Abstract
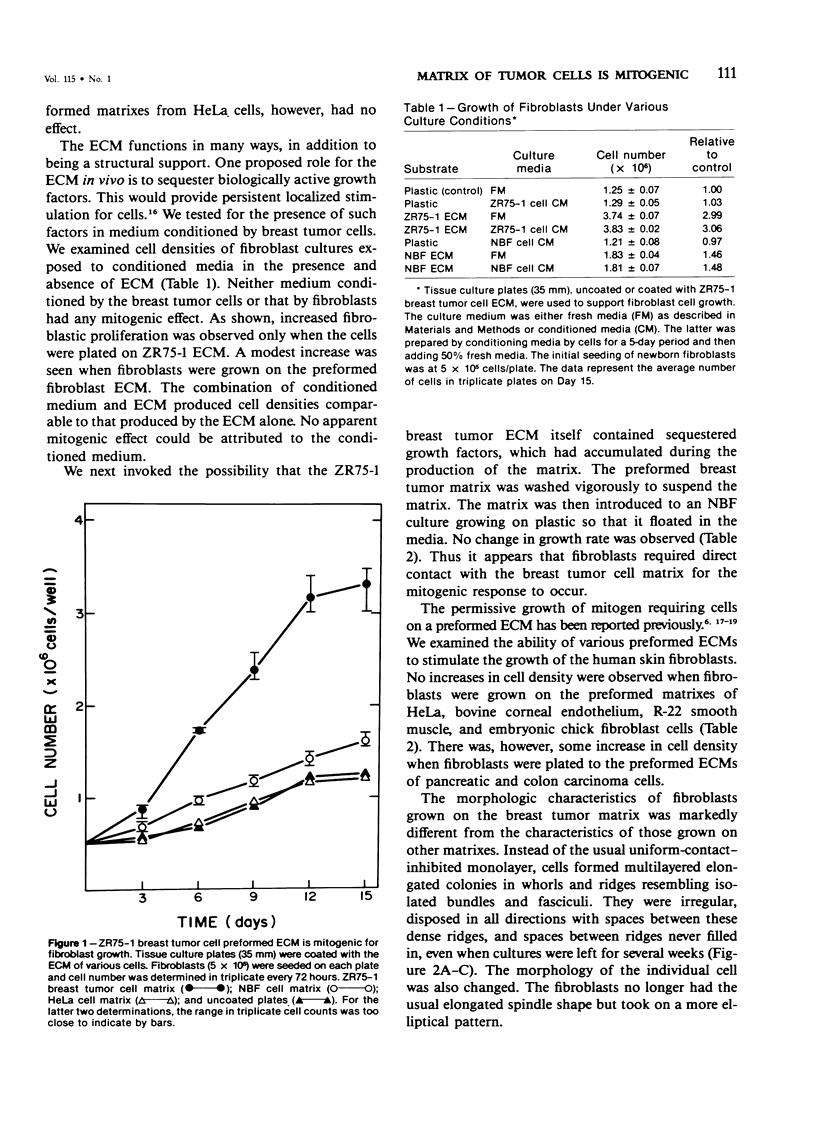
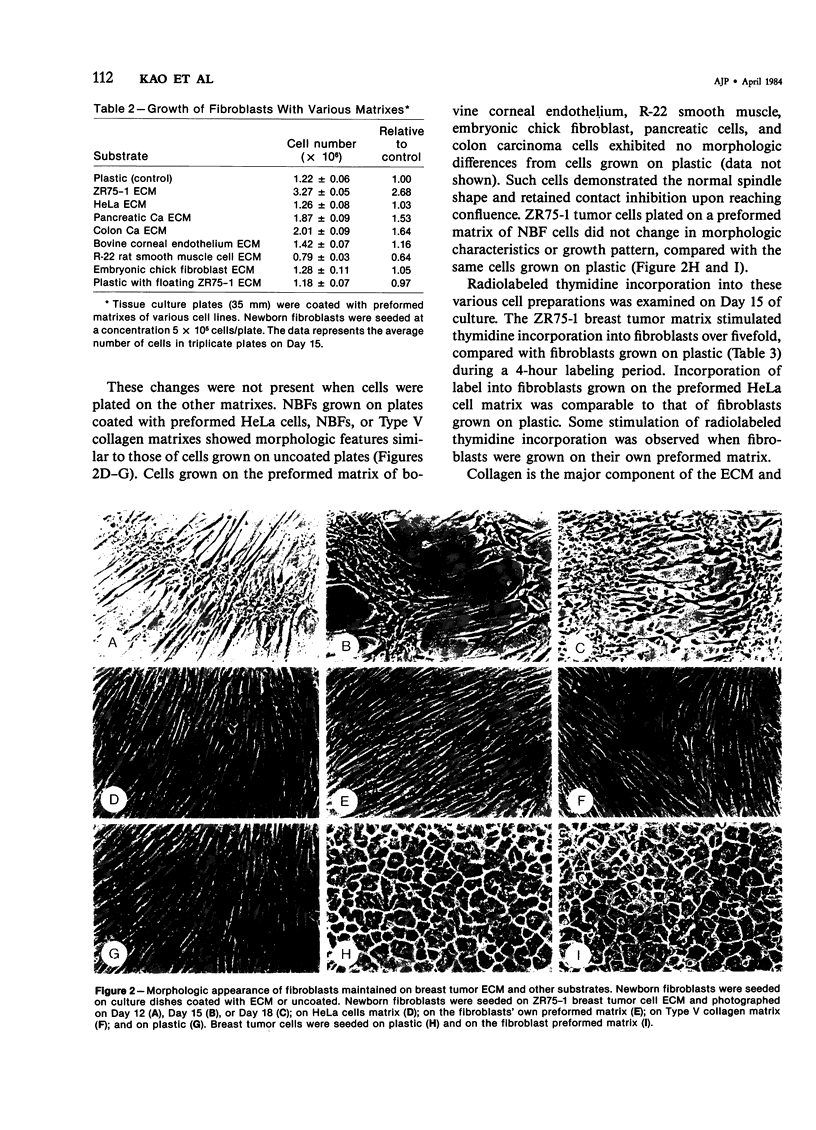
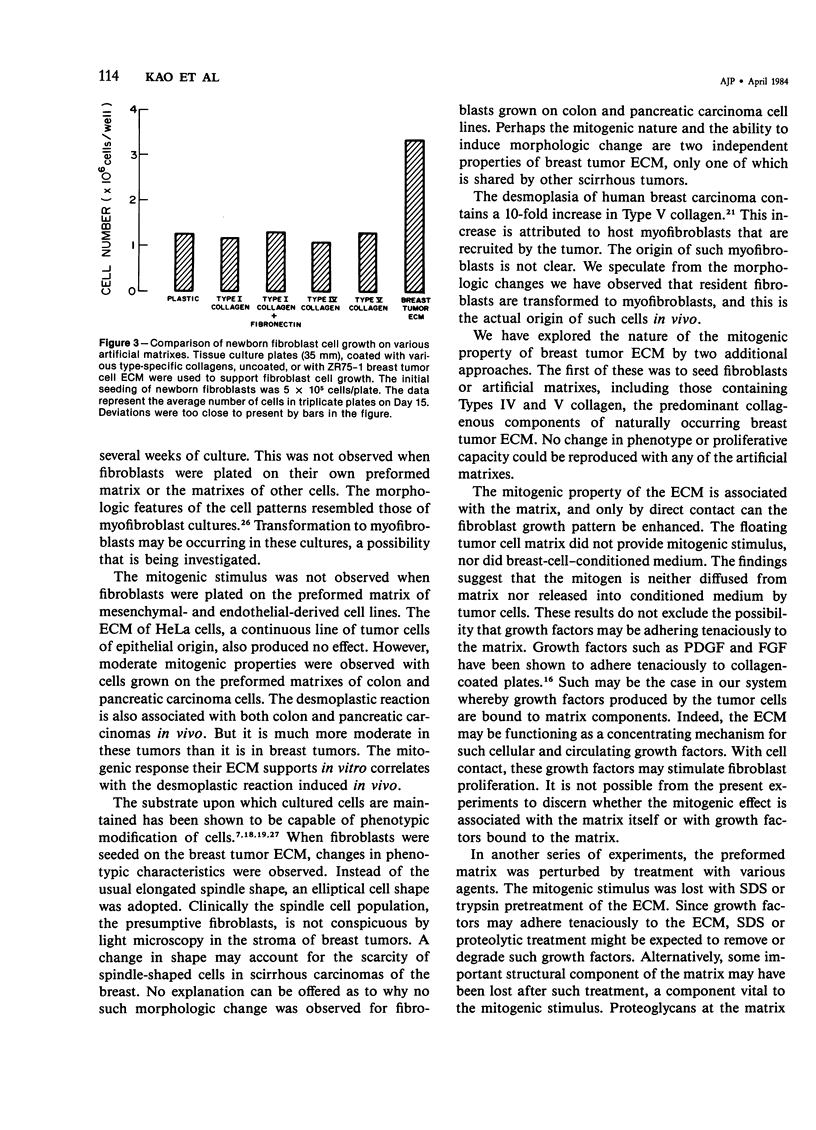
The basis of the scirrhous reaction to human breast carcinoma was investigated. When normal human skin fibroblasts were plated on the preformed extracellular matrix of human breast tumor cells, a remarkable series of changes was observed. The matrix of the tumor cells was mitogenic for the fibroblasts. An increased growth rate and a fourfold increase in cell density was observed. There was also a change in cell morphology and in the pattern in which the cells grew, with an apparent loss of contact inhibition. The spindle-shaped fibroblasts became more elliptical and grew in a series of whorls and dense ridges with spaces between them. These observations were made with the use of newborn foreskin fibroblasts and the matrix of an established line of human breast cancer cells, ZR75-1. No such effect was seen when fibroblasts were plated on their own preformed matrix, on the matrixes of other cell types, on various type-specific collagen gels, or on a combination of collagen and fibronectin or when fibroblasts were grown in media conditioned by the ZR75-1 cells. A floating tumor cell matrix added to the cell media also did not provide the mitogenic stimulus. Apparently, fibroblasts required direct contact with the tumor cell matrix for the mitogenic response to occur. In vivo, the matrix of breast tumor cells may modulate the growth and the morphology of host stromal cells. Collagen is a major synthetic product of fibroblasts. The stimulation of stromal cells to proliferate by adjacent breast tumor matrix may be the basis of the desmoplastic reaction, the intense fibrotic response associated with human breast cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Adnani M. S., Kirrane J. A., McGee J. O. Inappropriate production of collagen and prolyl hydroxylase by human breast cancer cells in vivo. Br J Cancer. 1975 Jun;31(6):653–660. doi: 10.1038/bjc.1975.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzopardi J. G., Laurini R. N. Elastosis in breast cancer. Cancer. 1974 Jan;33(1):174–183. doi: 10.1002/1097-0142(197401)33:1<174::aid-cncr2820330126>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Rao C. N., Grotendorst G. R., Liotta L. A. Increased content of Type V Collagen in desmoplasia of human breast carcinoma. Am J Pathol. 1982 Sep;108(3):276–283. [PMC free article] [PubMed] [Google Scholar]
- Buck R. C. The influence of contact guidance on the orientation of colonies of subcultured vascular smooth muscle cells. In Vitro. 1982 Sep;18(9):783–788. doi: 10.1007/BF02796502. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel L. W., Young N. A., Tralka T. S., Lippman M. E., O'Brien S. J., Joyce M. J. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978 Oct;38(10):3352–3364. [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Goldberg B. D. Binding of soluble type I collagen to fibroblasts: effects of thermal activation of ligand, ligand concentration, pinocytosis, and cytoskeletal modifiers. J Cell Biol. 1982 Dec;95(3):747–751. doi: 10.1083/jcb.95.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg B. Binding of soluble type I collagen molecules to the fibroblast plasma membrane. Cell. 1979 Feb;16(2):265–275. doi: 10.1016/0092-8674(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Delgado D., Vlodavsky I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4094–4098. doi: 10.1073/pnas.77.7.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Greenburg G., Birdwell C. R. Determination of cellular shape by the extracellular matrix and its correlation with the control of cellular growth. Cancer Res. 1978 Nov;38(11 Pt 2):4155–4171. [PubMed] [Google Scholar]
- Greene L. A., Tomita J. T., Varon S. Growth-stimulating activities of mouse submaxillary esteropeptidases on chick embryo fibroblasts in vitro. Exp Cell Res. 1971 Feb;64(2):387–395. doi: 10.1016/0014-4827(71)90092-9. [DOI] [PubMed] [Google Scholar]
- Jones P. A. Construction of an artificial blood vessel wall from cultured endothelial and smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1882–1886. doi: 10.1073/pnas.76.4.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P. A., Scott-Burden T., Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979 Jan;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. T., Wong M., Stern R. Elastin degradation by proteases from cultured human breast cancer cells. Biochem Biophys Res Commun. 1982 Mar 15;105(1):383–389. doi: 10.1016/s0006-291x(82)80056-9. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Tsao D., Hicks J., McIntyre L. J. Surface membrane glycoproteins of cultured human pancreatic cancer cells. Cancer. 1981 Mar 15;47(6 Suppl):1590–1596. doi: 10.1002/1097-0142(19810315)47:6+<1590::aid-cncr2820471422>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Kim Y. S., Tsao D., Siddiqui B., Whitehead J. S., Arnstein P., Bennett J., Hicks J. Effects of sodium butyrate and dimethylsulfoxide on biochemical properties of human colon cancer cells. Cancer. 1980 Mar 15;45(5 Suppl):1185–1192. doi: 10.1002/1097-0142(19800315)45:5+<1185::aid-cncr2820451324>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Stimulation of extracellular matrix synthesis in the developing cornea by glycosaminoglycans. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2310–2313. doi: 10.1073/pnas.71.6.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Engvall E. Complexing of fibronectin glycosaminoglycans and collagen. Biochim Biophys Acta. 1980 Aug 13;631(2):350–358. doi: 10.1016/0304-4165(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Smith J. C., Singh J. P., Lillquist J. S., Goon D. S., Stiles C. D. Growth factors adherent to cell substrate are mitogenically active in situ. Nature. 1982 Mar 11;296(5853):154–156. doi: 10.1038/296154a0. [DOI] [PubMed] [Google Scholar]
- Tseng S. C., Savion N., Gospodarowicz D., Stern R. Modulation of collagen synthesis by a growth factor and by the extracellular matrix: comparison of cellular response to two different stimuli. J Cell Biol. 1983 Sep;97(3):803–809. doi: 10.1083/jcb.97.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Lui G. M., Gospodarowicz D. Morphological appearance, growth behavior and migratory activity of human tumor cells maintained on extracellular matrix versus plastic. Cell. 1980 Mar;19(3):607–616. doi: 10.1016/s0092-8674(80)80037-7. [DOI] [PubMed] [Google Scholar]
- Wicha M. S., Lowrie G., Kohn E., Bagavandoss P., Mahn T. Extracellular matrix promotes mammary epithelial growth and differentiation in vitro. Proc Natl Acad Sci U S A. 1982 May;79(10):3213–3217. doi: 10.1073/pnas.79.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]




