interacts with the repeated amino acid motif of the human homologue of the yeast protein SRP1. Proc. Natl. Acad. Sci. USA 91:7633-7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuomo, C. A., S. A. Kirch, J. Gyuris, R. Brent, and M. A. Oettinger. 1994. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein Proc. Natl. Acad. Sci. USA 91:6156-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dingwall, C., S. V. Sharnick, and R. A. Laskey. 1982. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell 30:449-458. [DOI] [PubMed] [Google Scholar]
- 12.Fagerlund, R., K. Melén, L. Kinnunen, and I. Julkunen. 2002. Arginine/lysine-rich nuclear localization signals mediate interactions between dimeric STATs and importin alpha 5. J. Biol. Chem. 277:30072-30078. [DOI] [PubMed] [Google Scholar]
- 13.Fagerlund, R., L. Kinnunen, M. Kohler, I. Julkunen, and K. Melén. 2005. NF-κB is transported into the nucleus by importin α3 and importin α4. J. Biol. Chem. 280:15942-15951. [DOI] [PubMed] [Google Scholar]
- 14.Fontes, M. R., T. The, and B. Kobe. 2000. Structural basis of recognition of monopartite and bipartite nuclear localization sequences by mammalian importin-alpha. J. Mol. Biol. 297:1183-1194. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan, D., P. Palese, and M. Krystal. 1988. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J. Virol. 62:3020-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hatada, E., and R. Fukuda. 1992. Binding of influenza A virus NS1 protein to dsRNA in vitro. J. Gen. Virol. 73:3325-3329. [DOI] [PubMed] [Google Scholar]
- 17.Hiscox, J. A. 2002. The nucleolus—a gateway to viral infection? Arch. Virol. 147:1077-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalderon, D., B. L. Roberts, W. D. Richardson, and A. E. Smith. 1984. A short amino acid sequence able to specify nuclear location. Cell 39:499-509. [DOI] [PubMed] [Google Scholar]
- 19.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 20.Kawaoka, Y., O. T. Gorman, T. Ito, K. Wells, R. O. Donis, M. R. Castrucci, I. Donatelli, and R. G. Webster. 1998. Influence of host species on the evolution of the nonstructural (NS) gene of influenza A viruses. Virus Res. 55:143-156. [DOI] [PubMed] [Google Scholar]
- 21.Köhler, M., S. Ansieau, S. Prehn, A. Leutz, H. Haller, and E. Hartmann. 1997. Cloning of two novel human importin-alpha subunits and analysis of the expression pattern of the importin-alpha protein family. FEBS Lett. 417:104-108. [DOI] [PubMed] [Google Scholar]
- 22.Köhler, M., C. Speck, M. Christiansen, F. R. Bischoff, S. Prehn, H. Haller, D. Gorlich, and E. Hartmann. 1999. Evidence for distinct substrate specificities of importin α family members in nuclear protein import. Mol. Cell. Biol. 19:7782-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krug, R. M., and R. Soeiro. 1975. Studies on the intranuclear localization of influenza virus-specific proteins. Virology 64:378-387. [DOI] [PubMed] [Google Scholar]
- 24.Lanford, R. E., and J. S. Butel. 1984. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell 37:801-813. [DOI] [PubMed] [Google Scholar]
- 25.Li, S., J. Y. Min, R. M. Krug, and G. C. Sen. 2006. Binding of the influenza A virus NS1 protein to PKR mediates the inhibition of its activation by either PACT or double-stranded RNA. Virology 349:13-21. [DOI] [PubMed] [Google Scholar]
- 26.Li, Y., Z. Y. Chen, W. Wang, C. C. Baker, and R. M. Krug. 2001. The 3′-end-processing factor CPSF is required for the splicing of single-intron pre-mRNAs in vivo. RNA 7:920-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, J., P. A. Lynch, C. Y. Chien, G. T. Montelione, R. M. Krug, and H. M. Berman. 1997. Crystal structure of the unique RNA-binding domain of the influenza virus NS1 protein. Nat. Struct. Biol. 4:896-899. [DOI] [PubMed] [Google Scholar]
- 28.Lu, Y., M. Wambach, M. G. Katze, and R. M. Krug. 1995. Binding of the influenza virus NS1 protein to double-stranded RNA inhibits the activation of the protein kinase that phosphorylates the elF-2 translation initiation factor. Virology 214:222-228. [DOI] [PubMed] [Google Scholar]
- 29.Macken, C., H. Lu, J. Goodman, and L. Boykin. 2001. The value of a database in surveillance and vaccine selection, p. 103-106. In A. D. M. E. Osterhaus, N. Cox, and A. W. Hampson (ed.), Options for the control of influenza IV. Elsevier Science, Amsterdam, The Netherlands.
- 30.Malim, M. H., J. Hauber, R. Fenrick, and B. R. Cullen. 1988. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature 335:181-183. [DOI] [PubMed] [Google Scholar]
- 31.Matikainen, S., J. Siren, J. Tissari, V. Veckman, J. Pirhonen, M. Severa, Q. Sun, R. Lin, S. Meri, G. Uze, J. Hiscott, and I. Julkunen. 2006. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J. Virol. 80:3515-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melén, K., R. Fagerlund, J. Franke, M. Kohler, L. Kinnunen, and I. Julkunen. 2003. Importin alpha nuclear localization signal binding sites for STAT1, STAT2, and influenza A virus nucleoprotein. J. Biol. Chem. 278:28193-28200. [DOI] [PubMed] [Google Scholar]
- 33.Mibayashi, M., L. Martinez-Sobrido, Y. M. Loo, W. B. Cardenas, M. Gale, Jr., and A. Garcia-Sastre. 2007. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 81:514-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min, J. Y., and R. M. Krug. 2006. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: inhibiting the 2′-5′ oligo(A) synthetase/RNase L pathway. Proc. Natl. Acad. Sci. USA 103:7100-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori, Y., T. Okabayashi, T. Yamashita, Z. Zhao, T. Wakita, K. Yasui, F. Hasebe, M. Tadano, E. Konishi, K. Moriishi, and Y. Matsuura. 2005. Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J. Virol. 79:3448-3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nachury, M. V., U. W. Ryder, A. I. Lamond, and K. Weis. 1998. Cloning and characterization of hSRP1 gamma, a tissue-specific nuclear transport factor. Proc. Natl. Acad. Sci. USA 95:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakabayashi, H., K. Taketa, K. Miyano, T. Yamane, and J. Sato. 1982. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 42:3858-3863. [PubMed] [Google Scholar]
- 38.Nemeroff, M. E., S. M. Barabino, Y. Li, W. Keller, and R. M. Krug. 1998. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of CPSF and inhibits 3′ end formation of cellular pre-mRNAs. Mol. Cell 1:991-1000. [DOI] [PubMed] [Google Scholar]
- 39.Noah, D. L., K. Y. Twu, and R. M. Krug. 2003. Cellular antiviral responses against influenza A virus are countered at the posttranscriptional level by the viral NS1A protein via its binding to a cellular protein required for the 3′ end processing of cellular pre-mRNAS. Virology 307:386-395. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa, H., S. Inouye, F. I. Tsuji, K. Yasuda, and K. Umesone. 1995. Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc. Natl. Acad. Sci. USA 92:11899-11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson, M. O., K. Hingorani, and A. Szebeni. 2002. Conventional and nonconventional roles of the nucleolus. Int. Rev. Cytol. 219:199-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olson, M. O. 2004. Sensing cellular stress: another new function for the nucleolus? Sci. STKE 2004: pe10. [DOI] [PubMed] [Google Scholar]
- 43.Olson, M. O., and M. Dundr. 2005. The moving parts of the nucleolus. Histochem. Cell. Biol. 123:203-216. [DOI] [PubMed] [Google Scholar]
- 44.Pichlmair, A., O. Schulz, C. P. Tan, T. I. Naslund, P. Liljestrom, F. Weber, and C. Reis e Sousa. 2006. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997-1001. [DOI] [PubMed] [Google Scholar]
- 45.Qian, X. Y., F. Alonso-Caplen, and R. M. Krug. 1994. Two functional domains of the influenza virus NS1 protein are required for regulation of nuclear export of mRNA. J. Virol. 68:2433-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quaye, I. K., S. Toku, and T. Tanaka. 1996. Sequence requirement for nucleolar localization of rat ribosomal protein L31. Eur. J. Cell Biol. 69:151-155. [PubMed] [Google Scholar]
- 47.Ruggero, D., and P. P. Pandolfi. 2003. Does the ribosome translate cancer? Nat. Rev. Cancer 3:179-192. [DOI] [PubMed] [Google Scholar]
- 48.Seki, T., S. Tada, T. Katada, and T. Enomoto. 1997. Cloning of a cDNA encoding a novel importin-alpha homologue, Qip1: discrimination of Qip1 and Rch1 from hSrp1 by their ability to interact with DNA helicase Q1/RecQL. Biochem. Biophys. Res. Commun. 234:48-53. [DOI] [PubMed] [Google Scholar]
- 49.Shimizu, K., A. Iguchi, R. Gomyou, and Y. Ono. 1999. Influenza virus inhibits cleavage of the HSP70 pre-mRNAs at the polyadenylation site. Virology 254:213-219. [DOI] [PubMed] [Google Scholar]
- 50.Sirén, J., T. Imaizumi, T. Pietilä, R. Lin, J. Hiscott, D. L. Noah, R. M. Krug, D. Sarkar, P. B. Fisher, I. Julkunen, and S. Matikainen. 2006. RIG-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect. 8:2138-2144. [DOI] [PubMed] [Google Scholar]
- 51.Stark, L. A., and M. G. Dunlop. 2005. Nucleolar sequestration of RelA (p65) regulates NF-κB-driven transcription and apoptosis. Mol. Cell. Biol. 25:5985-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Summers, M. D., and G. E. Smith. 1986. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex. Agric. Exp. Station Bull. 1555:1-57. [Google Scholar]
- 53.Takeda, M., A. Pekosz, K. Shuck, L. H. Pinto, and R. A. Lamb. 2002. Influenza a virus M2 ion channel activity is essential for efficient replication in tissue culture. J. Virol. 76:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Timmers, A. C., R. Stuger, P. J. Schaap, J. van't Riet, and H. A. Raue. 1999. Nuclear and nucleolar localization of Saccharomyces cerevisiae ribosomal proteins S22 and S25. FEBS Lett. 452:335-340. [DOI] [PubMed] [Google Scholar]
- 55.Twu, K. Y., D. L. Noah, P. Rao, R. L. Kuo, and R. M. Krug. 2006. The CPSF30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 80:3957-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang, S. H., W. J. Syu, K. J. Huang, H. Y. Lei, C. W. Yao, C. C. King, and S. T. Hu. 2002. Intracellular localization and determination of a nuclear localization signal of the core protein of dengue virus. J. Gen. Virol. 83:3093-3102. [DOI] [PubMed] [Google Scholar]
- 57.Wang, W., K. Riedel, P. Lynch, C. Y. Chien, G. T. Montelione, and R. M. Krug. 1999. RNA binding by the novel helical domain of the influenza virus NS1 protein requires its dimer structure and a small number of specific basic amino acids. RNA 5:195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westaway, E. G., A. A. Khromykh, M. T. Kenney, J. M. Mackenzie, and M. K. Jones. 1997. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology 234:31-41. [DOI] [PubMed] [Google Scholar]
- 59.Yuan, W., J. M. Aramini, G. T. Montelione, and R. M. Krug. 2002. Structural basis for ubiquitin-like ISG 15 protein binding to the NS1 protein of influenza B virus: a protein-protein interaction function that is not shared by the corresponding N-terminal domain of the NS1 protein of influenza A virus. Virology 304:291-301. [DOI] [PubMed] [Google Scholar]