Abstract
An unidentified agent was cultured in primary monkey cells at the Los Angeles County Public Health Department from each of five stool specimens submitted from an outbreak of gastroenteritis. Electron microscopy and an adenovirus-specific monoclonal antibody confirmed this agent to be an adenovirus. Since viral titers were too low, complete serotyping was not possible. Using the DNase-sequence-independent viral nucleic acid amplification method, we identified several nucleotide sequences with a high homology to human adenovirus 41 (HAdV-41) and simian adenovirus 1 (SAdV-1). However, using anti-SAdV-1 sera, it was determined that this virus was serologically different than SAdV-1. Genomic sequencing and phylogenetic analysis confirmed that this new adenovirus was so divergent from the known human adenoviruses that it was not only a new type but also represented a new species (human adenovirus G). In a retrospective clinical study, this new virus was detected by PCR in one additional patient from a separate gastroenteritis outbreak. This study suggests that HAdV-52 may be one of many agents causing gastroenteritis of unknown etiology.
Viral gastroenteritis is defined as inflammation of the stomach and large and small intestines and is caused by infection with the following viruses: adenoviruses 40 and 41, norovirus, rotavirus, astrovirus, torovirus, picobirnavirus, and human parechovirus 1 (7, 32, 34). Gastroenteritis of unknown etiology is a serious problem in the United States and is estimated to cause 5,000 deaths annually (17). Unfortunately, information about deaths caused by gastroenteritis of unknown etiology is limited (17).
Adenoviruses were first isolated from civilians and Army recruits who had respiratory disease (21, 27). Epidemiological studies confirmed that adenoviruses are the cause of acute febrile respiratory disease among military recruits (14, 18). Since then, 51 human adenovirus (HAdV) serotypes have been characterized and classified according to their nucleic acid characteristics and homologies, hexon and fiber protein characteristics, and biological properties and placed in the genus Mastadenovirus (13). The 51 adenovirus serotypes that are known to infect humans are classified into six species (Human adenovirus A to F [HAdV-A to -F]) (5) and are known to cause gastroenteritis (HAdV-F; HAdV-40 and HAdV-41), acute febrile pharyngitis (HAdV-B and -C; HAdV-1 to -3 and HAdV-5 to -7), pharyngoconjunctival fever (HAdV-B; HAdV-3, HAdV-7, and HAdV-14), acute respiratory disease (HAdV-B and -E; HAdV-3, HAdV-4, HAdV-7, HAdV-14, and HAdV-21), pneumonia (HAdV-B, -C, and -E; HAdV-1 to -4 and HAdV-7), keratoconjunctivitis (HAdV-B and -D; HAdV- 8, HAdV-11, HAdV-19, and HAdV-37), pertussis-like syndrome (HAdV-C; HAdV-5), acute hemorrhagic cystitis (HAdV-B; HAdV-11 and HAdV-21), meningoencephalitis (HAdV-A, -B, and -D; HAdV-7, HAdV-12, and HAdV-32), and hepatitis (HAdV-C; HAdV-1, HAdV-2, and HAdV-5).
Adenoviruses have linear double-stranded DNA genomes that generally range from 26 to 45 kb and are encapsidated in an icosahedral protein shell (5). Through sequence analysis, it has been demonstrated that the genomes of all human adenoviruses have similar genetic organization (11, 24, 26).
In the past, human adenovirus serotype or species classification was defined by reactivity to different antibodies and by other biological properties (e.g., host specificity). Today, phylogenetics (based on phylogenetic calculations of informative viral proteins and on genomic organization) is the preferred and reliable method for classifying adenoviruses, as it demonstrates how viruses are related through molecular evolution (4, 19, 23).
In this study we sought to characterize an adenovirus that was isolated from the stool of a patient in a convalescent hospital who, along with four other patients, presented with gastroenteritis. Here we used DNase-sequence-independent viral nucleic acid amplification (DNase-SISPA) to obtain genetic information about the virus and performed a phylogenetic analysis. Near-complete viral genome sequencing, with genetic and phylogenetic analysis, was also performed.
MATERIALS AND METHODS
Study subjects.
An agent was cultured in primary monkey kidney cells at the Los Angeles County Public Health Department from five different stool specimens submitted from an outbreak of gastroenteritis at a convalescent home in Los Angeles County in February 2003. Based on the cytopathic effects and staining with an adenovirus-specific monoclonal antibody, the agent was identified as an adenovirus.
Viruses, cells, and neutralization test.
Five samples of virus grown in primary monkey kidney cells from stools obtained from clinical cases of gastroenteritis were sent by the Los Angeles Public Health Department to the California Department of Health Services Viral and Rickettsial Disease Laboratory (VRDL) in May 2003. Simian adenovirus 1 and hyperimmune rabbit antisera to simian adenovirus 1 were obtained from material archived at the VRDL. A-549 cells were from the VRDL in-house cell culture unit, and Graham 293 cells were obtained from the ATCC and maintained and prepared by the cell culture unit. The colorimetric neutralization test was previously described (9).
Electron microscopy.
Fluid from adenovirus-infected A-549 cells was prepared for negative staining by depositing a drop of concentrated virus suspension onto a 1% agarose surface and inverting a grid on the drop of viral suspension. When the fluid was completely absorbed into the agar, the grid was lifted off, stained with potassium phosphotungstate, and exposed to UV radiation for 10 min on each side. The grids were examined with a Hitachi 7500 electron microscope.
DNase-SISPA.
We screened cell-free medium from A-549 cells previously infected with the T03-2244 isolate of HAdV-52. One hundred μl of cell-free medium was filtered through a 0.22-μm filter (Ultrafree MC; Millipore, Bedford, MA) and treated with 250 U of DNase I (Roche Diagnostics, Mannheim, Germany) to remove contaminating double-stranded DNA. DNase I-resistant nucleic acids were purified using the MagNA Pure LC DNA isolation kit I (Roche Diagnostics, Mannheim, Germany). To detect viral DNA, double-stranded DNA was generated by incubating 50 μl of extracted DNA, 5 units of Klenow fragment (exo-; New England Biolabs, Beverly, MA), and 240 pmol of primer A (5-GTTTCCCAGTCACGATCNNNNNNNNN-3′) in 1× EcoPol buffer at 10°C for 5 min followed by 8 min at 37°C. Nucleic acid was subsequently amplified with primer B (5′-GTTTCCCAGTCACGATC-3′) in 10× Takara buffer under the following cycling conditions: 94°C for 21 s, 40°C for 30 s, 50°C for 30 s, and 72°C for 1 min, for 40 cycles. Next, 44 μl of PCR product was digested with the restriction enzyme Csp6.I (Fermentas, Hanover, MD). Adaptors composed of hybridized oligonucleotides NBam24 (5′-AGGCAACTGTGCTATCCGAGGGAG-3′) and NCsp11 (5′-TACTCCCTCGG-3′; 20 pmol) were then ligated to the restriction enzyme-digested DNA using T4 DNA ligase (Invitrogen, Carlsbad, CA) in 10× T4 DNA ligase buffer. Two μl of the ligation reaction mixture was used as a template in a 50-μl PCR mixture containing 25 pmol of NBam24, 10× Takara buffer, 10 mM deoxynucleoside triphosphates, and 2.5 units of Takara Ex Taq (Takara Bio, Shiga, Japan). The PCR mixture was heated to 72°C for 3 min before the addition of Takara Ex Taq. The mixture was then heated for an additional 5 min at 72°C to generate the primer-binding sites. Cycling conditions were as follows: 94°C for 1 min and 72°C for 3 min, for 40 cycles.
Analysis of DNase-SISPA-amplified DNA.
Amplified PCR products were analyzed by polyacrylamide gel electrophoresis (PAGE). The distinct DNA bands seen by PAGE were excised and pooled. DNA from the pooled PAGE bands from each sample was purified and subcloned into pCR-II-TOPO (Invitrogen, Carlsbad, CA). Because excised DNA bands were pooled prior to subcloning, we were unable to relate each sequence to a particular PAGE band. Plasmids containing inserts were purified and sequenced using the T7 sequencing primer. We sequenced our cloned and uncloned PCR fragments by using the BigDye terminator version 3.1 sequencing kit (Amersham Pharmacia Biotech, Piscataway, NJ) and an ABI Prism 3130X genetic analyzer (Applied BioSystems, Foster City, CA) according to the manufacturers’ instructions.
Amplification of the HAdV-52 genome.
To amplify regions of HAdV-52 flanking the sequences found with DNase-SISPA, we designed primers based on conserved adenovirus sequences generated with a nucleotide alignment of human adenovirus genomes from HAdV-A to -F (HAdV-12, NC_001460; HAdV-11, NC_004001; HAdV-2, NC_001405; HAdV-17, NC_002067; HAdV-4, NC_003266; HAdV-40, NC_001454) using Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA) and the sequences found with DNase-SISPA. All amplicons were then sequenced using primer walking. The 5′ end of the HAdV-52 genome was amplified and sequenced using a primer based on the first 28 bp of HAdV-40 (human adenovirus F, NC_001454) and our human adenovirus A to F alignment (fwd pradenoF1, 5′-CATCATCAATAATATACCTTAAAACTGG-3′, and rev pr543R, 5′-TCGCTSGCACTCAAGAGTGG-3′).
Patient specimens.
One hundred and twenty-five anonymized stool specimens collected from 22 norovirus (NoV)-positive and three NoV-negative gastroenteritis outbreaks in California, from 2004 to 2006, were obtained from the California Department of Health Services (Richmond, CA). These specimens were previously tested for NoV genotype I (GI) and GII by reverse transcription-PCR using primers targeting conserved regions of the virus. All stool samples from healthy asymptomatic patients were acquired from the David Grant USAF Medical Center in 2006. There were no replicate samples in this study. There is no age-related information with regard to any of these stool samples.
Specimen processing.
Stool specimens were processed in a biosafety level 2 hood. One hundred milligrams of stool was added to 900 μl of 0.85% saline. Stool was vortexed with glass beads and then boiled for 2 min. Next, the samples were centrifuged at 1,500 × g for 15 min and then allowed to settle for 10 min at room temperature. Samples were transferred to a 32-well plate for nucleic acid isolation.
Nucleic acid isolation.
Viral DNA was extracted from tissue culture and processed stool samples using the MagNA Pure LC DNA isolation kit I (Roche, Indianapolis, IN) according to the manufacturer's recommendations for the MagNA Pure LC automated nucleic acid extraction system.
Detection of HAdV-52 by PCR assay.
HAdV-52-positive stool samples were identified by performing PCR with 0.5 μl from nucleic acid extraction of stools. The oligonucleotide primer sequences used for detection of HAdV-52 were prAD5157, 5′-GTTTAGCGGGATCAAAAACC-3′, and prAD5892, 5′-GTGTTCCCCGAGTGGC-3′. Our reaction conditions were as follows: 0.5 μl (∼100 mg of stool) of extracted DNA in 5 μl of 10× Ex Taq buffer, 4 μl of 25 mM MgCl2, 4 μl of 10 mM deoxynucleoside triphosphate, 25 pmol of each primer, and 0.5 μl of Takara Ex Taq (Takara Bio, Shiga, Japan). Primers were designed by M. Jones. The PCR protocol was 95°C for 2 min, followed by 40 cycles of 95°C for 21 seconds, 58°C for 30 seconds, and 72°C for 20 seconds, and a final extension at 72°C for 7 min. Thermal cycling was carried out in a T3000 thermocycler (Biometra, Goettingen, Germany). The results were analyzed by electrophoresis with a 6.5% acrylamide gel stain with SYBR Green. This experiment was also performed independently at the VRDL in Richmond, CA.
Phylogenetic analysis of HAdV-52.
Predicted amino acid sequences of the longer adenoviral proteins (DNA polymerase, pTP, pIIIa, penton base, hexon, DNA binding protein, 100K) from HAdV-52 and from primate adenoviral genomes available from GenBank were aligned using the MultAlin program (8). Phylogenetic analyses of the alignment were performed with the PHYLIP program (Phylogeny Inference Package, version 3.65) (16). Briefly, Protdist (categories matrix) followed by Fitch (global rearrangements) programs were used (19). The tree was displayed using TreeView 1.5.2 (25) using simian adenovirus 3 as the outgroup. The validity of the tree topology obtained was tested by using bootstrap analysis (15) starting with Seqboot with 100 resamplings from the aligned sequences, followed by distance matrix calculations and the Consense program to calculate the most probable (consensus) tree.
Nucleotide sequence accession number.
The HAdV-52 full genome has been deposited with GenBank under accession number DQ923122. Other nucleotide sequences that were used in this study included the following: SAdV-1 (NC_006879), possum brushtail adenovirus hexon sequence (AAF65554.1), and porcine adenovirus 3 (NC_005869).
RESULTS
Study subjects.
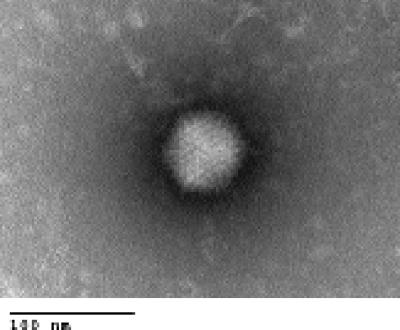
An agent was cultured in primary monkey cells at the Los Angeles County Public Health Department from five stool specimens from an outbreak of gastroenteritis at a convalescent home in Los Angeles County in February 2003. Based on the cytopathic effect and staining with an adenovirus-specific monoclonal antibody, the viral isolates were sent to the VRDL for more thorough characterization. There, the isolates were grown in A-549 cells and confirmed to be adenoviruses by staining with an adenovirus-specific monoclonal antibody as well as electron microscopy (Fig. 1). Complete serotyping was not possible because the viral titers were too low.
FIG. 1.
Transmission electron micrograph of isolated HAdV-52. Note that the virus has a typical icosahedral shape and is roughly 70 nm, which is typical of adenoviruses (29). Bar, 100 nm.
Molecular detection of new adenovirus.
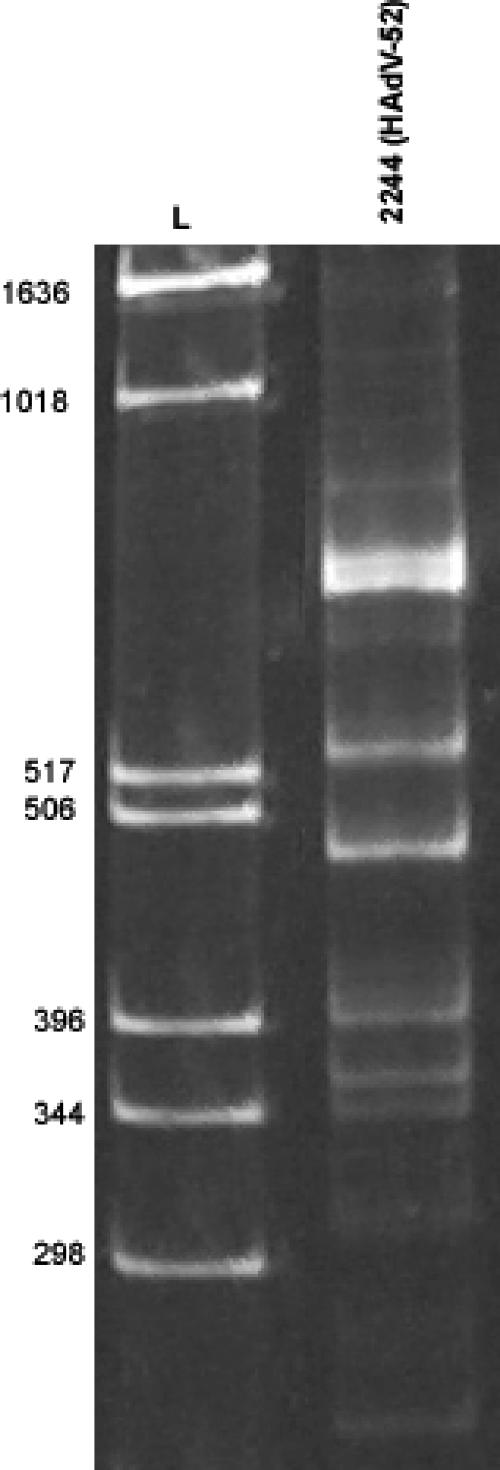
Different segments of this unknown adenovirus genome were amplified via PCR, using DNase-SISPA (2, 29) (see Materials and Methods). This method is useful in that it does not require prior sequence knowledge. The PCR products were analyzed by PAGE (Fig. 2).
FIG. 2.
DNase-SISPA of HAdV-52 from DNA-extracted tissue culture sample 2244. PCR products were analyzed on a 6.5% acrylamide gel. Lane L, molecular weight markers.
Bands generated using DNase-SISPA were sequenced, and similarity to known viruses was then tested using BLASTn (for nucleic acid similarity) and tBLASTx (for protein similarity to the DNA database translated in six open reading frames). Prior to the sequencing of SAdV-1, the first batch of HAdV-52 sequences blasted (via BLASTn) against the GenBank database had low similarity to HAdV-41, with E scores greater than 0.005. However, these sequences had high homology to HAdV-41 when using tBLASTx, with E scores of less than 1.0 × 10−20. After SAdV-1 was added to the GenBank database, the second set of HAdV-52 sequences showed high homology to SAdV-1 (greater than 90% nucleotide identity) as well as HAdV-41 (greater than 80% nucleotide identity), with E scores of less than 1.0 × 10−20 using both BLASTn and tBLASTx.
Amplification and sequencing of the new adenovirus.
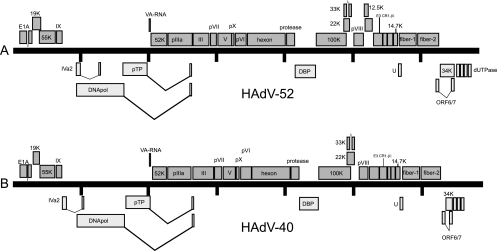
To amplify and sequence the remainder of the novel adenovirus genome, which we have provisionally named HAdV-52, since there are 51 known serotypes, we used several different strategies (see Materials and Methods). First, we amplified regions of the genome between the sequences that we found with DNase-SISPA. To find sequences outside of this area, we designed primers based on the sequences that we found as well as conserved parts of adenovirus sequence alignments using the Primer Premier 5.0 software (Premier Biosoft International, Palo Alto, CA). This enabled us to perform long-range PCR and sequence larger sections of the HAdV-52 genome. The resulting near-full-length genome sequence was 34,250 bp in length, contained 36 open reading frames, and had a genetic organization similar to other mastadenoviruses (Fig. 3). Furthermore, the overall G/C content was 55.1%.
FIG. 3.
Gene organization in HAdV-52 (A) and HAdV-40 (B). Each genome is represented by a central black horizontal line marked at 5-kbp intervals. Protein-encoding regions are shown as boxes. Dark gray boxes above the black line represent open reading frames (ORFs) that are encoded on the positive strand. Light gray boxes underneath the black line represent ORFs that are encoded on the negative strand. Note that HAdV-40 does not have a 12.5K homolog, and the first ORF (dUTPase) present in HAdV-52 is missing, too.
Phylogenetic analysis of HAdV-52.
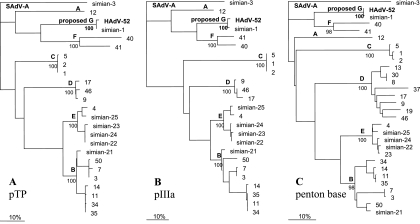
Phylogenetic analysis based on any protein (fitting the appropriate criteria of length of sequence, adequate conservation, and not the result of gene duplications, thus making possible the identification of the amino acids with common origin) demonstrated that HAdV-52 was a new adenovirus type, and the most similar adenovirus was SAdV-1 (Fig. 4). The most similar HAdV types were members of species HAdV-F (HAdV-40 and -41) and HAdV-A (HAdV-12, -18, and -31). Still, these two HAdV species clusters were at least as far from HAdV-52 (and SAdV-1) as they were from each other. This phylogenetic distance is greater than that between any other established HAdV species. The tree topology, concerning HAdV-52, was practically the same with any tested protein; thus, the possibility that HAdV-52 is the product of recombination is highly unlikely. The clustering of the HAdV serotypes as shown on the phylogenetic tree was confirmed by maximum bootstrap values.
FIG. 4.
Phylogenetic analysis of HAdV-52 is based on the predicted amino acid sequences of pTP (A), pIIIa (B), and penton base (C) from selected adenoviruses. Numbers denote human adenovirus serotypes. HAdV-52 (in bold) shows the new isolate. Simian adenoviruses are designated by the word simian and their serotype number. Note that simian adenoviruses 1 and 3 are from Old World monkeys, while SAdV-21 to -25 were isolated from chimpanzees. All of these trees are unrooted. SAdV-3 was chosen as an outgroup, as earlier studies suggested it to be the most ancient from the fully sequenced primate adenoviruses (21, 22). Official human adenovirus species are designated by the letter used for their subsequent lettering, e.g., F for human adenovirus F. Simian adenovirus A is shown by its abbreviated name, SAdV-A. The newly proposed HAdV species (containing a monkey and a human AdV type) is designated “proposed G” (in bold). Bootstrap values (for 100 data sets) are shown for the accepted and the presently proposed species.
Viral neutralization.
At the VRDL, HAdV-52 failed to replicate in high enough titers in HFD, PMK, and A-549 cells to be comprehensively typed by neutralization. Since HAdV-52 and SAdV-1 are genetically similar, we wanted to make sure that we had not isolated SAdV-1 in humans, and so we cultured enough virus to neutralize HAdV-52 and SAdV-1 with antisera to SAdV-1. SAdV-1 and HAdV-52 grew to titers of 104.5 and 103.5, respectively. Antisera to SAdV-1 neutralized 320 tissue culture infective doses of SAdV-1 at a dilution of 1:256 and showed no neutralization of HAdV-52 at a dilution of equal to or less than 1:32. These results demonstrated that HAdV-52 and SAdV-1 were serologically different.
Evidence of other HAdV-52 infections.
We looked for evidence of HAdV-52 in other gastroenteritis outbreaks by using a PCR screening assay which detected the presence of a 735-bp fragment in the hexon gene of HAdV-52. The stool samples that we tested came from two different sites. NoV-positive and -negative stools from 22 NoV-positive and three NoV-negative gastroenteritis outbreaks were acquired from the VRDL, and stool samples from healthy individuals were collected from the David Grant USAF Medical Center. One out of 66 NoV-negative stool samples that we examined was PCR positive for HAdV-52 (Table 1). This was confirmed by sequencing. To rule out PCR contamination, this result was successfully repeated at the VRDL. The results were identical. Not one of the 86 stool samples from healthy individuals that we examined was positive for HAdV-52 (Table 1).
TABLE 1.
Detection of HAdV-52 in stool samples collected from NoV-positive, NoV-negative (but suffering from gastroenteritis), and healthy individuals
| Subjects | No. tested | No. positive |
|---|---|---|
| Norovirus positive | 59 | 0 |
| Norovirus negative | 66 | 1 |
| Healthy asymptomatic | 86 | 0 |
Since the five stool samples from the original gastroenteritis outbreak were unavailable, we wanted to further investigate the possibility that the original batch of primary monkey kidney cells was contaminated. When we checked with the primary monkey kidney cell supplier, they reported that no other customers complained of adenovirus contamination from that lot and that they do not check for adenoviruses unless more than one customer complains. Unfortunately, they did not have any cells from the original lot of primary monkey kidney cells to test. However, we were able to detect HAdV-52 from tissue culture samples that were individually inoculated with stool from the other four members of the original outbreak by using the above-mentioned PCR assay (data not shown). The amplicons were confirmed as HAdV-52 by sequencing.
Molecular characteristics of human adenovirus 52.
There were 36 open reading frames in the HAdV-52 sequence, of which 11 were coded for on the negative strand. Typical of most adenovirus genomes, there were two repeat regions at the 5′ and 3′ ends that were 32 bp each. In addition, we found a single virus-associated RNA gene in the HAdV-52 genome. In contrast, simian adenoviruses 1, 2, 7, 11, 12, and 19 all have one virus-associated RNA gene in their genomes. Interestingly, HAdV-52 also has two fiber genes, which HAdV-40 and HAdV-41 have as well. In fact, HAdV-40 and HAdV-41 (the only members of the human adenovirus F species) are the only human adenoviruses that have two fiber genes and are known to cause gastroenteritis, the ailment with which the five patients who were infected with HAdV-52 presented.
The HAdV-52 genome contains 16 genes that are preserved in all adenoviruses studied to date, including the partly sequenced fish adenovirus (23). Specifically, it contains an E1A gene, the E1B 55K and 19K genes, one E4 34K gene (duplicated in some nonprimate mastadenoviruses and in all atadenoviruses), and proteins IX and V, occurring only in mastadenoviruses (11). Interestingly, the E3 region of HAdV-52 is similar to that found in SAdV-1 and in the members of HAdV-A. Furthermore, the penton base protein of HAdV-52 has the αv integrin-binding motif RGD that is missing from HAdV-40 and -41.
In the past, serology and more recently molecular biology and sequence analysis of the hexon gene have been used to classify human adenoviruses into six subgenera later named species (3, 6, 20, 32). To further characterize HAdV-52, we compared the predicted amino acid sequence from the hexon gene of HAdV-52 to the hexon genes of the reference strains from each HAdV species (Table 2). We also used the hexon gene of HAdV-41 in our analysis, because it is known to cause gastroenteritis. To simplify our analysis, we used the classification system devised by Crawford-Miksza and Schnurr (10), which uses HAdV-2 as a positional reference.
TABLE 2.
Comparison of amino acid identity in conserved and variable loop regions of HAdV hexon proteins (HAdV-A to -F) and SAdV-1 to HAdV-52
| Species | HAdV serotype | % Amino acid identity in region
|
||||
|---|---|---|---|---|---|---|
| C1 (1-131)a | L1 (132-332) | C2 (333-425) | L2 (426-476) | C3 (477-968) | ||
| HAdV-A | 12 | 96.2 | 63.1 | 93.5 | 41.3 | 85.3 |
| HAdV-B | 11 | 94.7 | 51.6 | 93.5 | 37.0 | 81.8 |
| HAdV-C | 2 | 93.9 | 57.3 | 93.5 | 50.0 | 82.4 |
| HAdV-D | 17 | 96.9 | 46.5 | 92.5 | 37.0 | 80.3 |
| HAdV-E | 4 | 96.2 | 46.5 | 94.6 | 47.8 | 80.8 |
| HAdV-F | 40 | 98.5 | 59.9 | 97.8 | 41.3 | 85.7 |
| 41 | 98.5 | 67.5 | 97.8 | 67.4 | 93.8 | |
| SAdV-1 | 99 | 69.6 | 98.9 | 76.6 | 98.2 | |
The numbers in parentheses correspond to the amino acid number in HAdV-2.
The predicted amino acid sequence for the HAdV-52 hexon protein has 920 amino acids, which makes it one of the smallest hexon proteins among HAdVs. The smallest human adenovirus hexon proteins are from HAdV-12 and -18, both 919 amino acids (11), while the largest HAdV hexon is that of HAdV-2, with 968 amino acids (11). The predicted hexon amino acid sequence of SAdV-1 was similar to that of HAdV-52 (Table 2). Of the human adenovirus hexon proteins to which we compared HAdV-52, HAdV-41 was most similar in amino acid identity (Table 2). The constant regions 1, 2, and 3 of HAdV-41 had 98.5, 97.8, and 93.1% amino acid identities with the constant regions of HAdV-52, respectively. The first (L1) and second (L2) loops of HAdV-41 were also closely related in amino acid identity, 67.5 and 67.4%, respectively, to HAdV-52. Interestingly, there were tyrosine residues at positions 830 and 831, while five of six other reference strains have a tyrosine residue at position 830 (Fig. 5). To determine how unique this sequence was, we compared it to all known adenovirus hexon protein sequences. Every mastadenovirus hexon sequence contained a gap at this position in the alignment or, rarely, a phenylalanine in the preceding position and tyrosine at position 831 (data not shown). The only exception is porcine adenovirus 3, which has a tyrosine at position 831 (Fig. 5). This is supplementary evidence suggesting that HAdV-52 is noticeably divergent from the other human adenoviruses. Taken together with the phylogenetic analysis, the genome organization differences, and the serological data, these data strengthen the proposal to classify HAdV-52 as a new species (proposed Human adenovirus G).
FIG. 5.
Alignment of a selected region of HAdV-52 and adenovirus hexon sequences. Letters in parentheses denote the human species. Porcine AdV-3 is porcine adenovirus 3, and BrushTail-Pos AdV is brush tail possum adenovirus. Tyrosine at position 831 is highlighted in boldface and yellow.
DISCUSSION
All human adenoviruses belong to the Mastadenovirus genus. Previously serotyped into 51 serotypes, they have been classified into six “groups” and later “subgenera” from A to F. These are now at the taxonomic level of species and correctly named Human adenovirus A to F (6). Here we characterized a novel human adenovirus, the 52nd type, that most likely merits the establishment of a new HAdV species which we propose to be named species Human adenovirus G. Phylogenetically, the closest AdV to HAdV-52 is SAdV-1, which is another member of this newly proposed species.
In the past, G/C content had been used to classify the current HAdV species. The differences between the G/C content of HAdV-52 (55.1%) and SAdV-1 (55%) and HAdV-40 and -41 (51.2 and 50.8%, respectively) are significant enough to differentiate these viruses into two species.
The most variable and thus most informative component of adenovirus genomes (including HAdVs) are the E3 and E4 regions, because they show unique differences (33). The sequence coding for E3 12.5K and the dUTPase found in HAdV-52 are missing in HAdV-40 and -41. The deletion of these two genes from the HAdV-F members makes them quite different from HAdV-52 and SAdV-1 as well as the other primate adenoviruses. Another striking difference between HAdV-52 and other adenoviruses is the absence of the 19K protein sequence in the E3 gene in HAdV-52 and its presence in HAdV-B, -C, and -D.
Adenovirus entry occurs by attachment of the fiber knob to different receptors on the surface of susceptible cells and subsequent internalization by the interaction between the penton base and cellular αv integrins. Both HAdV-52 and SAdV-1 have an integrin-binding motif. In contrast, HAdV-40 and -41 lack this motif (1, 12). This unique feature of the two HAdV-F species members is further evidence that they are distinct from the proposed HAdV-G members.
HAdV-52 has a single VA RNA, while most HAdV-B types and all HAdV-C, -D, and -E types (including the chimpanzee adenoviruses) have two of them. HAdV-A and -F types and all Old World monkey adenoviruses have a single VA RNA.
The existence or lack of the above-mentioned genes and integrin-binding motif as single shared derived characteristics supports the phylogenetic trees in Fig. 4, which cluster all primate adenoviruses, then the Old World monkey adenoviruses and HAdV-A and -F, and HAdV-52. The two fiber genes cluster HAdV-F and the proposed HAdV-G. Finally, HAdV-40 and -41 are clustered by two genes, and the RGD motif is deleted.
Multiple genome content differences necessitate a need to establish a species for HAdV-52 and SAdV-1 separate from the six HAdV species and SAdV-A. The species barriers are human-made classification schemes; however, they are necessary to reflect the important molecular evolutionary differences between the different viruses. In our case, the question of whether or not to establish a separate species for HAdV-52 and SAdV-1 is open for discussion, but their described phylogenetic distances (at least as great as those differentiating the other HAdV species), the different genome organizations (two missing genes and the lack of the integrin-binding site in HAdV-40 and -41), the different G/C contents of the proposed members of these virus clusters, and host specificity all seem to strengthen the proposal for a new species with a separate evolutionary past.
Recent phylogenetic analysis of the Adenoviridae family demonstrated that primate adenoviruses (all mastadenoviruses), including the newly sequenced chimpanzee (SAdV-21 to -25) and Old World monkey (SAdV-1 and -3) serotypes (4, 11, 23, 24, 28), are monophyletic. It is clear that several chimpanzee adenovirus serotypes belong to HAdV-E and -B, while the monkey adenovirus SAdV-3 forms an independent species more ancient than the other primate species (23). Species HAdV-A and -F are close to the monkey adenovirus types; thus, their relatively close phylogenetic relation is clear. HAdV-52 also belongs to these human adenoviruses with a clear Old World monkey origin. However, our phylogenetic analysis demonstrates that HAdV-52 is separated from HAdV-A and -F, thus, the establishment of a new species for this virus and its close relative SAdV-1. As HAdV-52 was isolated from humans, we propose the name Human adenovirus G for this new species. Note that this is the case with all the other primate adenovirus species that contain the chimpanzee adenovirus types studied thus far. They are all named “human adenovirus” species. While HAdV-52 is very similar to the Old World monkey adenovirus SAdV-1, virus neutralization and phylogenetic analysis clearly show that they are sufficiently different to form separate adenovirus types. Nevertheless, it is clear that SAdV-1 and HAdV-52 belong to the same species.
We also unsuccessfully attempted to amplify HAdV-52 using a multiplex assay that amplifies the fiber gene of adenoviruses A through F (35) (data not shown). Sequence analysis demonstrates that the primers would not produce an amplicon based on the number of mismatches (data not shown). The best forward primer (AdC1) matches 17 of 22 bases in HAdV-52. However, only 9 of 20 nucleotides in the reverse primer (AdC2) match the HAdV-52 sequence (data not shown). These data are consistent with HAdV-52 being a member of a new species.
We were unable to conclusively determine whether HAdV-52 was the causative agent of gastroenteritis in the five patients who were sick. To do this would require an animal model as well as a system in which to grow the virus. However, it must be noted that no other agent was found. We analyzed 59 norovirus-positive cases with known etiology and 66 norovirus-negative cases with unknown etiology from eight different norovirus outbreaks for the presence of HAdV-52 via PCR. Using the same assay, we also analyzed 86 healthy stool samples for the presence of HAdV-52. HAdV-52 was present in one sample with unknown etiology. Whether or not HAdV-52 causes gastroenteritis has not been determined here. Further investigation is under way.
Since HAdV-52 was genetically similar to SAdV-1 and was grown in primary monkey kidney cells, there was a possibility that HAdV-52 was SAdV-1 or a similar simian adenovirus. However, our results clearly demonstrated that antisera to SAdV-1, which neutralized SAdV-1 quite well, had a weak ability to neutralize HAdV-52 at a dilution of less than or equal to 1:32. In addition, we were able to amplify HAdV-52 from a human fecal sample in a separate gastroenteritis outbreak (Table 1). These results demonstrated that HAdV-52 is serologically different than SAdV-1 and is of human origin.
The presence of the tyrosine residue at position 831 in the hexon gene is intriguing. The fact that HAdV-52 is the only human adenovirus that contains a tyrosine residue at position 831 is further proof of the distinctness of HAdV-52 from all of the known human adenoviruses. Another hypothesis is that this is the result of a recent zoonotic infection. This would seem highly plausible in light of the fact that SAdV-1 is so closely related to HAdV-52 (Fig. 4). However, the hexon gene of SAdV-1 also lacks Y831, making the latter hypothesis less likely. Yet another possibility is that this is due to a sequencing error. The sequence that encodes the tyrosine residues was sequenced seven times, always yielding the same sequence. Therefore, the tyrosines at positions 830 and 831 are not the results of sequencing artifacts. As Y831 is also found in porcine adenovirus 3, yet not in other mastadenoviruses, this small characteristic also suggests that HAdV-52 is divergent from the other human serotypes. Future sequencing of other adenoviruses will determine whether Old World monkey adenoviruses (other than SAdV-1) have such an insertion.
Here we identified and characterized a new virus, HAdV-52, that was cultured from the stool of a patient presenting with gastroenteritis. HAdV-52 represents a new type and a new species of the family Adenoviridae.
Acknowledgments
The work reported herein was performed under U.S. Air Force Surgeon General-approved Clinical Investigation no. FDG20040024E and FDG20050026E and partially supported by Hungarian Research Fund grant T043422 as well as the Blood Systems Foundation.
The views expressed in this material are those of the authors and do not reflect the official policy or position of the U.S. Government, the Department of Defense, or the Department of the Air Force.
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Albinsson, B., and A. H. Kidd. 1999. Adenovirus type 41 lacks an RGD αv-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 64:125-136. [DOI] [PubMed] [Google Scholar]
- 2.Allander, T., S. U. Emerson, R. E. Engle, R. H. Purcell, and J. Bukh. 2001. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc. Natl. Acad. Sci. USA 98:11609-11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, A., and V. Mautner. 1994. Phylogenetic relationships among adenovirus serotypes. Virology 205:438-452. [DOI] [PubMed] [Google Scholar]
- 4.Benkö, M., and B. Harrach. 2003. Molecular evolution of adenoviruses. Curr. Top. Microbiol. Immunol. 272:3-35. [DOI] [PubMed] [Google Scholar]
- 5.Benkö, M., B. Harrach, G. W. Both, W. C. Russell, B. M. Adair, E. Adam, J. C. de Jong, M. Hess, M. Johnson, A. Kajon, A. H. Kidd, H. D. Lehmkuhl, Q.-G. Li, V. Mautner, P. Pring-Akerblom, and G. Wadell. 2005. Family Adenoviridae, p. 213-228. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy. VIIIth Report of the International Committee on Taxonomy of Viruses. Elsevier, New York, NY.
- 6.Benkö, M., B. Harrach, and W. C. Russell. 2000. Family Adenoviridae, p. 227-238. In M. H. V. Van Regenmortel, C. M. Fauquet, and D. H. L. Bishop (ed.), Virus taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, New York, NY.
- 7.Clark, B., and M. McKendrick. 2004. A review of viral gastroenteritis. Curr. Opin. Infect. Dis. 17:461-469. [DOI] [PubMed] [Google Scholar]
- 8.Corpet, F. 1998. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crawford-Miksza, L. K., and D. P. Schnurr. 1994. Quantitative colorimetric microneutralization assay for characterization of adenoviruses. J. Clin. Microbiol. 32:2331-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawford-Miksza, L. K., and D. P. Schnurr. 1996. Adenovirus serotype evolution is driven by illegitimate recombination in the hypervariable regions of the hexon protein. Virology 224:357-367. [DOI] [PubMed] [Google Scholar]
- 11.Davison, A. J., M. Benko, and B. Harrach. 2003. Genetic content and evolution of adenoviruses. J. Gen. Virol. 84:2895-2908. [DOI] [PubMed] [Google Scholar]
- 12.Davison, A. J., E. A. Telford, M. S. Watson, K. McBride, and V. Mautner. 1993. The DNA sequence of adenovirus type 40. J. Mol. Biol. 234:1308-1316. [DOI] [PubMed] [Google Scholar]
- 13.De Jong, J. C., A. G. Wermenbol, M. W. Verweij-Uijterwaal, K. W. Slaterus, P. Wertheim-Van Dillen, G. J. Van Doornum, S. H. Khoo, and J. C. Hierholzer. 1999. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 37:3940-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dingle, J. H., and A. D. Langmuir. 1968. Epidemiology of acute, respiratory disease in military recruits. Am. Rev. Respir. Dis. 976(Suppl.):1-65. [DOI] [PubMed] [Google Scholar]
- 15.Felsenstein, J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783-791. [DOI] [PubMed] [Google Scholar]
- 16.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 17.Frenzen, P. D. 2003. Mortality due to gastroenteritis of unknown etiology in the United States. J. Infect. Dis. 187:441-452. [DOI] [PubMed] [Google Scholar]
- 18.Ginsberg, H. S., E. Gold, W. S. Jordan, Jr., S. Katz, G. F. Badger, and J. H. Dingle. 1955. Relation of the new respiratory agents to acute respiratory diseases. Am. J. Public Health 45:915-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrach, B., and M. Benkö. 1998. Phylogenetic analysis of adenovirus sequences. Proof of the necessity of establishing a third genus in the Adenoviridae family, p. 309-339. In W. S. M. Wold (ed.), Adenovirus methods and protocols, vol. 21. Humana Press Inc., Totowa, NJ. [Google Scholar]
- 20.Hierholzer, J. C., R. Wigand, L. J. Anderson, T. Adrian, and J. W. Gold. 1988. Adenoviruses from patients with AIDS: a plethora of serotypes and a description of five new serotypes of subgenus D types (43-47). J. Infect. Dis. 158:804-813. [DOI] [PubMed] [Google Scholar]
- 21.Hilleman, M. R., and J. H. Werner. 1954. Recovery of new agent from patients with acute respiratory illness. Proc. Soc. Exp. Biol. Med. 851:183-188. [DOI] [PubMed] [Google Scholar]
- 22.Jones, M. S., A. Kapoor, V. V. Lukashov, P. Simmonds, F. Hecht, and E. Delwart. 2005. New DNA viruses identified in patients with acute viral infection syndrome. J. Virol. 79:8230-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovács, G. M., A. J. Davison, A. N. Zakhartchouk, and B. Harrach. 2004. Analysis of the first complete genome sequence of an Old World monkey adenovirus reveals a lineage distinct from the six human adenovirus species. J. Gen. Virol. 85:2799-2807. [DOI] [PubMed] [Google Scholar]
- 24.Kovács, G. M., B. Harrach, A. N. Zakhartchouk, and A. J. Davison. 2005. The complete genome sequence of simian adenovirus 1-an Old World monkey adenovirus with two fiber genes. J. Gen. Virol. 86:1681-1686. [DOI] [PubMed] [Google Scholar]
- 25.Kovacs, G. M., S. E LaPatra, J. C. D'Halluin, and M. Benko. 2003. Phylogenetic analysis of the hexon and protease genes of a fish adenovirus isolated from white sturgeon (Acipenser transmontanus) supports the proposal for a new adenovirus genus. Virus Res. 981:27-34. [DOI] [PubMed] [Google Scholar]
- 26.Lauer, K. P., I. Llorente, E. Blair, J. Seto, V. Krasnov, A. Purkayastha, S. E. Ditty, T. L. Hadfield, C. Buck, C. Tibbetts, and D. Seto. 2004. Natural variation among human adenoviruses: genome sequence and annotation of human adenovirus serotype 1. J. Gen. Virol. 85:2615-2625. [DOI] [PubMed] [Google Scholar]
- 27.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 28.Reddy, P. S., S. Ganesh, N. J. Knowles, M. Kaleko, S. Connelly, and A. Bristol. 2006. Complete sequence and organization of the human adenovirus serotype 46 genome. Virus Res. 116:119-128. [DOI] [PubMed] [Google Scholar]
- 29.Rowe, W. P., R. J. Huebner, L. K. Gilmore, R. H. Parrott, and T. G. Ward. 1953. Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc. Soc. Exp. Biol. Med. 84:570-573. [DOI] [PubMed] [Google Scholar]
- 30.Roy, S., G. Gao, D. S. Clawson, L. H. Vandenberhe, S. F. Farina, and J. M. Wilson. 2004. Complete nucleotide sequences and genome organization of four chimpanzee adenoviruses. Virology 324:361-372. [DOI] [PubMed] [Google Scholar]
- 31.San Martin, C., and B. M. Burnett. 2003. Structural studies on adenoviruses. Curr. Top. Microbiol. Immunol. 272:57-94. [DOI] [PubMed] [Google Scholar]
- 32.Uhnoo, I., G. Wadell, L. Svensson, and M. Johansson. 1983. Two new serotypes of enteric adenovirus causing infantile diarrhoea. Dev. Biol. Standard. 53:311-318. [PubMed] [Google Scholar]
- 33.Ursu, K., B. Harrach, K. Matiz, and M. Benkö. 2004. DNA sequencing and analysis of the right-hand part of the genome of the unique bovine adenovirus type 10. J. Gen. Virol. 85:593-601. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelmi, I., E. Roman, and A. Sanchez-Fauquier. 2003. Viruses causing gastroenteritis. Clin. Microbiol. Infect. 9:247-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu, W., M. C. McDonough, and D. D. Erdman. 2000. Species-specific identification of human adenoviruses by a multiplex PCR assay. J. Clin. Microbiol. 38:4114-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]