Abstract
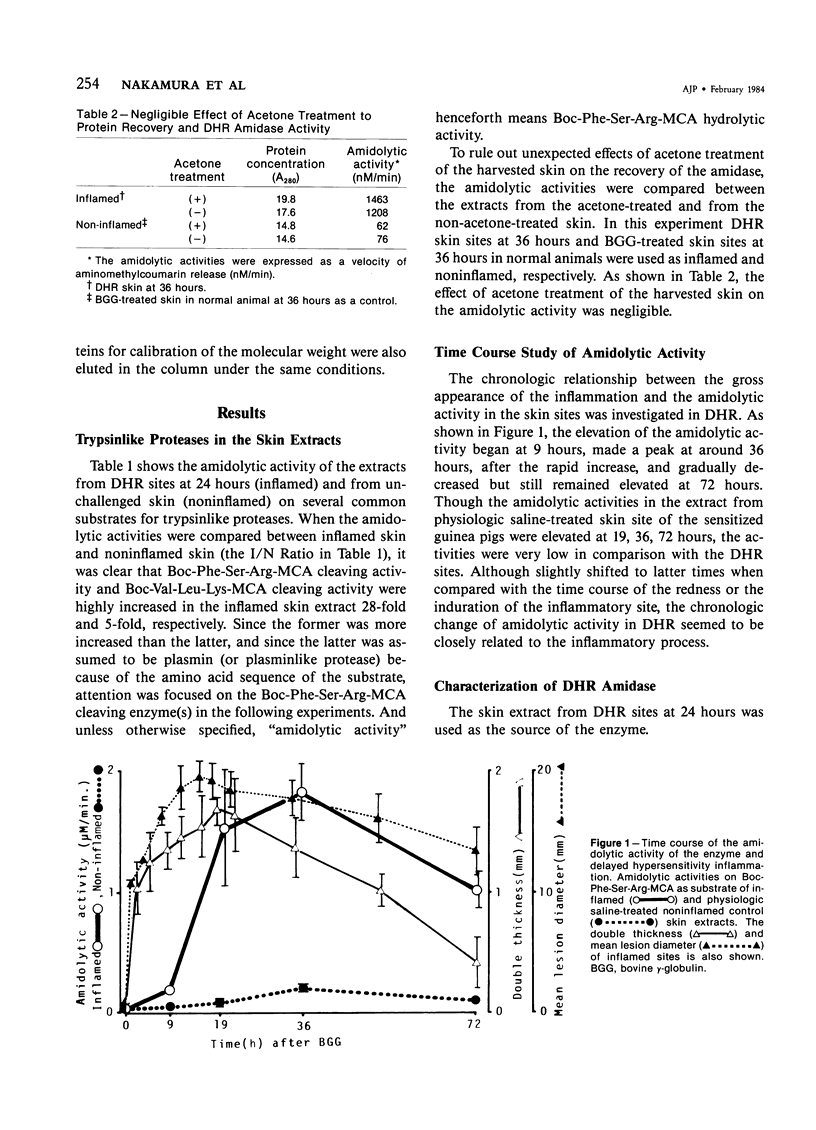
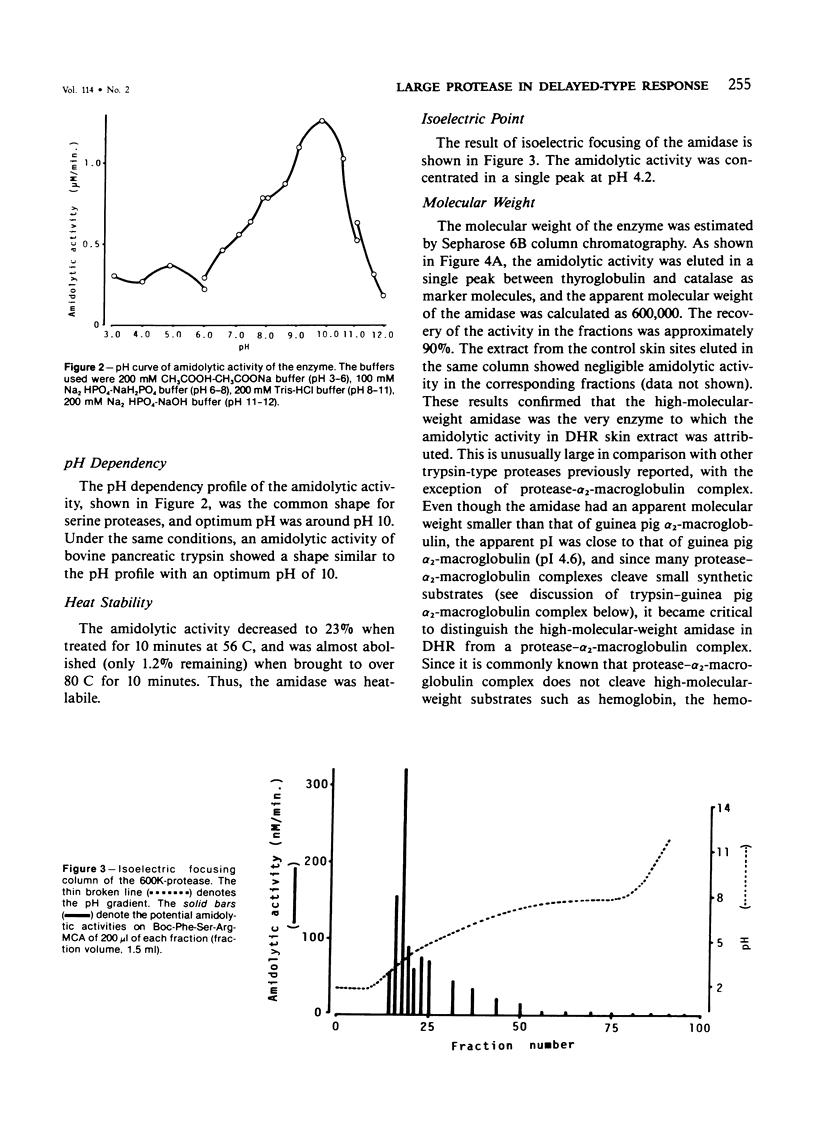
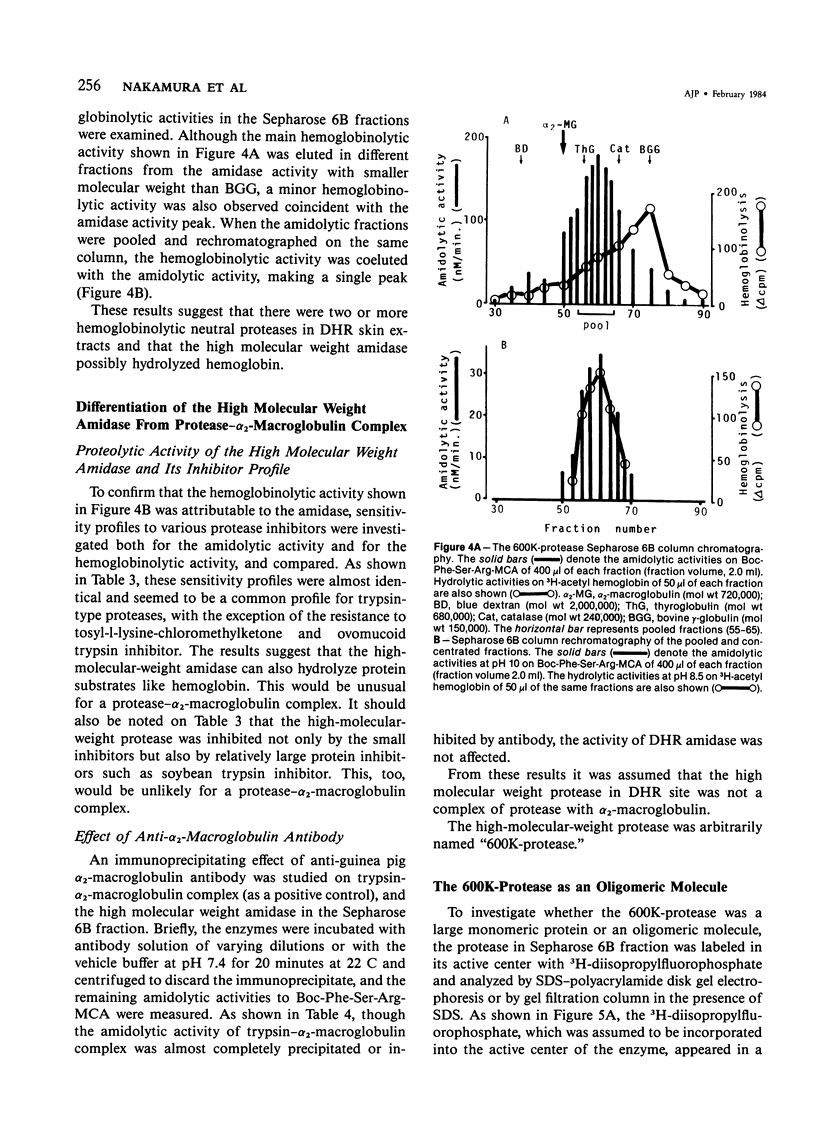
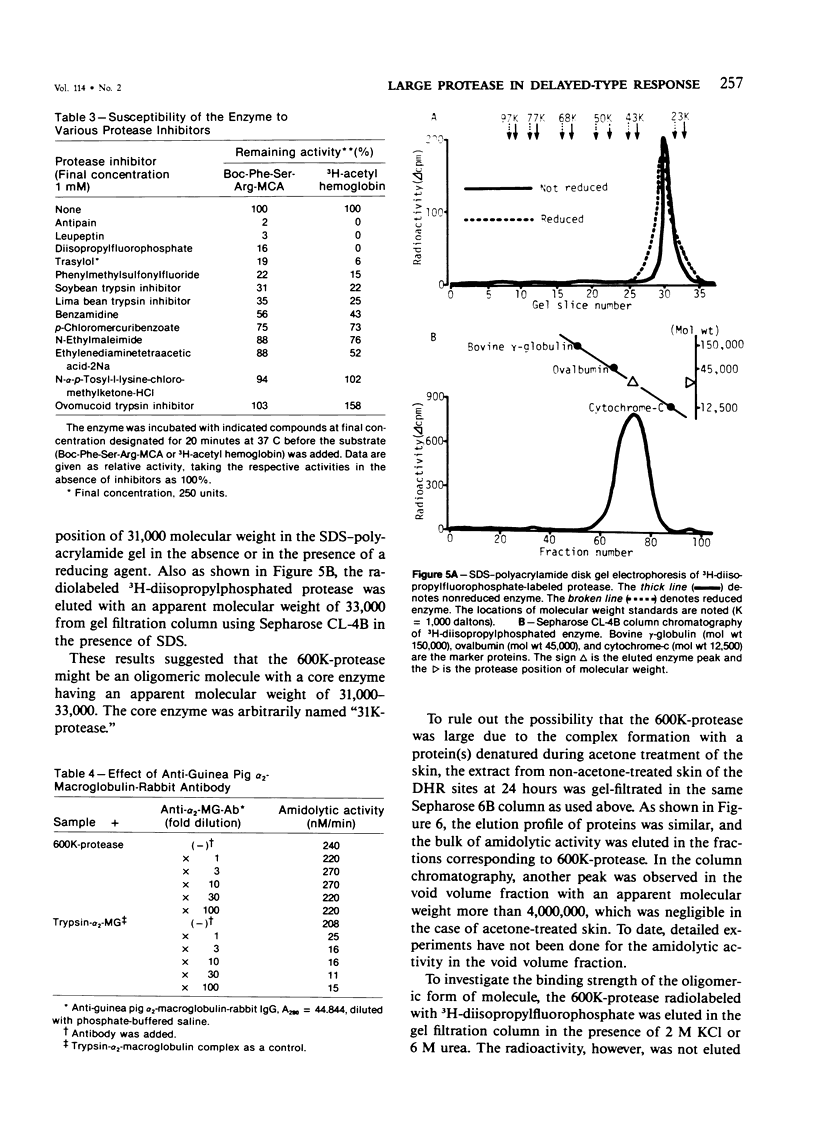
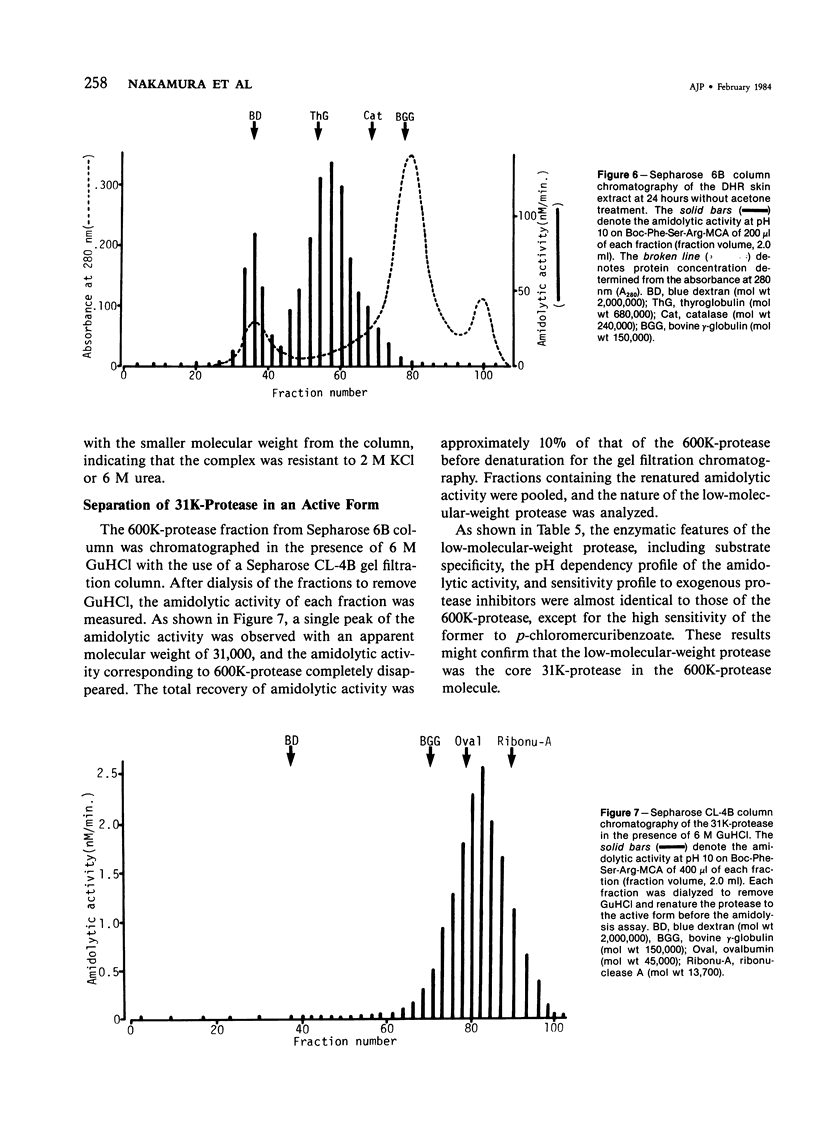
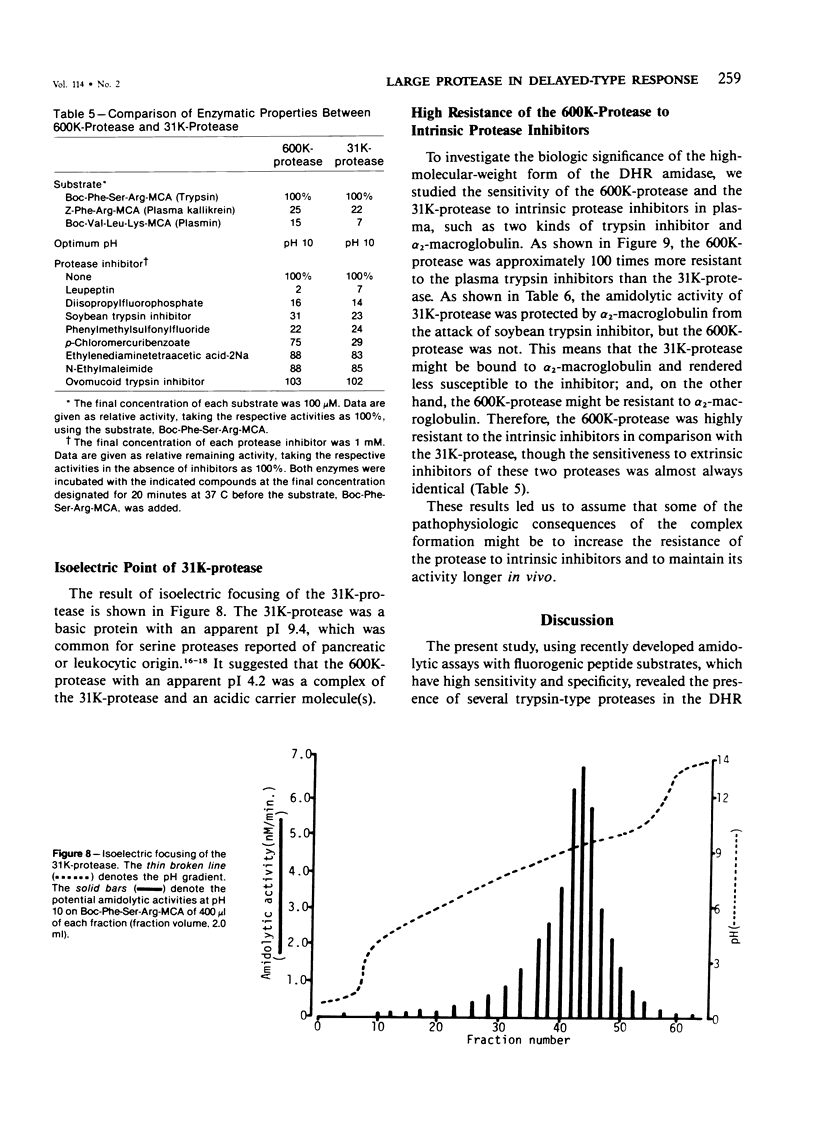
A trypsinlike protease was extracted from the delayed hypersensitivity skin sites in guinea pigs. Extractable amounts of the enzyme were chronologically paralleled with the gross appearance of the inflammation, and the maximum activity from the inflamed sites at 24-36 hours was about 20 times stronger than that from normal skin, suggesting a potential role in the pathogenesis of the delayed hypersensitivity reaction. The enzyme, which suitably hydrolyzed t-butyloxycarbonyl-phenylalanyl-seryl-arginine 4-methylcoumaryl-7-amide, was partially purified by isoelectric focusing or by gel filtration. The enzyme demonstrated a single peak of activity on the former column with an apparent isoelectric point of 4.2, and in the latter it showed an apparent molecular weight of 600,000 (600K-protease). When incubated with 3H-diisopropylfluorophosphate, the enzyme lost all amidolytic activity and yielded a single band of radioactivity in polyacrylamide disk gel electrophoresis in the presence of sodium dodecyl sulfate, and a single peak of radioactivity in gel filtration, both having an apparent molecular weight of 31,000-33,000 (31K-protease). That the 600K-protease might be a complex with alpha 2-macroglobulin was ruled out. The 31K-protease was separated from the 600K-protease by gel filtration in the presence of 6 M guanidine hydrochloride, and was renatured to an active form. An apparent isoelectric point of the 31K-protease observed was 9.4, suggesting that the 600K-protease may be a complex of 31K-protease with an acidic carrier molecule(s). Both proteases, 31K- and 600K-protease, had identical substrate specificity, a pH profile of amidolytic activity, and susceptibility to exogenous protease inhibitors. However, when sensitivities to intrinsic protease inhibitors in guinea pig plasma, two kinds of trypsin inhibitor, and alpha 2-macroglobulin were compared, the 600K-protease was at least 100 times more resistant than the 31K-protease. It was supposed that one of the pathophysiologically significant functions of the complex formation might be to maintain the enzyme activity longer in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Botham G. M., Woodward A. W., Lucy J. A. Calcium-activated thiol-proteinase activity in the fusion of rat erythrocytes induced by benzyl alcohol. Biochem J. 1980 Dec 15;192(3):829–836. doi: 10.1042/bj1920829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980 Jun 1;187(3):909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskola J., Fräki J. E. Lymphocyte stimulation in vitro by proteinases and its augmentation with a proteinase binding factor from human skin. Arch Dermatol Res. 1978 Nov 10;263(2):223–226. doi: 10.1007/BF00446444. [DOI] [PubMed] [Google Scholar]
- Fräki J. E., Hopsu-Havu V. K. Human skin proteases. Differential extraction of proteases and of endogenous protease inhibitors. Arch Dermatol Forsch. 1972;242(4):329–342. [PubMed] [Google Scholar]
- Fräki J. E., Junnila S. K., Hopsu-Havu V. K. Proteinase binding and enhancing factor from human skin. Arch Dermatol Res. 1979 Mar 31;264(2):185–191. doi: 10.1007/BF00431130. [DOI] [PubMed] [Google Scholar]
- Hatcher V. B., Lazarus G. S., Levine N., Burk P. G., Yost F. J., Jr Characterization of a chemotactic and cytotoxic proteinase from human skin. Biochim Biophys Acta. 1977 Jul 8;483(1):160–171. doi: 10.1016/0005-2744(77)90018-3. [DOI] [PubMed] [Google Scholar]
- Hayashi H. A review on the natural mediators of inflammatory leucotaxis. Acta Pathol Jpn. 1982;32 (Suppl 2):271–284. [PubMed] [Google Scholar]
- Hille M. B., Barrett A. J., Dingle J. T., Fell H. B. Microassay for cathepsin D shows an unexpected effedt of cycloheximide on limb-bone rudiments in organ culture. Exp Cell Res. 1970 Aug;61(2):470–472. doi: 10.1016/0014-4827(70)90476-3. [DOI] [PubMed] [Google Scholar]
- Huttunen R., Korhonen L. K. New trypsin- and chymotrypsin-binding factor in rat plasma. Ann Med Exp Biol Fenn. 1973;51(4):145–150. [PubMed] [Google Scholar]
- Ishiura S., Murofushi H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. I. Purification and characterization. J Biochem. 1978 Jul;84(1):225–230. doi: 10.1093/oxfordjournals.jbchem.a132111. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Sugita H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. II. Substrate specificity. J Biochem. 1979 Aug;86(2):579–581. doi: 10.1093/oxfordjournals.jbchem.a132558. [DOI] [PubMed] [Google Scholar]
- Kambara T., Hashimoto M. An alkaline protease in the delayed hypersensitivity skin lesions in the guinea-pigs. Experientia. 1979 Aug 15;35(8):1110–1112. doi: 10.1007/BF01949973. [DOI] [PubMed] [Google Scholar]
- Kambara T., Katsuya H., Maeda S. Experimental study on the mechanisms of delayed hypersensitivity reaction, with special reference to emigration and function of mononuclear cells. Acta Pathol Jpn. 1972 Aug;22(3):465–476. doi: 10.1111/j.1440-1827.1972.tb01845.x. [DOI] [PubMed] [Google Scholar]
- Kambara T., Kawagoe T., Nakamura T. An alkaline proteinase which has inflammatory activity isolated from guinea pig lymphoid cells. Biochim Biophys Acta. 1982 May 27;716(2):224–231. doi: 10.1016/0304-4165(82)90272-0. [DOI] [PubMed] [Google Scholar]
- Kambara T., Ueda K., Maeda S. The chemical mediation of delayed hypersensitivity skin reactions. I. Purification of a macrophage-chemotactic factor from bovien gamma-globulin-induced skin reactions in guinea pigs. Am J Pathol. 1977 May;87(2):359–374. [PMC free article] [PubMed] [Google Scholar]
- Kato H., Adachi N., Ohno Y., Iwanaga S., Takada K., Sakakibara S. New fluorogenic peptide substrates for plasmin. J Biochem. 1980 Jul;88(1):183–190. [PubMed] [Google Scholar]
- Kobayashi S., Nagasawa S. Protease inhibitors in guinea pig serum. I. Isolation of two functionally different trypsin inhibitors from guinea pig serum. Biochim Biophys Acta. 1974 Apr 11;342(2):372–381. doi: 10.1016/0005-2795(74)90093-2. [DOI] [PubMed] [Google Scholar]
- Koida M., Walter R. Post-proline cleaving enzyme. Purification of this endopeptidase by affinity chromatography. J Biol Chem. 1976 Dec 10;251(23):7593–7599. [PubMed] [Google Scholar]
- Kozono K., Yamamoto T., Kambara T. A proteaselike permeability factor in guinea pig skin: contact activation of the latent permeability factor and its nature as a prekallikrein activator. Am J Pathol. 1980 Sep;100(3):619–632. [PMC free article] [PubMed] [Google Scholar]
- Marossy K. Interaction of the chymotrypsin- and elastase-like enzymes of the human granulocyte with glycosaminoglycans. Biochim Biophys Acta. 1981 Jun 15;659(2):351–361. doi: 10.1016/0005-2744(81)90061-9. [DOI] [PubMed] [Google Scholar]
- Melloni E., Sparatore B., Salamino F., Michetti M., Pontremoli S. Cytosolic calcium dependent proteinase of human erythrocytes: formation of an enzyme-natural inhibitor complex induced by Ca2+ ions. Biochem Biophys Res Commun. 1982 Jun 15;106(3):731–740. doi: 10.1016/0006-291x(82)91772-7. [DOI] [PubMed] [Google Scholar]
- Morita T., Kato H., Iwanaga S., Takada K., Kimura T. New fluorogenic substrates for alpha-thrombin, factor Xa, kallikreins, and urokinase. J Biochem. 1977 Nov;82(5):1495–1498. doi: 10.1093/oxfordjournals.jbchem.a131840. [DOI] [PubMed] [Google Scholar]
- Murachi T., Tanaka K., Hatanaka M., Murakami T. Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv Enzyme Regul. 1980;19:407–424. doi: 10.1016/0065-2571(81)90026-1. [DOI] [PubMed] [Google Scholar]
- Rothschild A. M. Hydrolysis of arginine and tyrosine esters in mast cells exposed to epinephrine. Biochem Pharmacol. 1980 Feb;29(3):419–427. doi: 10.1016/0006-2952(80)90522-5. [DOI] [PubMed] [Google Scholar]
- Song C. W., Tabachnick J., McCarron D. J., Jr A trypsin-like protease in guinea pig skin. Experientia. 1969 Oct 15;25(10):1063–1064. doi: 10.1007/BF01901431. [DOI] [PubMed] [Google Scholar]
- Sugita H., Ishiura S., Suzuki K., Imahori K. Inhibition of epoxide derivatives on chicken calcium-activated neutral protease (CANP) in vitro and in vivo. J Biochem. 1980 Jan;87(1):339–341. doi: 10.1093/oxfordjournals.jbchem.a132742. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Ishiura S., Takahashi-Nakamura M., Katamoto T., Suzuki K., Imahori K. Studies on a Ca2+-activated neutral proteinase of rabbit skeletal muscle. II. Characterization of sulfhydryl groups and a role of Ca2+ ions in this enzyme. J Biochem. 1981 Nov;90(5):1405–1411. doi: 10.1093/oxfordjournals.jbchem.a133606. [DOI] [PubMed] [Google Scholar]
- Ueda K., Kambara T. The chemical mediation of delayed hypersensitivity skin reaction. II. Characterization of a macrophage-chemotactic factor from bovine gamma-globulin-induced skin reaction in guinea pigs. Am J Pathol. 1978 Jul;92(1):241–252. [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Kawaguchi T., Okamoto T., Kambara T. The chemical mediation of delayed hypersensitivity skin reactions: III. Purification and characterization of a precursor protein for macrophage-chemotactic factor in normal guinea pig plasma. Am J Pathol. 1982 Sep;108(3):291–298. [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by lymphokine-activated macrophages. Science. 1975 Jan 24;187(4173):261–263. doi: 10.1126/science.163038. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Cochrane C. G. A protease-like permeability factor in guinea pig skin: immunologic identity with plasma Hageman factor. Am J Pathol. 1982 May;107(2):127–134. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Cochrane C. G. Guinea pig Hageman factor as a vascular permeability enhancement factor. Am J Pathol. 1981 Nov;105(2):164–175. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kambara T. A protease-like permeability factor in the guinea pig skin. 1. Partial purification and characterization. Biochim Biophys Acta. 1978 Apr 19;540(1):55–64. doi: 10.1016/0304-4165(78)90434-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kozono K., Kambara T. A protease-like permeability factor in the guinea pig skin. 2. In vitro activation of the latent form permeability factor by weakly acidic phosphate buffer. Biochim Biophys Acta. 1978 Aug 17;542(2):222–231. doi: 10.1016/0304-4165(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kozono K., Okamoto T., Kato H., Kambara T. Purification of guinea-pig plasma prekallikrein. Activation by prekallikrein activator derived from guinea-pig skin. Biochim Biophys Acta. 1980 Aug 7;614(2):511–525. doi: 10.1016/0005-2744(80)90240-5. [DOI] [PubMed] [Google Scholar]
- Yoshimoto T., Orlowski R. C., Walter R. Postproline cleaving enzyme: identification as serine protease using active site specific inhibitors. Biochemistry. 1977 Jun 28;16(13):2942–2948. doi: 10.1021/bi00632a022. [DOI] [PubMed] [Google Scholar]



