Abstract
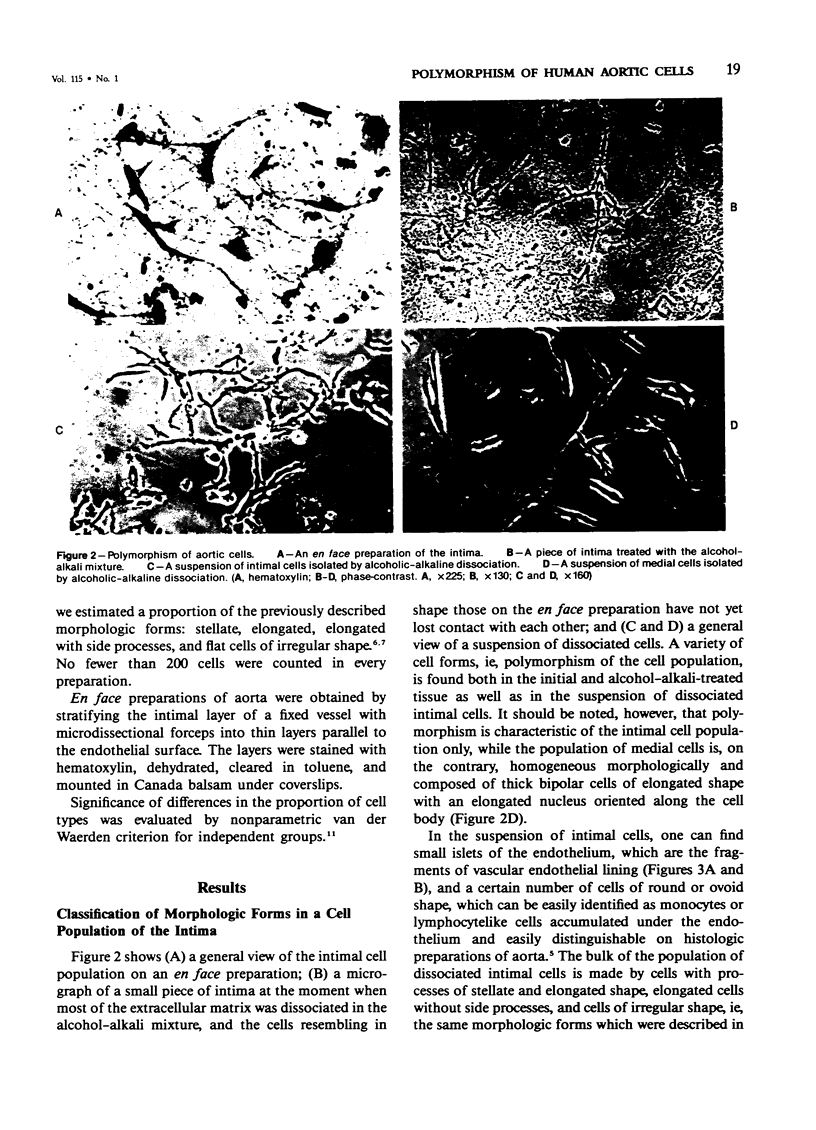
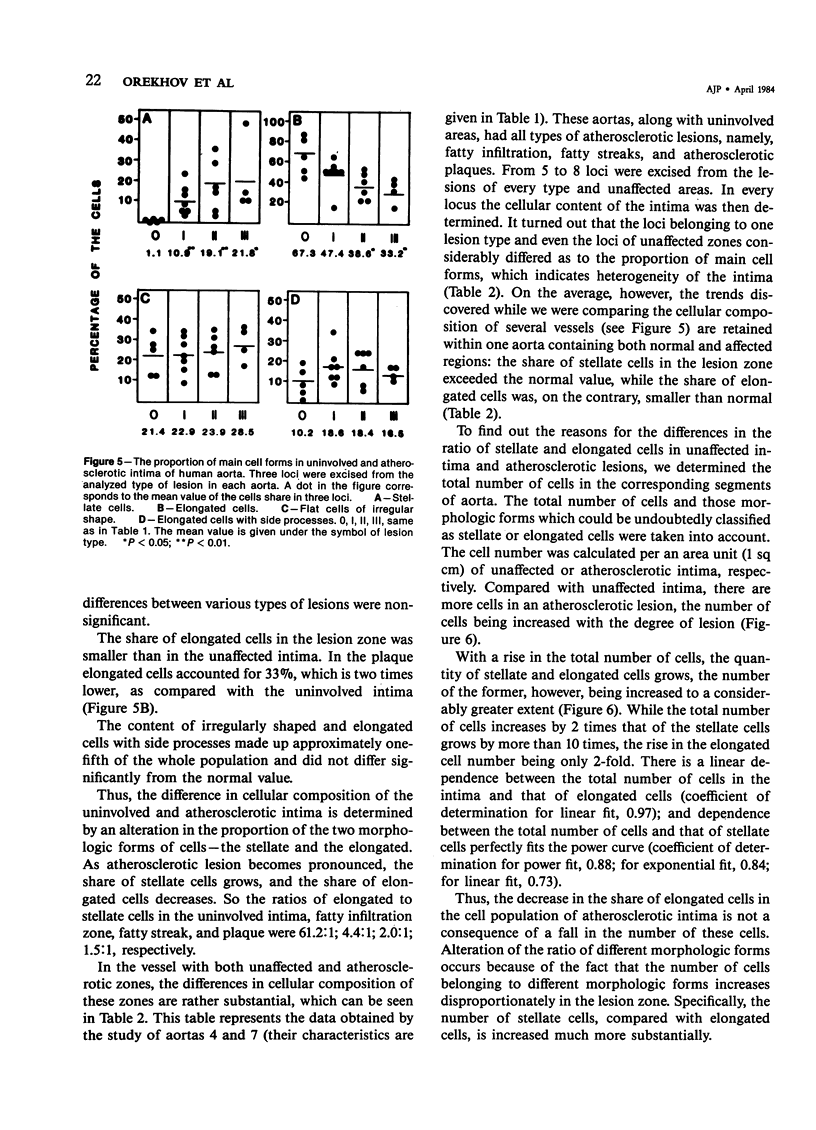
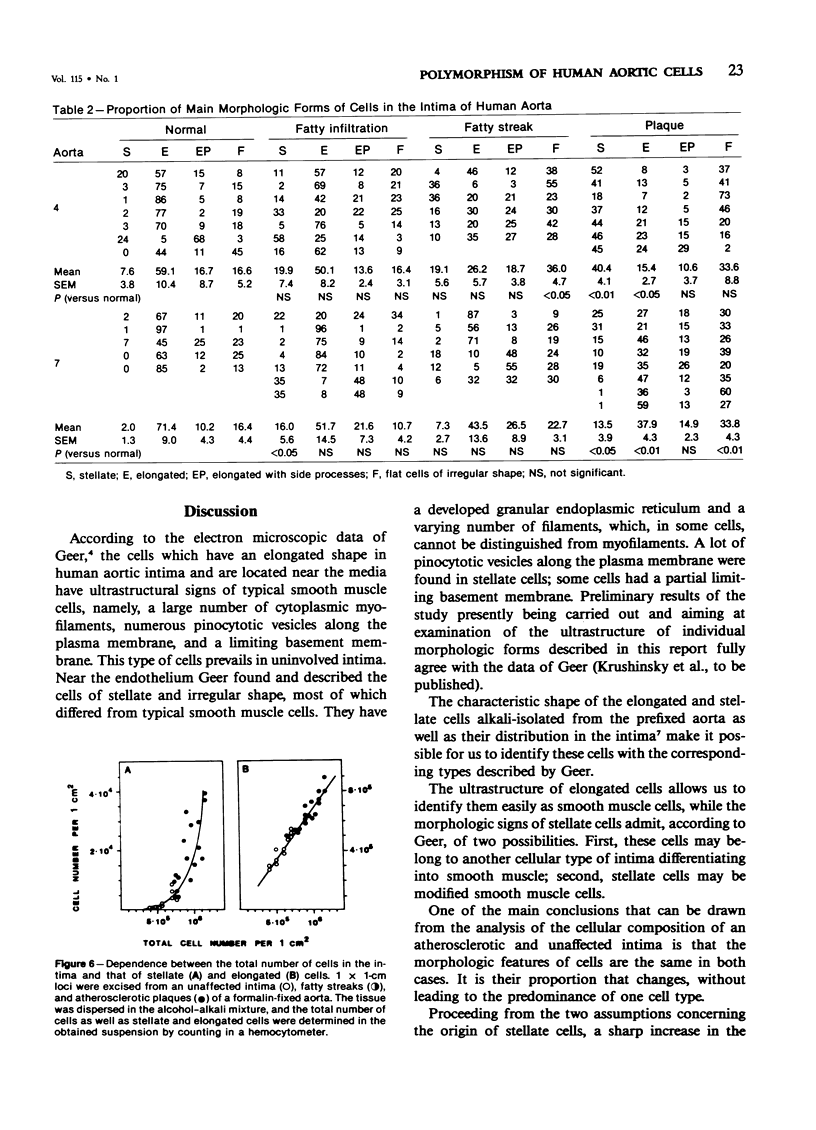
Alcoholic-alkaline dissociation was used in the study of cellular composition of human aorta. Cells were isolated from an uninvolved intima and intima with different types of atherosclerotic lesions: fatty infiltration, fatty streak, and atherosclerotic plaque. In the isolated suspension we evaluated the ratio of four previously described morphologic forms of cells: stellate, elongated, elongated with side processes, and flat cells of irregular shape. It was demonstrated that the quota of stellate cells in an atherosclerotic lesion considerably exceeds that of the normal intima. For elongated cells the opposite is true. The other two cell forms are represented in the uninvolved and atherosclerotic intima in approximately equal proportions. Alteration of the ratio of different morphologic forms occurs because of the fact that the number of cells belonging to different morphologic forms increases disproportionately in the lesion zone. Specifically, the number of stellate cells is increased much more substantially, compared with elongated cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer J. C. Fine structure of human aortic intimal thickening and fatty streaks. Lab Invest. 1965 Oct;14(10):1764–1783. [PubMed] [Google Scholar]
- KHAVKIN T. N. O razviti ateroskleroticheskikh izmenenii aorty. Arkh Patol. 1950 Sep-Oct;12(5):23–33. [PubMed] [Google Scholar]
- Krushinsky A. V., Orekhov A. N., Smirnov V. N. Stellate cells in the intima of human aorta. Application of alkaline dissociation method in the analysis of the vessel wall cellular content. Acta Anat (Basel) 1983;117(3):266–269. [PubMed] [Google Scholar]
- Orekhov A. N., Kosykh V. A., Repin V. S., Smirnov V. N. Cell proliferation in normal and atherosclerotic human aorta. II. Autoradiographic observation on deoxyribonucleic acid synthesis in primary cell culture. Lab Invest. 1983 Jun;48(6):749–754. [PubMed] [Google Scholar]
- Schönfelder M. Orthologie und Pathologie der Langhans-Zellen der Aortenintima des Menschen. Pathol Microbiol (Basel) 1969;33(3):129–145. [PubMed] [Google Scholar]
- Smith E. B., Evans P. H., Downham M. D. Lipid in the aortic intima. The correlation of morphological and chemical characteristics. J Atheroscler Res. 1967 Mar-Apr;7(2):171–186. doi: 10.1016/s0368-1319(67)80079-6. [DOI] [PubMed] [Google Scholar]
- Smith E. B. The relationship between plasma and tissue lipids in human atherosclerosis. Adv Lipid Res. 1974;12(0):1–49. doi: 10.1016/b978-0-12-024912-1.50008-9. [DOI] [PubMed] [Google Scholar]
- Tertov V. V., Orekhov A. N., Repin V. S., Smirnov V. N. Dibutyryl cyclic AMP decreases proliferative activity and the cholesteryl ester content in cultured cells of atherosclerotic human aorta. Biochem Biophys Res Commun. 1982 Dec 31;109(4):1228–1233. doi: 10.1016/0006-291x(82)91908-8. [DOI] [PubMed] [Google Scholar]