Abstract
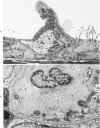
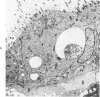
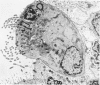
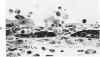
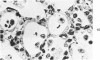
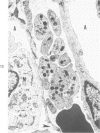
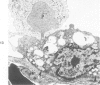
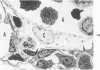
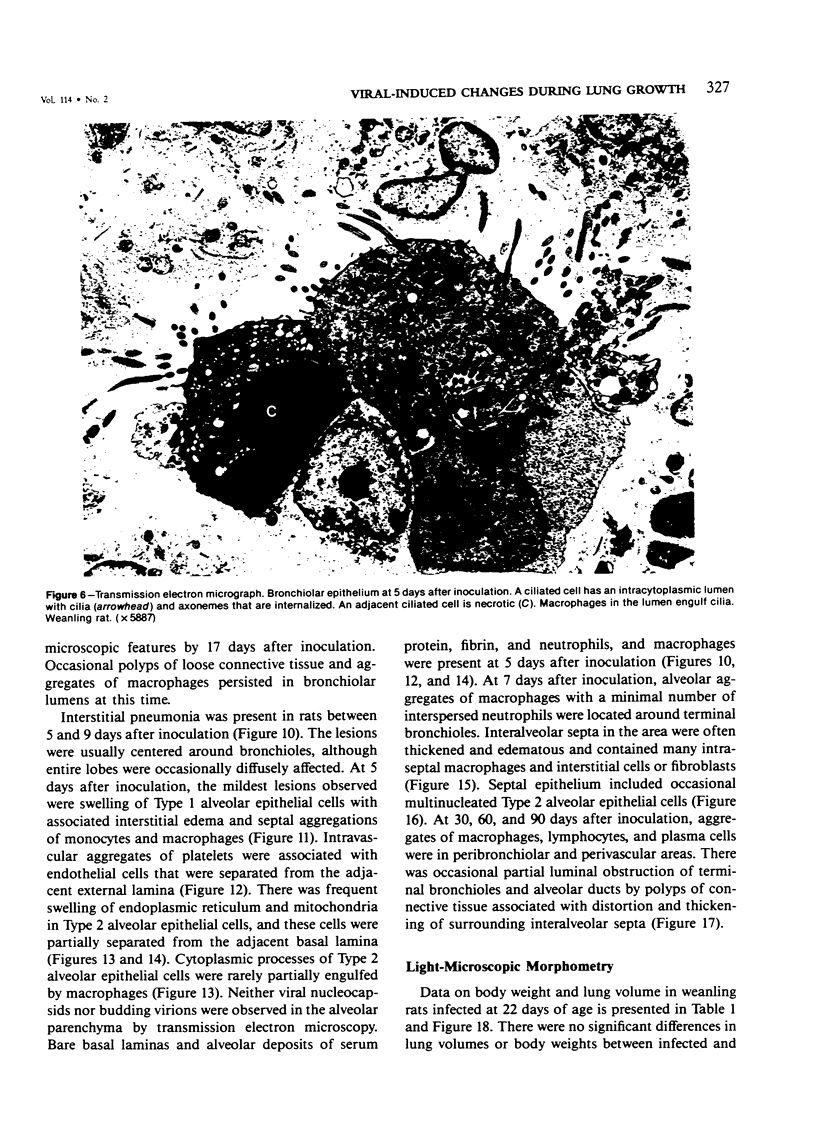
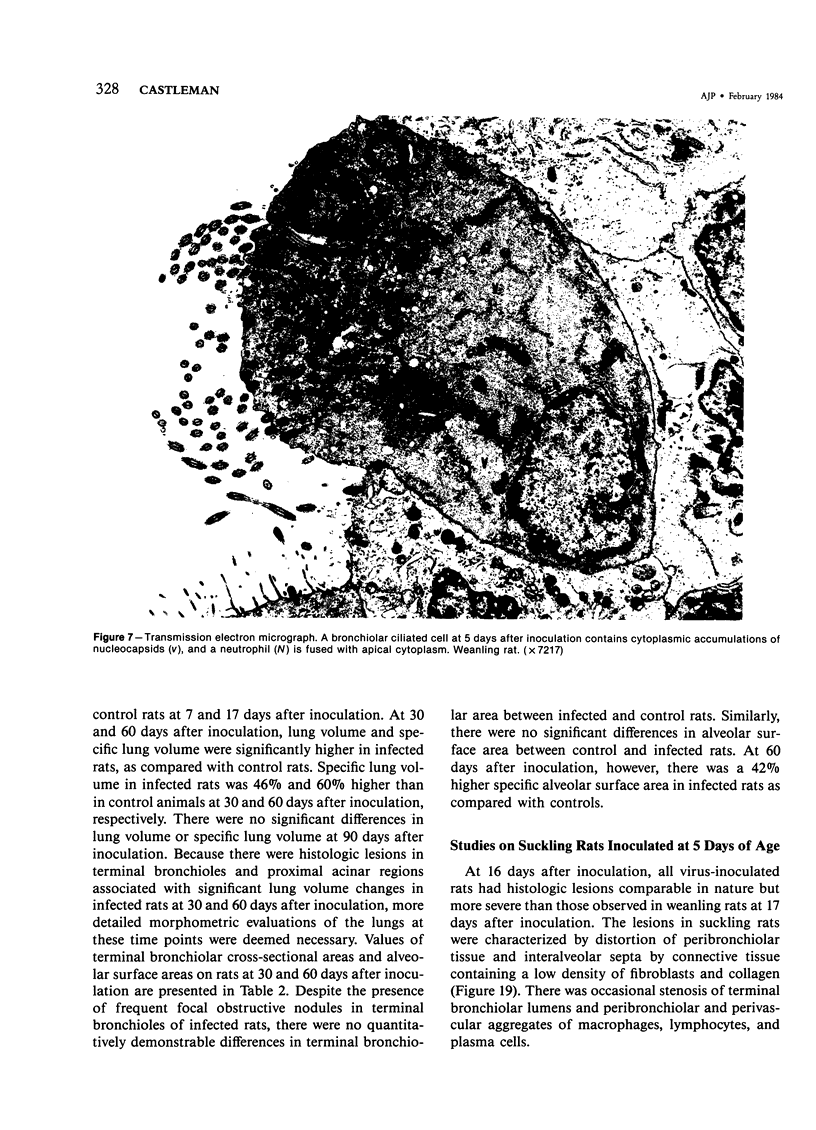
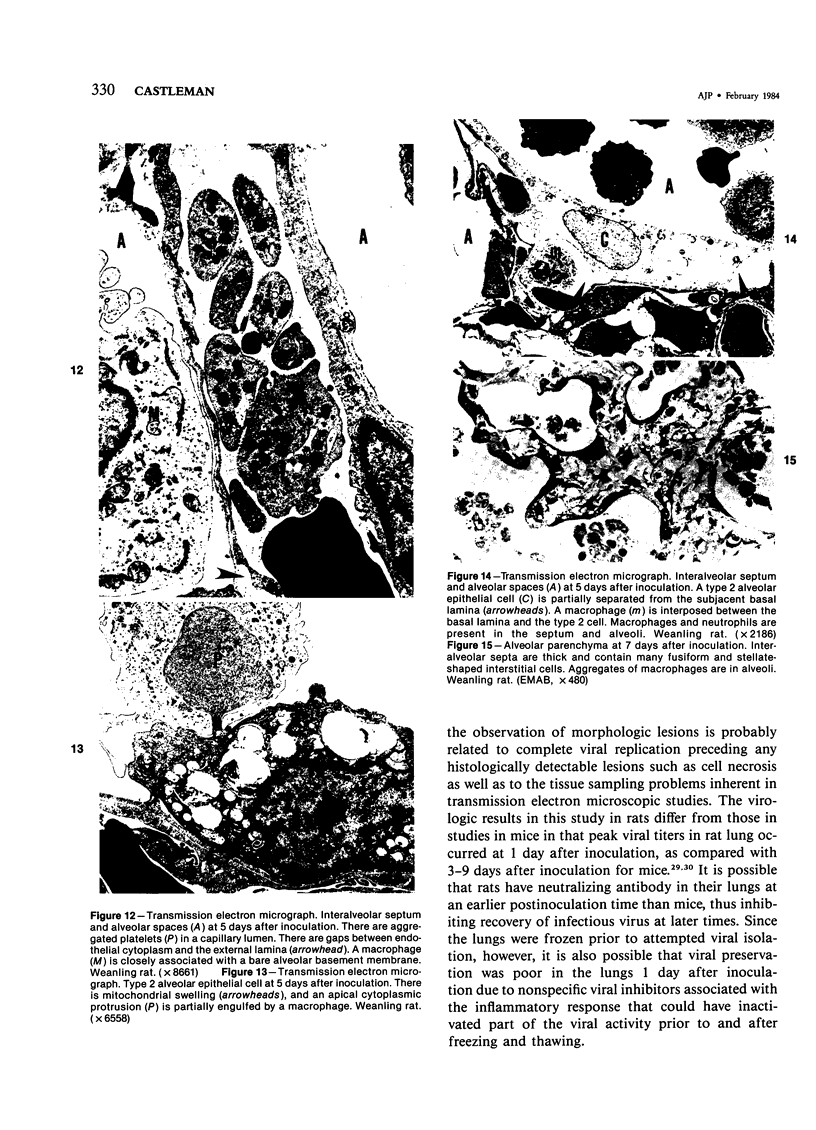
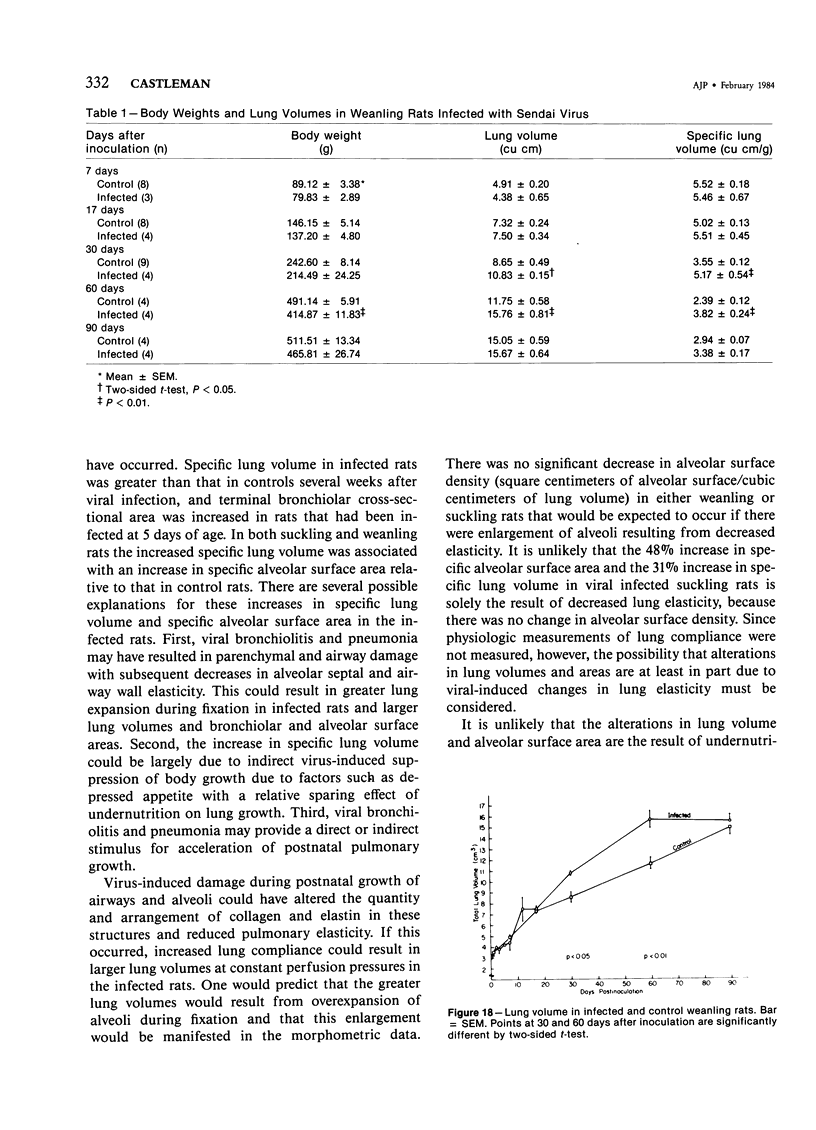
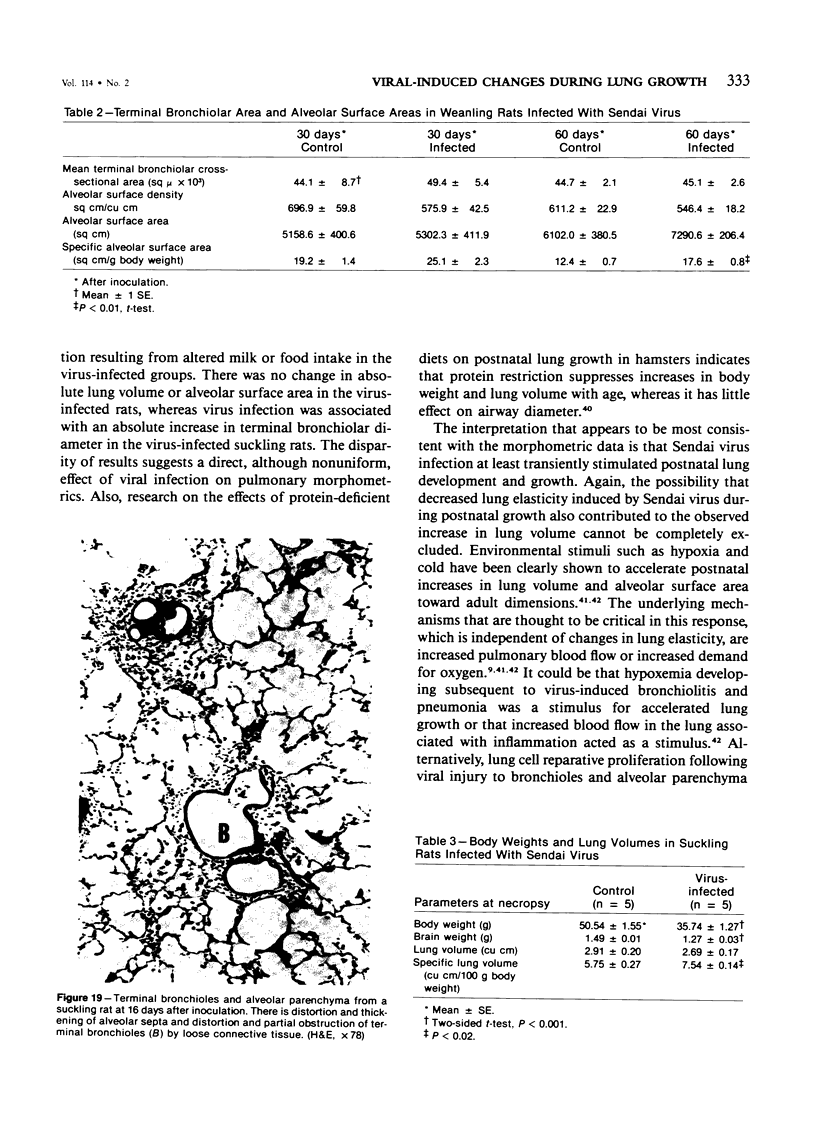
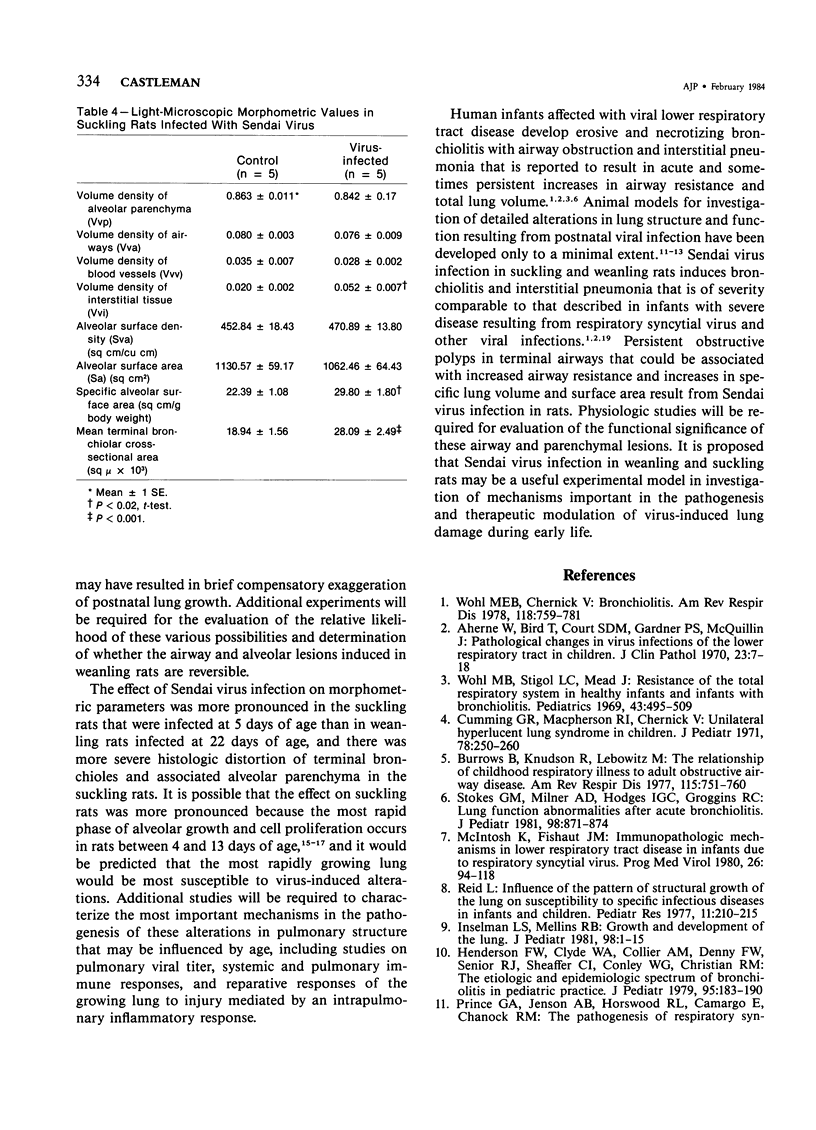
The ultrastructural and morphometric effects of viral respiratory disease during postnatal lung growth were examined in weanling (22-day-old) and suckling (5-day-old) rats infected with parainfluenza Type 1 (Sendai) virus. Viral nucleocapsids and budding virions were identified by transmission electron microscopy in ciliated cells, mucous cells, and nonciliated bronchiolar epithelial cells of weanling rats at 5 days after inoculation and were associated with epithelial necrosis and erosion as well as hyperplasia of nonciliated bronchiolar epithelial cells. Interstitial pneumonia characterized in early stages by swelling and sloughing of Type 1 and Type 2 alveolar epithelial cells was also present at 5 and 7 days after inoculation. Lesions persisting at 30, 60, and 90 days after inoculation included multifocal connective tissue polyps in terminal bronchioles that partially obstructed bronchiolar lumens. Specific lung volume was greater (P less than 0.01) in weanling rats at 30 and 60 days following viral infection than in control rats, and specific alveolar surface area was 42% greater (P less than 0.01) in infected rats at 60 days after inoculation. Suckling rats infected during a phase of rapid postnatal lung growth at 5 days of age had 33% greater (P less than 0.02) specific alveolar surface area and 48% greater (P less than 0.001) mean terminal bronchiolar cross-sectional area when compared with control rats at 22 days of age. The results indicate that viral pulmonary infection during early life can induce acute and persistent alterations in pulmonary structure that could adversely affect lung function.
Full text
PDF
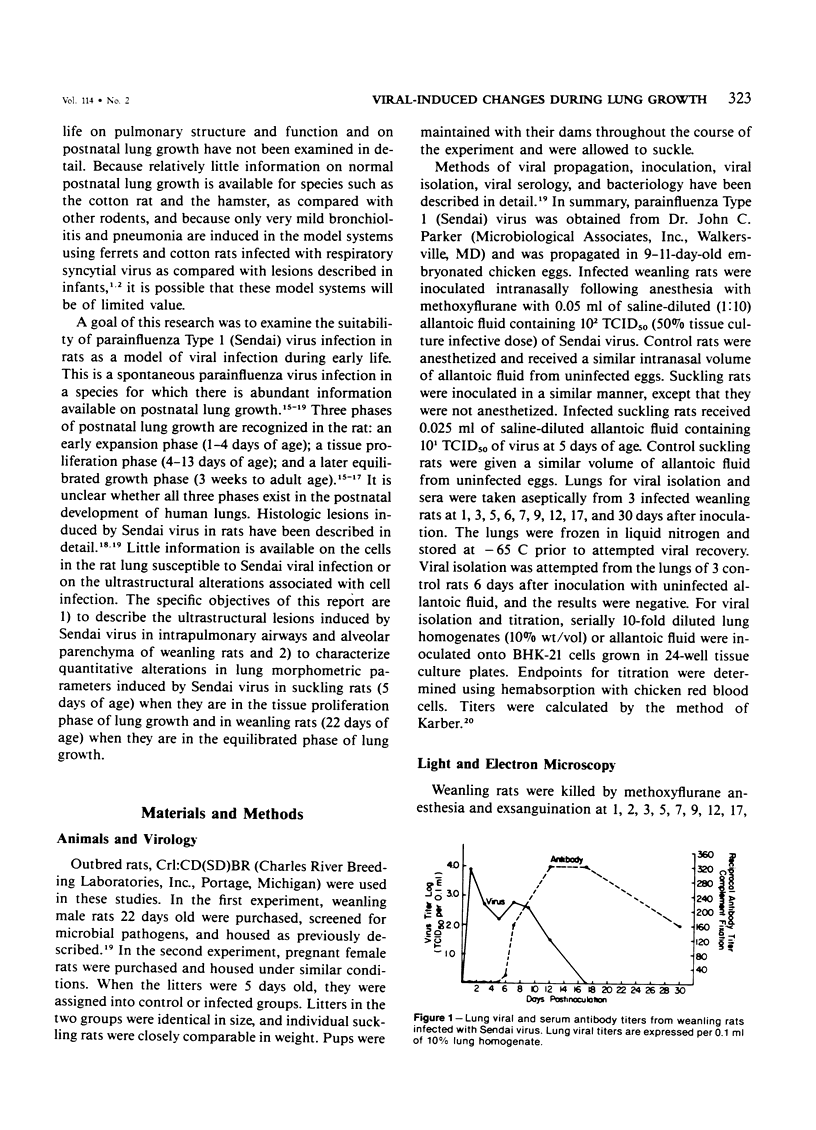
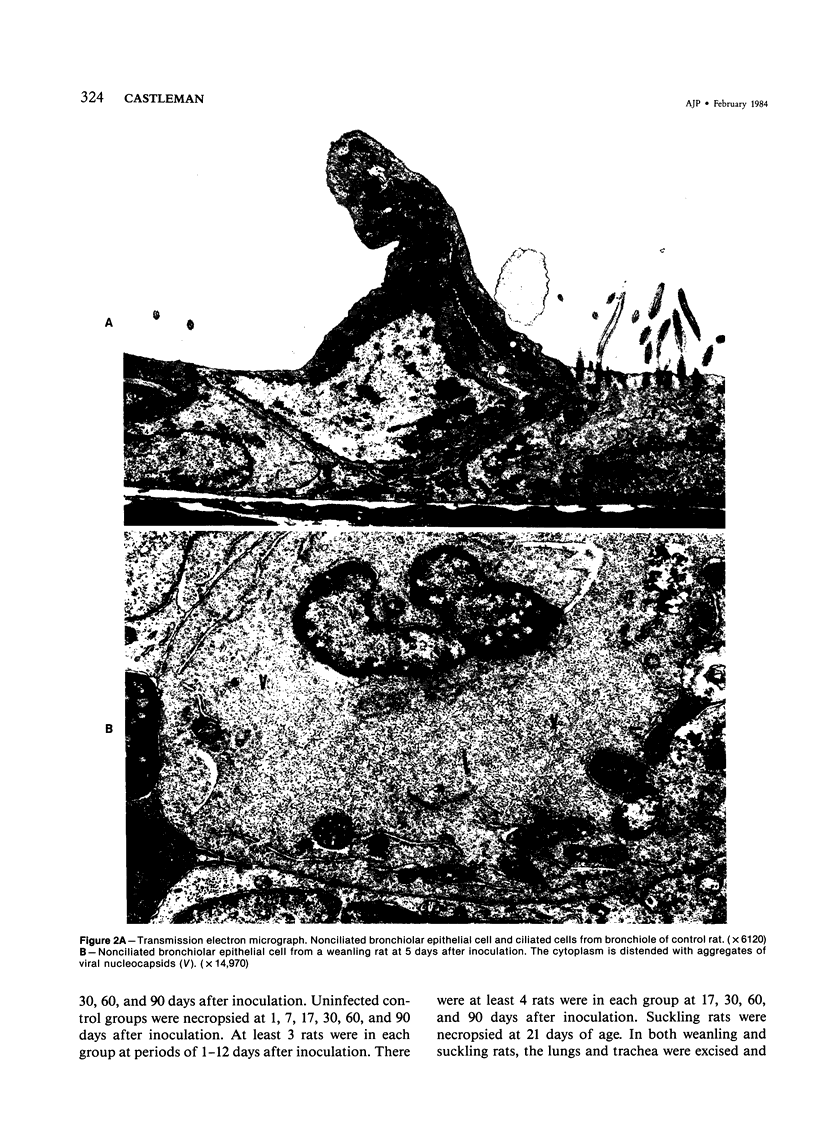





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aherne W., Bird T., Court S. D., Gardner P. S., McQuillin J. Pathological changes in virus infections of the lower respiratory tract in children. J Clin Pathol. 1970 Feb;23(1):7–18. doi: 10.1136/jcp.23.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Darraji A. M., Cutlip R. C., Lehmkuhl H. D., Graham D. L. Experimental infection of lambs with bovine respiratory syncytial virus and Pasteurella haemolytica: pathologic studies. Am J Vet Res. 1982 Feb;43(2):224–229. [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody J. S., Vaccaro C. Postnatal formation of alveoli: interstitial events and physiologic consequences. Fed Proc. 1979 Feb;38(2):215–223. [PubMed] [Google Scholar]
- Brownstein D. G., Smith A. L., Johnson E. A. Sendai virus infection in genetically resistant and susceptible mice. Am J Pathol. 1981 Nov;105(2):156–163. [PMC free article] [PubMed] [Google Scholar]
- Burri P. H., Dbaly J., Weibel E. R. The postnatal growth of the rat lung. I. Morphometry. Anat Rec. 1974 Apr;178(4):711–730. doi: 10.1002/ar.1091780405. [DOI] [PubMed] [Google Scholar]
- Burri P. H. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974 Sep;180(1):77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Burrows B., Knudson R. J., Lebowitz M. D. The relationship of childhood respiratory illness to adult obstructive airway disease. Am Rev Respir Dis. 1977 May;115(5):751–760. doi: 10.1164/arrd.1977.115.5.751. [DOI] [PubMed] [Google Scholar]
- Carthew P., Sparrow S. Sendai virus in nude and germ-free rats. Res Vet Sci. 1980 Nov;29(3):289–292. doi: 10.1016/S0034-5288(18)32629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Schwartz L. W., Tyler W. S. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol. 1980 Mar;98(3):811–840. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L. Respiratory tract lesions in weanling outbred rats infected with Sendai virus. Am J Vet Res. 1983 Jun;44(6):1024–1031. [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr Model systems for studying the pathogenesis of infections causing bronchiolitis in man. Pediatr Res. 1977 Mar;11(3 Pt 2):243–246. [PubMed] [Google Scholar]
- Cumming G. R., Macpherson R. I., Chernick V. Unilateral hyperlucent lung syndrome in children. J Pediatr. 1971 Feb;78(2):250–260. doi: 10.1016/s0022-3476(71)80008-2. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976 Sep;35(3):246–257. [PubMed] [Google Scholar]
- Fantone J. C., Kunkel S. L., Ward P. A. Chemotactic mediators in neutrophil-dependent lung injury. Annu Rev Physiol. 1982;44:283–293. doi: 10.1146/annurev.ph.44.030182.001435. [DOI] [PubMed] [Google Scholar]
- Henderson F. W., Clyde W. A., Jr, Collier A. M., Denny F. W., Senior R. J., Sheaffer C. I., Conley W. G., 3rd, Christian R. M. The etiologic and epidemiologic spectrum of bronchiolitis in pediatric practice. J Pediatr. 1979 Aug;95(2):183–190. doi: 10.1016/s0022-3476(79)80647-2. [DOI] [PubMed] [Google Scholar]
- Hfreere R. H., Weibel E. R. Stereologic techniques in microscopy. J R Microsc Soc. 1967;87(1):25–34. doi: 10.1111/j.1365-2818.1967.tb04489.x. [DOI] [PubMed] [Google Scholar]
- Inselman L. S., Mellins R. B. Growth and development of the lung. J Pediatr. 1981 Jan;98(1):1–15. doi: 10.1016/s0022-3476(81)80524-0. [DOI] [PubMed] [Google Scholar]
- Kauffman S. L., Burri P. H., Weibel E. R. The postnatal growth of the rat lung. II. Autoradiography. Anat Rec. 1974 Sep;180(1):63–76. doi: 10.1002/ar.1091800108. [DOI] [PubMed] [Google Scholar]
- Lechner A. J., Banchero N. Lung morphometry in guinea pigs acclimated to cold during growth. J Appl Physiol Respir Environ Exerc Physiol. 1980 May;48(5):886–891. doi: 10.1152/jappl.1980.48.5.886. [DOI] [PubMed] [Google Scholar]
- Lechner A. J., Banchero N. Lung morphometry in guinea pigs acclimated to hypoxia during growth. Respir Physiol. 1980 Nov;42(2):155–169. doi: 10.1016/0034-5687(80)90112-7. [DOI] [PubMed] [Google Scholar]
- Lum H., Schwartz L. W., Dungworth D. L., Tyler W. S. A comparative study of cell renewal after exposure to ozone or oxygen. Response of terminal bronchiolar epithelium in the rat. Am Rev Respir Dis. 1978 Aug;118(2):335–345. doi: 10.1164/arrd.1978.118.2.335. [DOI] [PubMed] [Google Scholar]
- Matsuba K., Thurlbeck W. M. The number and dimensions of small airways in nonemphysematous lungs. Am Rev Respir Dis. 1971 Oct;104(4):516–524. doi: 10.1164/arrd.1971.104.4.516. [DOI] [PubMed] [Google Scholar]
- McIntosh K., Fishaut J. M. Immunopathologic mechanisms in lower respiratory tract disease of infants due to respiratory syncytial virus. Prog Med Virol. 1980;26:94–118. [PubMed] [Google Scholar]
- Mills J. Effects of Sendai virus infection on function of cultured mouse alveolar macrophages. Am Rev Respir Dis. 1979 Dec;120(6):1239–1244. doi: 10.1164/arrd.1979.120.6.1239. [DOI] [PubMed] [Google Scholar]
- Moe J. B., Osburn B. I., Schwartz L. W., Anderson J., Johnson K. P. Experimental adenovirus SV-20 pneumonia in fetal rhesus monkeys. Pathologic and virologic studies. Lab Invest. 1979 Sep;41(3):211–219. [PubMed] [Google Scholar]
- Prince G. A., Jenson A. B., Horswood R. L., Camargo E., Chanock R. M. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978 Dec;93(3):771–791. [PMC free article] [PubMed] [Google Scholar]
- Prince G. A., Porter D. D. The pathogenesis of respiratory syncytial virus infection in infant ferrets. Am J Pathol. 1976 Feb;82(2):339–352. [PMC free article] [PubMed] [Google Scholar]
- Reid L. Influence of the pattern of structural growth of lung on susceptibility to specific infectious diseases in infants and children. Pediatr Res. 1977 Mar;11(3 Pt 2):210–215. [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970 Jun;26(1):57–60. [PubMed] [Google Scholar]
- Shaw J. O., Henson P. M., Henson J., Webster R. O. Lung inflammation induced by complement-derived chemotactic fragments in the alveolus. Lab Invest. 1980 May;42(5):547–558. [PubMed] [Google Scholar]
- Slauson D. O. The mediation of pulmonary inflammatory injury. Adv Vet Sci Comp Med. 1982;26:99–153. [PubMed] [Google Scholar]
- Stokes G. M., Milner A. D., Hodges I. G., Groggins R. C. Lung function abnormalities after acute bronchiolitis. J Pediatr. 1981 Jun;98(6):871–874. doi: 10.1016/s0022-3476(81)80577-x. [DOI] [PubMed] [Google Scholar]
- Ward J. M., Houchens D. P., Collins M. J., Young D. M., Reagan R. L. Naturally-occurring Sendai virus infection of athymic nude mice. Vet Pathol. 1976;13(1):36–46. doi: 10.1177/030098587601300105. [DOI] [PubMed] [Google Scholar]
- Weibel E. R. A simplified morphometric method for estimating diffusing capacity in normal and emphysematous human lungs. Am Rev Respir Dis. 1973 Apr;107(4):579–588. doi: 10.1164/arrd.1973.107.4.579. [DOI] [PubMed] [Google Scholar]
- Wohl M. E., Chernick V. State of the art: bronchiolitis. Am Rev Respir Dis. 1978 Oct;118(4):759–781. doi: 10.1164/arrd.1978.118.4.759. [DOI] [PubMed] [Google Scholar]
- Wohl M. E., Stigol L. C., Mead J. Resistance of the total respiratory system in healthy infants and infants with bronchiolitis. Pediatrics. 1969 Apr;43(4):495–509. [PubMed] [Google Scholar]
- Zurcher C., Burek J. D., Van Nunen M. C., Meihuizen S. P. A naturally occurring epizootic caused by Sendai virus in breeding and aging rodent colonies. I. Infection in the mouse. Lab Anim Sci. 1977 Dec;27(6):955–962. [PubMed] [Google Scholar]