Abstract
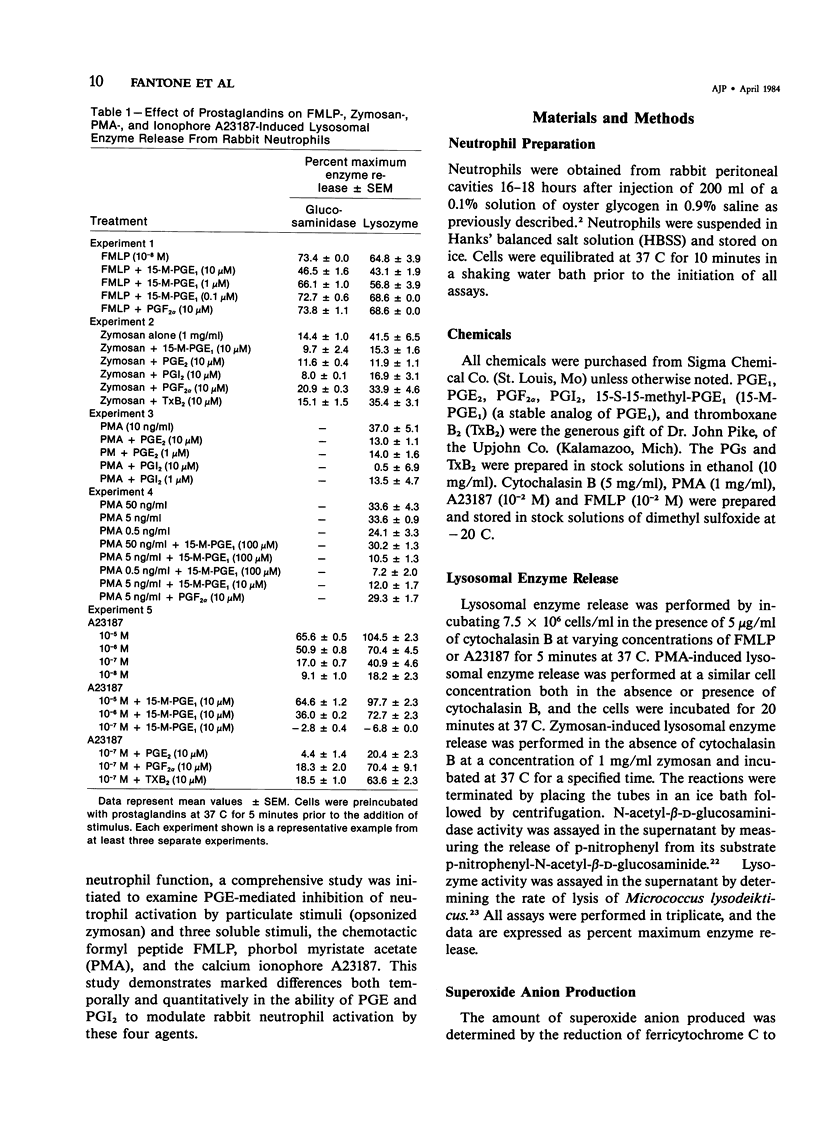
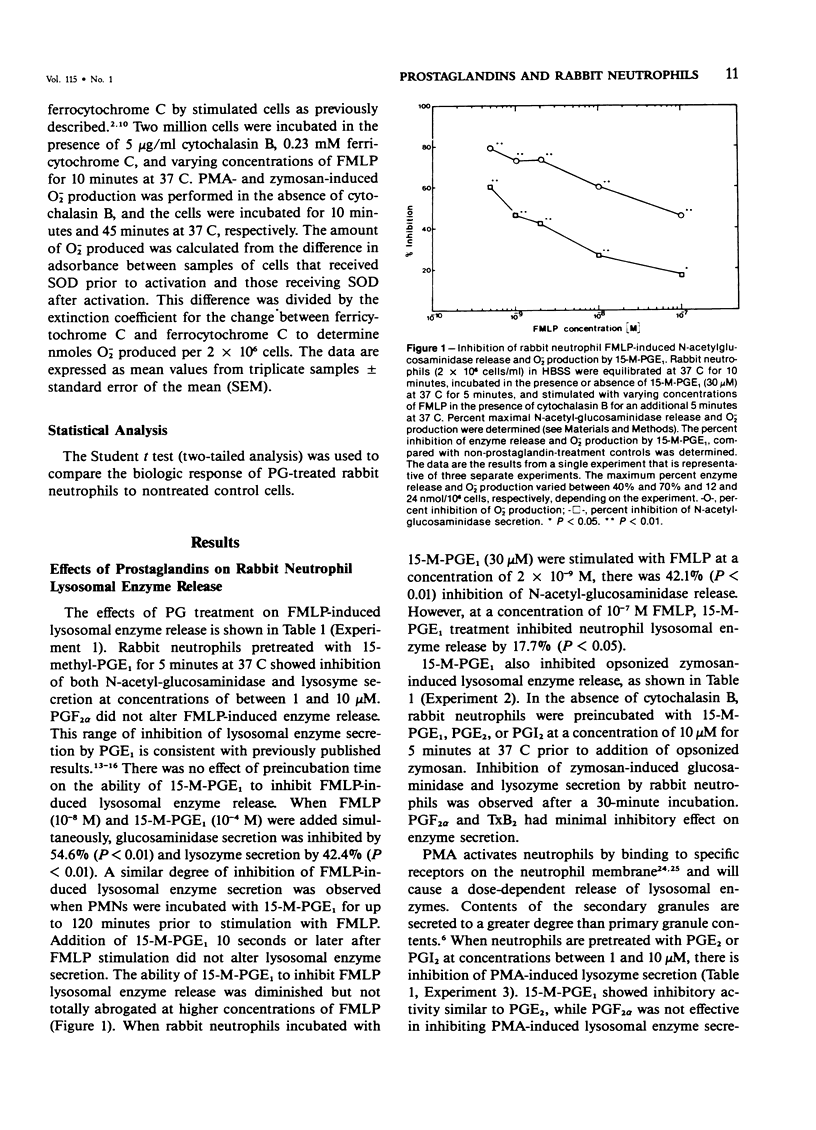
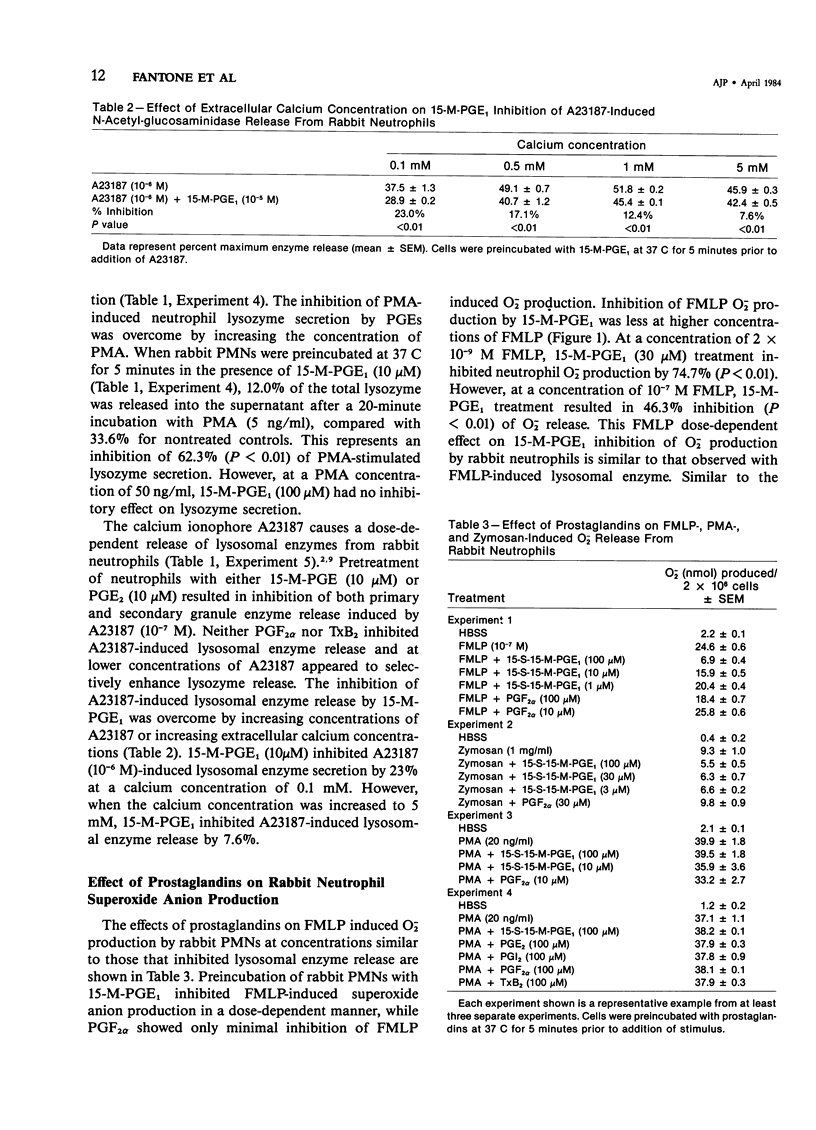
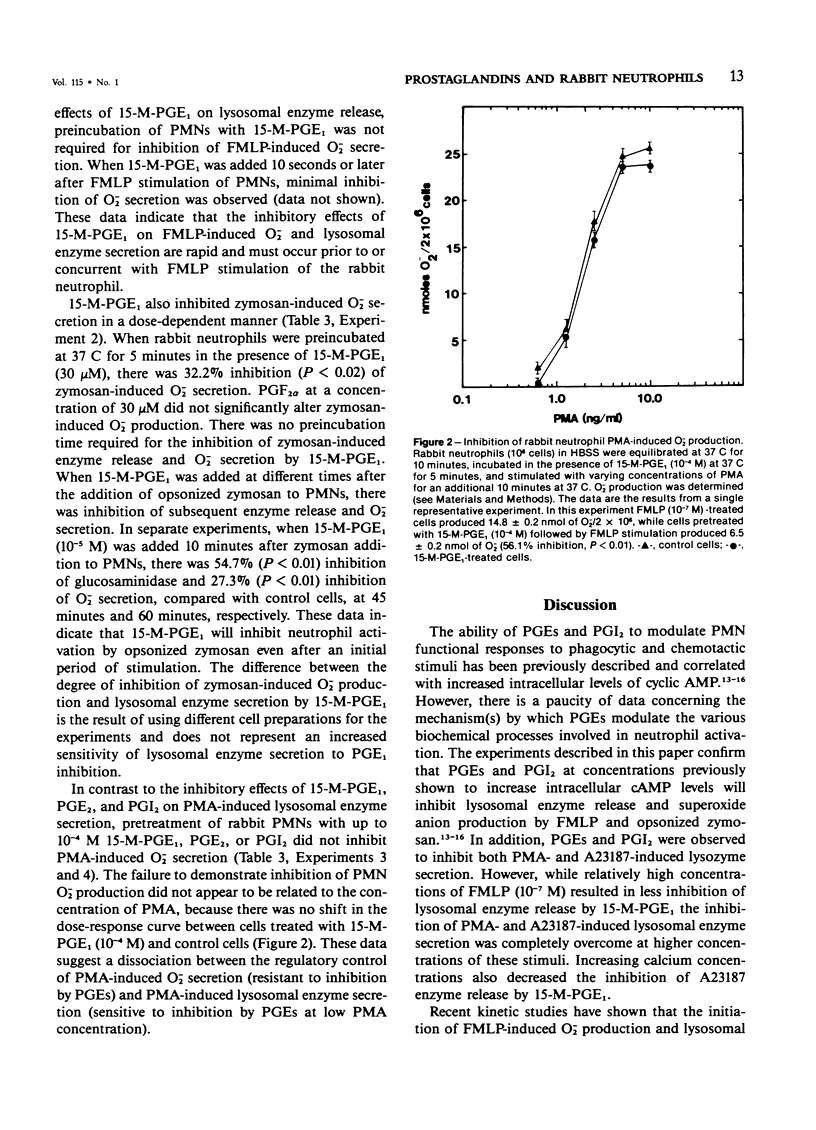
Prostaglandins (PGs) of the E series and PGI2 have been shown to inhibit acute inflammatory reactions in vivo and polymorphonuclear leukocyte (PMN), chemotaxis, lysosomal enzyme release, and superoxide anion (O-2) production in vitro. This inhibition of neutrophil stimulation by PGEs and PGI2 has been correlated with their ability to increase intracellular cyclic adenosine monophosphate (cAMP) levels. However, the mechanism(s) by which PGEs and PGI2 alter the complex biochemical and biophysical events associated with stimulus-response coupling in the neutrophil are not clear. It is reported here that both PGEs and PGI2 in micromolar concentrations inhibit formyl-methionyl-leucyl-phenylalanine (FMLP)- and zymosan-induced lysosomal enzyme secretion and superoxide anion production in a dose-dependent manner. No preincubation time of PMNs with the prostaglandins is required for inhibition. Addition of PGEs 10 seconds or later after FMLP stimulation does not alter the biologic response of the neutrophils to the stimulus, suggesting that the prostaglandin inhibition effects early events associated with stimulus-response coupling in the neutrophil. Prostaglandin inhibition of lysosomal enzyme release by the calcium ionophore A23187 was overcome by increasing the extracellular ionophore and/or calcium concentration, suggesting that PGs may modulate intracellular free calcium levels in a manner similar to that observed with platelets. Inhibition of phorbol myristate acetate (PMA)-induced neutrophil lysosomal enzyme secretion by PGEs and PGI2 was overcome by increasing concentrations of PMA. However, neither PGEs nor PGI2 altered O-2 production by PMA-treated neutrophils. These data indicate a dissociation between PMA-stimulated O-2 production and lysosomal enzyme release. These findings are consistent with the hypothesis that inhibition of neutrophil stimulation by PGEs and PGI2 is a result of increased intracellular cyclic AMP levels and modulation of calcium-dependent events. In addition, the data indicate that there are at least two mechanisms by which PMNs can be stimulated to produce O-2, one inhibited by PGEs and PGI2 and a second independent of prostaglandin modulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Robinson J. M., Lazdins J. K., Briggs R. T., Karnovsky M. J., Karnovsky M. L. Comparative aspects of oxidative metabolism of neutrophils from human blood and guinea pig peritonea: magnitude of the respiratory burst, dependence upon stimulating agents, and localization of the oxidases. J Cell Physiol. 1980 Dec;105(3):541–545. doi: 10.1002/jcp.1041050319. [DOI] [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Cohen H. J., Newburger P. E., Chovaniec M. E., Whitin J. C., Simons E. R. Opsonized zymosan-stimulated granulocytes-activation and activity of the superoxide-generating system and membrane potential changes. Blood. 1981 Nov;58(5):975–982. [PubMed] [Google Scholar]
- Cutler L., Rodan G., Feinstein M. B. Cytochemical localization of adenylate cyclase and of calcium ion, magnesium ion-activated ATPases in the dense tubular system of human blood platelets. Biochim Biophys Acta. 1978 Sep 6;542(3):357–371. doi: 10.1016/0304-4165(78)90367-7. [DOI] [PubMed] [Google Scholar]
- DeChatelet L. R., Shirley P. S., Johnston R. B., Jr Effect of phorbol myristate acetate on the oxidative metabolism of human polymorphonuclear leukocytes. Blood. 1976 Apr;47(4):545–554. [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., White J. G., Holmes B. Specific degranulation of human polymorphonuclear leukocytes. Nature. 1974 Mar 22;248(446):347–348. doi: 10.1038/248347a0. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Kunkel S. L., Ward P. A. Suppression of human polymorphonuclear function after intravenous infusion of prostaglandin E1. Prostaglandins Med. 1981 Aug;7(2):195–198. doi: 10.1016/0161-4630(81)90062-8. [DOI] [PubMed] [Google Scholar]
- Fantone J. C., Kunkel S. L., Ward P. A., Zurier R. B. Suppression by prostaglandin E1 of vascular permeability induced by vasoactive inflammatory mediators. J Immunol. 1980 Dec;125(6):2591–2596. [PubMed] [Google Scholar]
- Fantone J. C., Marasco W. A., Elgas L. J., Ward P. A. Anti-inflammatory effects of prostaglandin E1: in vivo modulation of the formyl peptide chemotactic receptor on the rat neutrophil. J Immunol. 1983 Apr;130(4):1495–1497. [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Horn J. K., Kaplan H. B., Weissmann G. Calcium-induced lysozyme secretion from human polymorphonuclear leukocytes. Biochem Biophys Res Commun. 1974 Sep 23;60(2):807–812. doi: 10.1016/0006-291x(74)90312-x. [DOI] [PubMed] [Google Scholar]
- Goodwin B. J., Weinberg J. B. Receptor-mediated modulation of human monocyte, neutrophil, lymphocyte, and platelet function by phorbol diesters. J Clin Invest. 1982 Oct;70(4):699–706. doi: 10.1172/JCI110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F. The regulation of lipomodulin, a phospholipase inhibitory protein, in rabbit neutrophils by phosphorylation. J Biol Chem. 1981 Aug 10;256(15):7730–7733. [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Nishizuka Y. Cooperative roles of various membrane phospholipids in the activation of calcium-activated, phospholipid-dependent protein kinase. J Biol Chem. 1981 Jul 25;256(14):7146–7149. [PubMed] [Google Scholar]
- Kunkel S. L., Fantone J. C., Ward P. A., Zurier R. B. Modulation of inflammatory reactions by prostaglandins. Prog Lipid Res. 1981;20:633–640. doi: 10.1016/0163-7827(81)90118-1. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Thrall R. S., Kunkel R. G., McCormick J. R., Ward P. A., Zurier R. B. Suppression of immune complex vasculitis in rats by prostaglandin. J Clin Invest. 1979 Nov;64(5):1525–1529. doi: 10.1172/JCI109611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käser-Glanzmann R., Jakäbovä M., George J. N., Lüscher E. F. Stimulation of calcium uptake in platelet membrane vesicles by adenosine 3',5'-cyclic monophosphate and protein kinase. Biochim Biophys Acta. 1977 May 2;466(3):429–440. doi: 10.1016/0005-2736(77)90336-4. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Johnston R. B., Jr Effect of anti-inflammatory drugs and agents that elevate intracellular cyclic AMP on the release of toxic oxygen metabolites by phagocytes: studies in a model of tissue-bound IgG. Clin Immunol Immunopathol. 1978 Apr;9(4):482–490. doi: 10.1016/0090-1229(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Lehrer R. I., Cohen L. Receptor-mediated regulation of superoxide production in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest. 1981 Nov;68(5):1314–1320. doi: 10.1172/JCI110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone G., Thomas L. L., Lichtenstein L. M. The role of agonists that activate adenylate cyclase in the control of cAMP metabolism and enzyme release by human polymorphonuclear leukocytes. J Immunol. 1980 Nov;125(5):2277–2283. [PubMed] [Google Scholar]
- Morinelli T. A., Niewiarowski S., Kornecki E., Figures W. R., Wachtfogel Y., Colman R. W. Platelet aggregation and exposure of fibrinogen receptors by prostaglandin endoperoxide analogues. Blood. 1983 Jan;61(1):41–49. [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Changes in ionic movements across rabbit polymorphonuclear leukocyte membranes during lysosomal enzyme release. Possible ionic basis for lysosomal enzyme release. J Cell Biol. 1977 Dec;75(3):635–649. doi: 10.1083/jcb.75.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Dechatelet L. R., McCall C. E., Bass D. A. Neutrophil aggregation: evidence for a different mechanism of action by phorbol myristate acetate. Proc Soc Exp Biol Med. 1980 Nov;165(2):225–232. doi: 10.3181/00379727-165-40962. [DOI] [PubMed] [Google Scholar]
- Razin E., Globerson A. The effect of various prostaglandins on plasma membrane receptors and function of mouse macrophages. Adv Exp Med Biol. 1979;114:415–419. doi: 10.1007/978-1-4615-9101-6_68. [DOI] [PubMed] [Google Scholar]
- Rivkin I., Rosenblatt J., Becker E. L. The role of cyclic AMP in the chemotactic responsiveness and spontaneous motility of rabbit peritoneal neutrophils. The inhibition of neutrophil movement and the elevation of cyclic AMP levels by catecholamines, prostaglandins, theophylline and cholera toxin. J Immunol. 1975 Oct;115(4):1126–1134. [PubMed] [Google Scholar]
- Roos D., Bot A. A., van Schaik M. L., de Boer M., Daha M. R. Interaction between human neutrophils and zymosan particles: the role of opsonins and divalent cations. J Immunol. 1981 Feb;126(2):433–440. [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simchowitz L., Spilberg I. Generation of superoxide radicals by human peripheral neutrophils activated by chemotactic factor. Evidence for the role of calcium. J Lab Clin Med. 1979 Apr;93(4):583–593. [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. Ligand/receptor internalization: a spectroscopic analysis and a comparison of ligand binding, cellular response, and internalization by human neutrophils. J Cell Biochem. 1982;20(2):193–202. doi: 10.1002/jcb.240200210. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Sano K., Nishizuka Y. Counteraction of calcium-activated, phospholipid-dependent protein kinase activation by adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in platelets. J Biochem. 1982 Jan;91(1):403–406. doi: 10.1093/oxfordjournals.jbchem.a133700. [DOI] [PubMed] [Google Scholar]
- Tran P. L., Ter-Minassian-Saraga L., Madelmont G., Castagna M. Tumor promoter 12-O-tetradecanoylphorbol 13-acetate alters state, fluidity and hydration of 1,2-diacyl-sn-glycero-3-phosphocholine bilayers. Biochim Biophys Acta. 1983 Jan 5;727(1):31–38. doi: 10.1016/0005-2736(83)90365-6. [DOI] [PubMed] [Google Scholar]
- WOOLLEN J. W., HEYWORTH R., WALKER P. G. Studies on glucosaminidase. 3. Testicular N-acetyl-beta-glucosaminidase and N-acetyl-beta-galactosaminidase. Biochem J. 1961 Jan;78:111–116. doi: 10.1042/bj0780111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabucchi G., Romeo D. The dissociation of exocytosis and respiratory stimulation in leucocytes by ionophores. Biochem J. 1976 May 15;156(2):209–213. doi: 10.1042/bj1560209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurier R. B., Weissmann G., Hoffstein S., Kammerman S., Tai H. H. Mechanisms of lysosomal enzyme release from human leukocytes. II. Effects of cAMP and cGMP, autonomic agonists, and agents which affect microtubule function. J Clin Invest. 1974 Jan;53(1):297–309. doi: 10.1172/JCI107550. [DOI] [PMC free article] [PubMed] [Google Scholar]



