Abstract
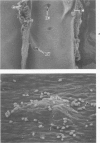
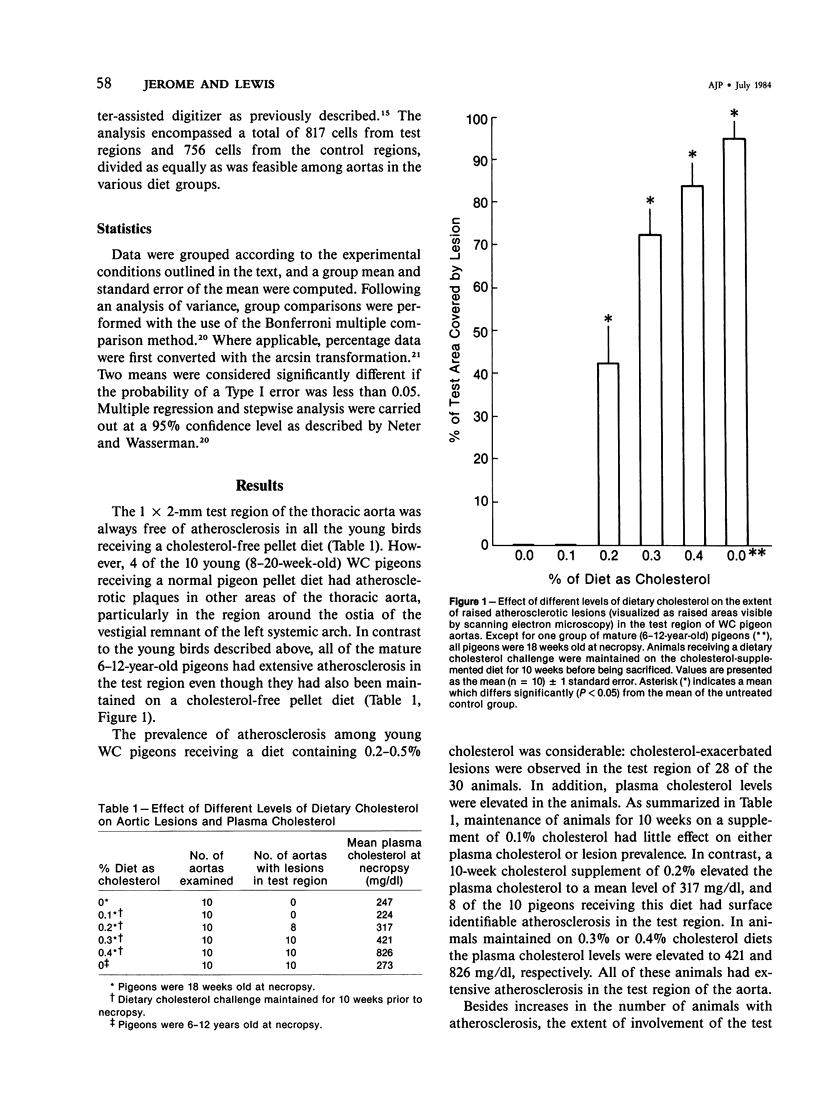
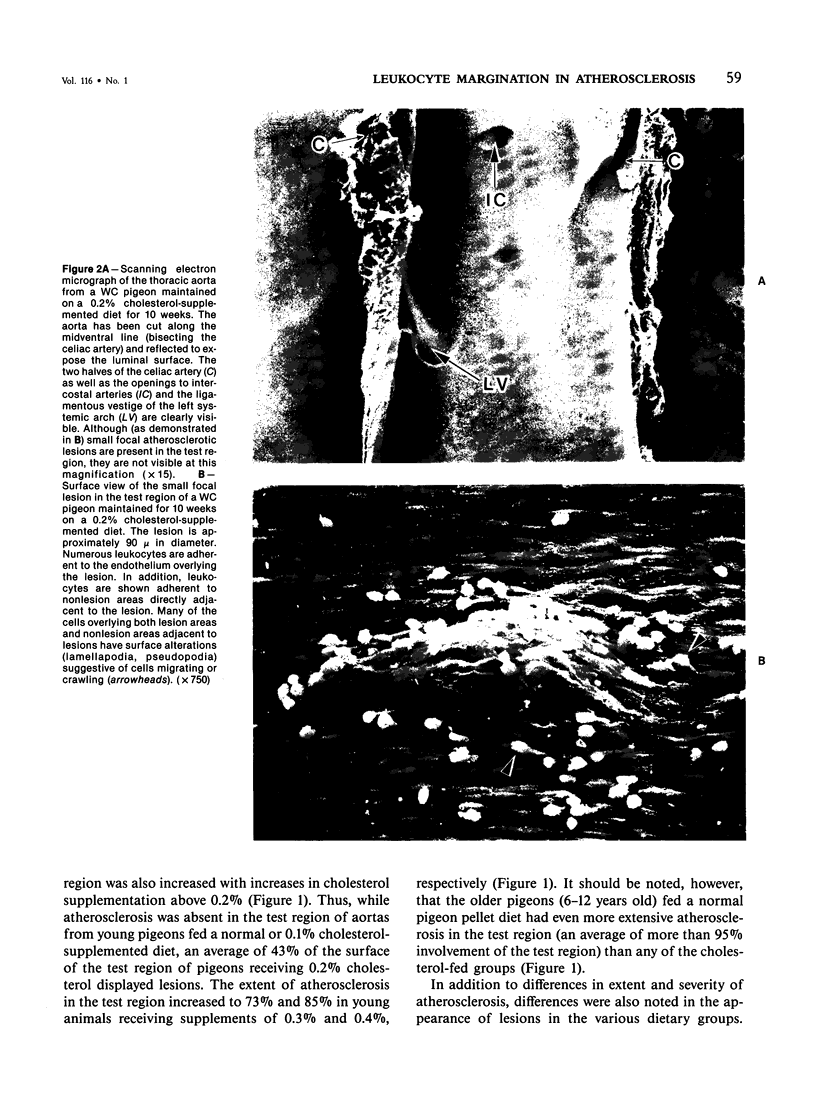
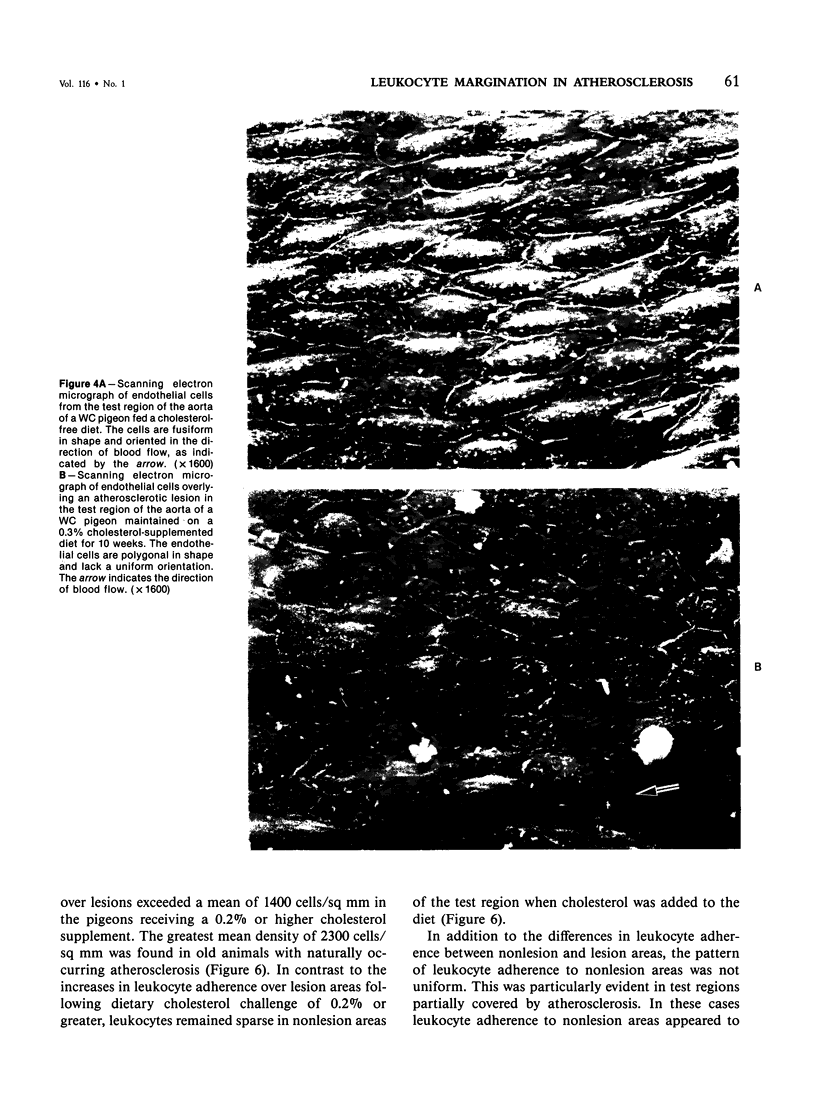
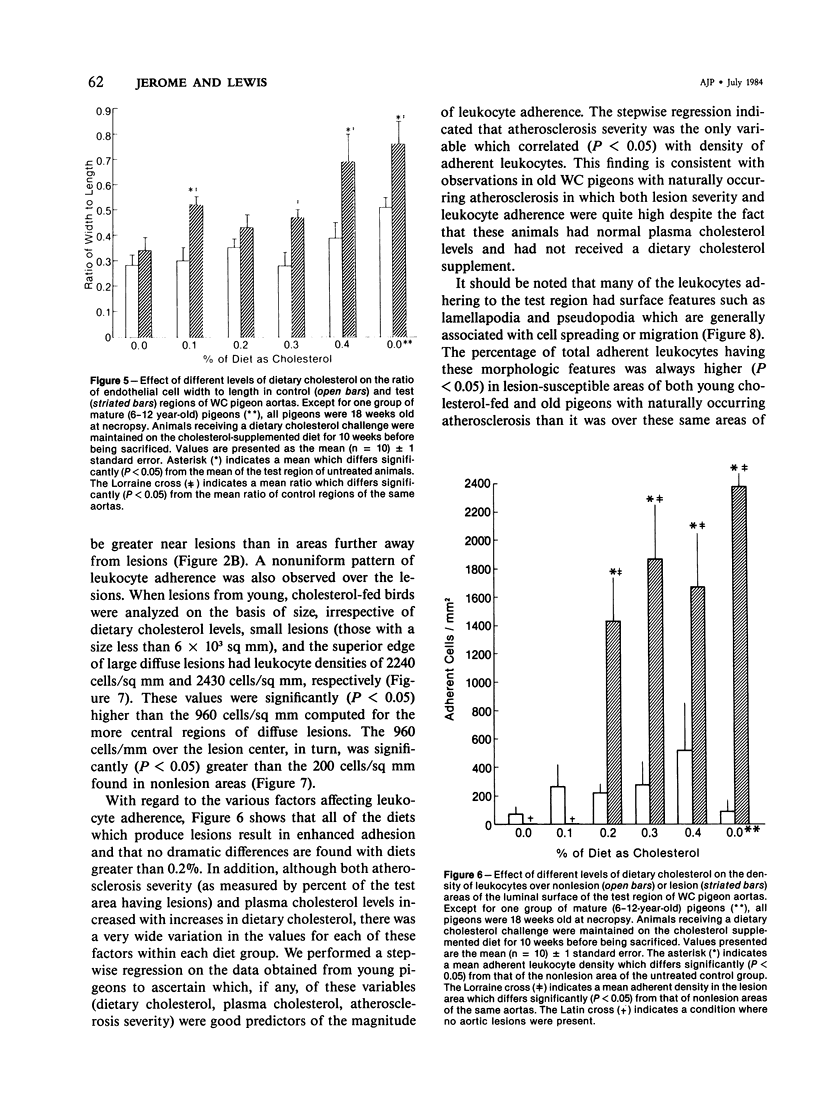
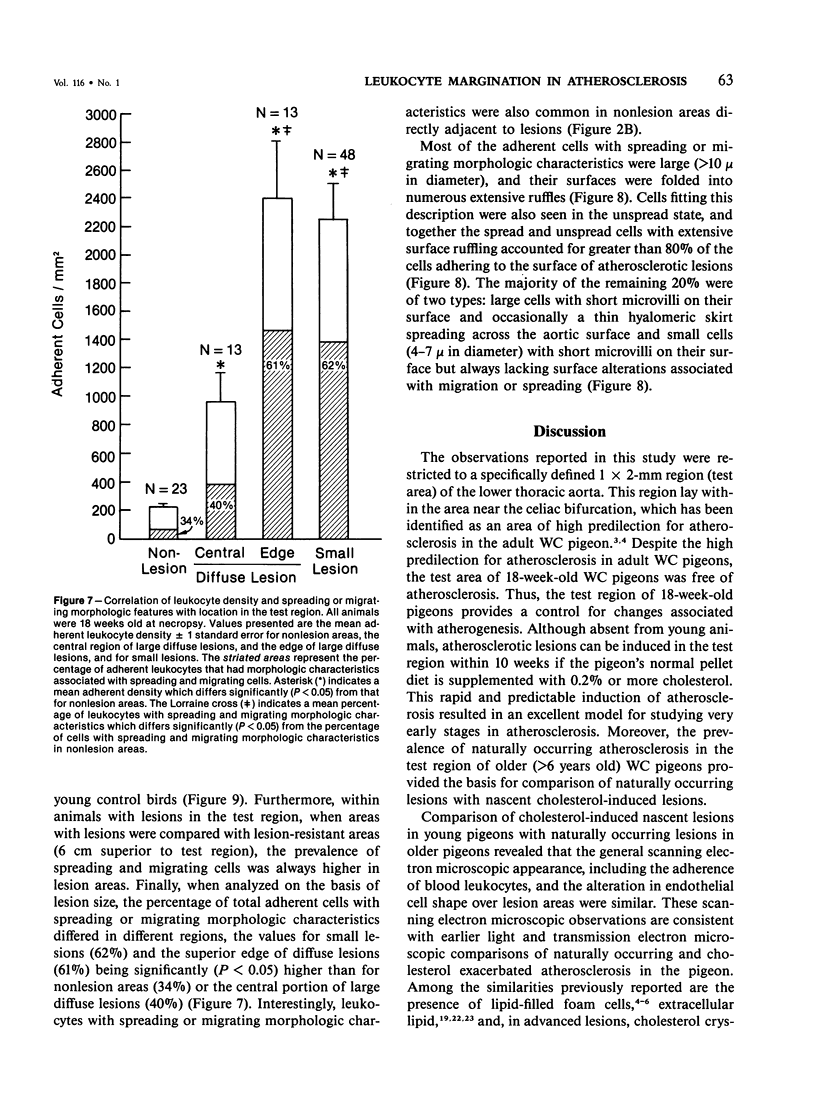
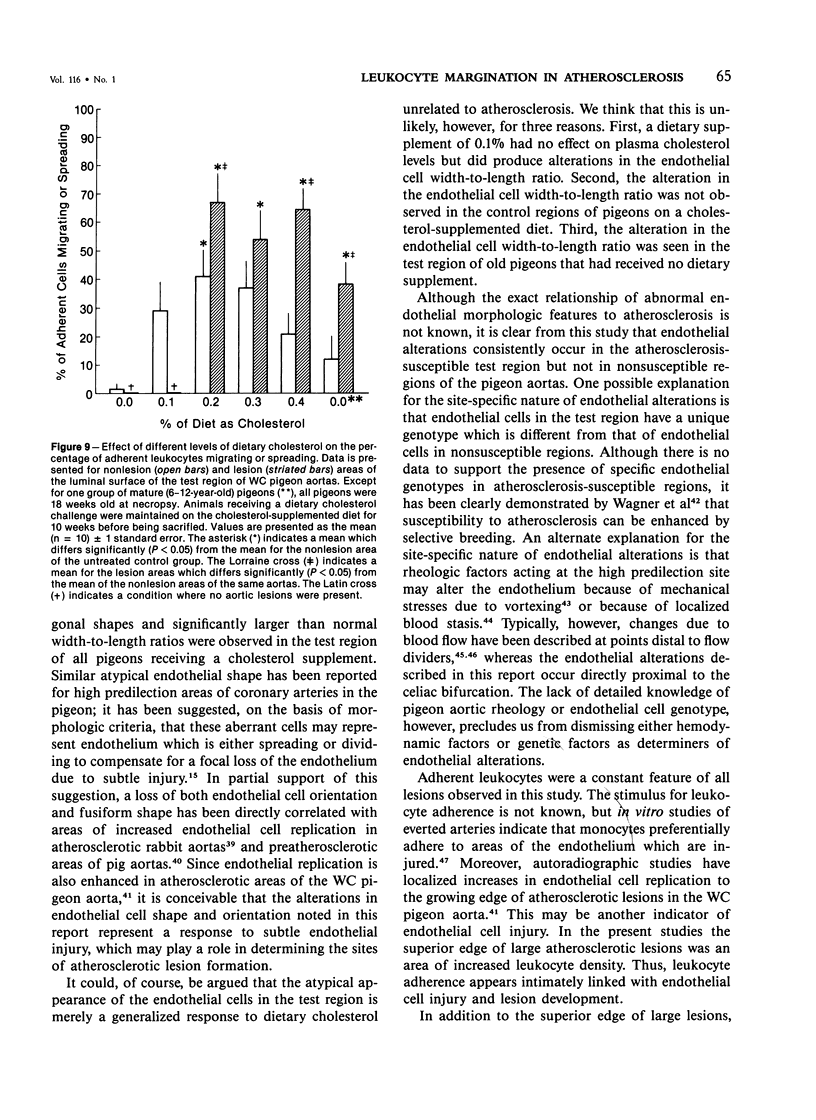
The addition of 0.2% or more cholesterol to the diet of young White Carneau pigeons produced atherosclerotic lesions within 10 weeks in a 2-sq mm area of the lower thoracic aorta. Concurrent with lesion development, a shift in the shape of endothelial cells from fusiform to polygonal was noted. This shift changed the ratio of endothelial cell width to length from 0.34 in pigeons receiving a control diet to 0.50 in pigeons receiving cholesterol. In contrast, endothelial cells in an atherosclerosis-resistant region 6 cm superior to the test region retained their fusiform shape despite the addition of cholesterol to the pigeon's diet. Cholesterol diets also increased adherence of leukocytes to the luminal surface of the aorta. This was most prevalent at the edge of large lesions (2430 +/- 180 cells/sq mm) and over small lesions (2240 +/- 150 cells/sq mm). Leukocyte adherence was also increased in the central region of large lesions (960 +/- 140 cells/sq mm). In addition, leukocyte activation, as evidence by crawling or spreading cells, was increased almost twofold over small lesions and the edge of large lesions when compared with adherent cells over nonlesion areas.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B. Detection of macrophages in atherosclerotic lesions with cytochrome oxidase. Br J Exp Pathol. 1976 Feb;57(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- Bell F. P., Adamson I. L., Schwartz C. J. Aortic endothelial permeability to albumin: focal and regional patterns of uptake and transmural distribution of 131I-albumin in the young pig. Exp Mol Pathol. 1974 Feb;20(1):57–68. doi: 10.1016/0014-4800(74)90043-4. [DOI] [PubMed] [Google Scholar]
- Bell F. P., Gallus A. S., Schwartz C. J. Focal and regional patterns of uptake and the transmural distribution of 131-I-fibrinogen in the pig aorta in vivo. Exp Mol Pathol. 1974 Apr;20(2):281–292. doi: 10.1016/0014-4800(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Björkerud S. Reaction of the aortic wall of the rabbit after superficial, longitudinal, mechanical trauma. Virchows Arch A Pathol Pathol Anat. 1969;347(3):197–210. doi: 10.1007/BF00543107. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. B. ATHEROSCLEROSIS--SPONTANEOUS AND INDUCED. Adv Lipid Res. 1963;1:211–252. [PubMed] [Google Scholar]
- CLARKSON T. B., LOFLAND H. B. Effects of cholesterol-fat diets on pigeons susceptible and resistant to atherosclerosis. Circ Res. 1961 Jan;9:106–109. doi: 10.1161/01.res.9.1.106. [DOI] [PubMed] [Google Scholar]
- CLARKSON T. B., PRICHARD R. W., NETSKY M. G., LOFLAND H. B. Atherosclerosis in pigeons; its spontaneous occurrence and resemblance to human atherosclerosis. AMA Arch Pathol. 1959 Aug;68(2):143–147. [PubMed] [Google Scholar]
- Caplan B. A., Schwartz C. J. Increased endothelial cell turnover in areas of in vivo Evans Blue uptake in the pig aorta. Atherosclerosis. 1973 May-Jun;17(3):401–417. doi: 10.1016/0021-9150(73)90031-2. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., King J. S., Jr, Lofland H. B., Feldner M. A., Bullock B. C. Pathologic characteristics and composition of diet-aggravated atherosclerotic plaques during "regression". Exp Mol Pathol. 1973 Dec;19(3):267–283. doi: 10.1016/0014-4800(73)90059-2. [DOI] [PubMed] [Google Scholar]
- Clarkson T. B., Lofland H. B., Jr Response of pigeon arteries to cholesterol as a function of time. Arch Pathol. 1967 Nov;84(5):513–516. [PubMed] [Google Scholar]
- Clarkson T. B., Middleton C. C., Prichard R. W., Lofland H. B. Naturally-occurring atherosclerosis in birds. Ann N Y Acad Sci. 1965 Sep 8;127(1):685–693. doi: 10.1111/j.1749-6632.1965.tb49435.x. [DOI] [PubMed] [Google Scholar]
- Cooke P. H., Smith S. C. Smooth muscle cells: the source of foam cells in atherosclerotic white Carneau pigeons. Exp Mol Pathol. 1968 Apr;8(2):171–189. doi: 10.1016/0014-4800(68)90014-2. [DOI] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Fox J. A., Hugh A. E. Localization of atheroma: a theory based on boundary layer separation. Br Heart J. 1966 May;28(3):388–399. doi: 10.1136/hrt.28.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaton E., Wolman M. The role of smooth muscle cells and hematogenous macrophages in atheroma. J Pathol. 1977 Oct;123(2):123–128. doi: 10.1002/path.1711230208. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977 Nov;89(2):313–334. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol. 1981 May;103(2):191–200. [PMC free article] [PubMed] [Google Scholar]
- Glagov S. Mechanical stresses on vessels and the non-uniform distribution of atherosclerosis. Med Clin North Am. 1973 Jan;57(1):63–77. doi: 10.1016/s0025-7125(16)32302-1. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Björnheden T., Bylock A., Bondjers G. Fc-dependent binding of monocytes to areas with endothelial injury in the rabbit aorta. Exp Mol Pathol. 1981 Jun;34(3):264–280. doi: 10.1016/0014-4800(81)90044-7. [DOI] [PubMed] [Google Scholar]
- Hassler O. The origin of the cells constituting arterial intima thickening. An experimental autoradiographic study with the use of H3-thymidine. Lab Invest. 1970 Apr;22(4):286–293. [PubMed] [Google Scholar]
- Helin P., Lorenzen I., Garbarsch C., Matthiessen M. E. Arteriosclerosis in rabbit aorta induced by mechanical dilatation. Biochemical and morphological studies. Atherosclerosis. 1971 May-Jun;13(3):319–331. doi: 10.1016/0021-9150(71)90075-x. [DOI] [PubMed] [Google Scholar]
- Jerome W. G., Lewis J. C., Taylor R. G., White M. S. Concurrent endothelial cell turnover and leukocyte margination in early atherosclerosis. Scan Electron Microsc. 1983;(Pt 3):1453–1459. [PubMed] [Google Scholar]
- Lee K. T., Lee K. J., Lee S. K., Imai H., O'Neal R. M. Poorly differentiated subendothelial cells in swine aortas. Exp Mol Pathol. 1970 Aug;13(1):118–129. doi: 10.1016/0014-4800(70)90089-4. [DOI] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. H., Fry D. L. Canine hyperlipoproteinemia and atherosclerosis. Accumulation of lipid by aortic medial cells in vivo and in vitro. Am J Pathol. 1977 Apr;87(1):205–226. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R. Immunologic arterial injury in atherogenesis. Ann N Y Acad Sci. 1976;275:210–227. doi: 10.1111/j.1749-6632.1976.tb43355.x. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- PRICHARD R. W., CLARKSON T. B., GOODMAN H. O., LOFLAND H. B. AORTIC ATHEROSCLEROSIS IN PIGEONS AND ITS COMPLICATIONS. Arch Pathol. 1964 Mar;77:244–257. [PubMed] [Google Scholar]
- Reidy M. A., Bowyer D. E. Scanning electron microscopy of arteries. The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis. 1977 Feb;26(2):181–194. doi: 10.1016/0021-9150(77)90101-0. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Wight T. N., Smith S. C., Brannigan D. Spontaneous atherosclerosis in pigeons. A model system for studying metabolic parameters associated with atherogenesis. Am J Pathol. 1972 Apr;67(1):1–22. [PMC free article] [PubMed] [Google Scholar]
- Schwartz S. M., Gajdusek C. M., Selden S. C., 3rd Vascular wall growth control: the role of the endothelium. Arteriosclerosis. 1981 Mar-Apr;1(2):107–126. doi: 10.1161/01.atv.1.2.107. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M. Role of endothelial integrity in atherosclerosis. Artery. 1980;8(4):305–314. [PubMed] [Google Scholar]
- Silkworth J. B., McLean B., Stehbens W. E. The effect of hypercholesterolemia on aortic endothelium studied en face. Atherosclerosis. 1975 Nov-Dec;22(3):335–348. doi: 10.1016/0021-9150(75)90015-5. [DOI] [PubMed] [Google Scholar]
- Somer J. B., Schwartz C. J. Focal 3 H-cholesterol uptake in the pig aorta. Atherosclerosis. 1971 May-Jun;13(3):293–304. doi: 10.1016/0021-9150(71)90073-6. [DOI] [PubMed] [Google Scholar]
- St Clair R. W. Metabolic changes in the arterial wall associated with atherosclerosis in the pigeon. Fed Proc. 1983 May 15;42(8):2480–2485. [PubMed] [Google Scholar]
- Stary H. C., Strong J. P. The fine structure of nonatherosclerotic intimal thickening, of developing, and of regressing atherosclerotic lesions at the bifurcation of the left coronary artery. Adv Exp Med Biol. 1976;67(00):89–108. doi: 10.1007/978-1-4614-4618-7_5. [DOI] [PubMed] [Google Scholar]
- Tesar G. E., Kottke B. A. Location and sequence of atherosclerotic plaque formation in white Carneau and show racer pigeons: reevaluation and redefinition. Arch Pathol Lab Med. 1978 Nov;102(11):581–586. [PubMed] [Google Scholar]
- Wagner W. D., Clarkson T. B., Feldner M. A., Prichard R. W. The development of pigeon strains with selected atherosclerosis characteristics. Exp Mol Pathol. 1973 Dec;19(3):304–319. doi: 10.1016/0014-4800(73)90062-2. [DOI] [PubMed] [Google Scholar]
- Weber G., Fabbrini P., Resi L. On the presence of a concanavalin-A reactive coat over the endothelial aortic surface and its modifications during early experimental cholesterol atherogenesis in rabbits. Virchows Arch A Pathol Pathol Anat. 1973 Jun 29;359(4):299–307. doi: 10.1007/BF00548601. [DOI] [PubMed] [Google Scholar]
- Weibel E. R., Kistler G. S., Scherle W. F. Practical stereological methods for morphometric cytology. J Cell Biol. 1966 Jul;30(1):23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. S., Lewis J. C., Taylor R. G. Surface characteristics of adherent avian cells; extrapolation of in vitro characteristics to cells on atherosclerotic lesions. Artery. 1982;11(1):33–46. [PubMed] [Google Scholar]