Abstract
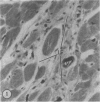
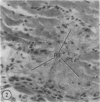
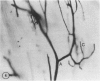
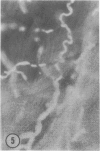
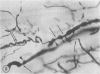
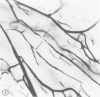
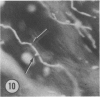
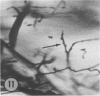
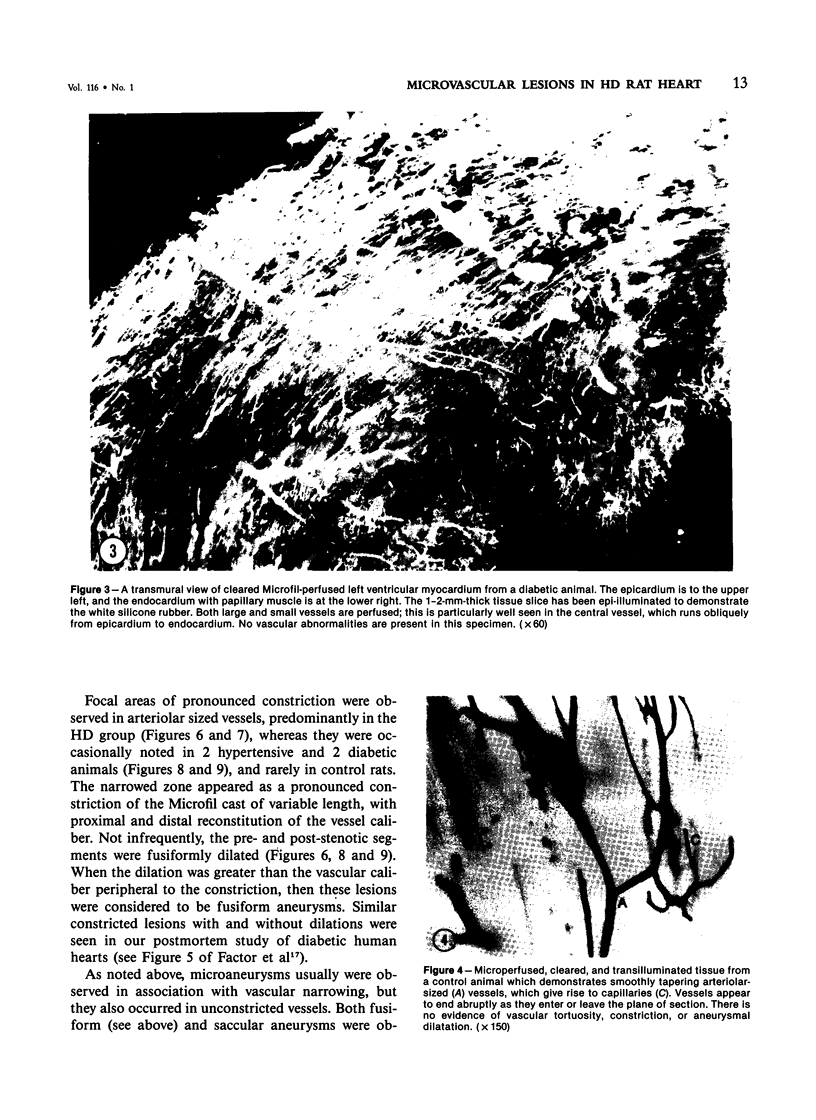
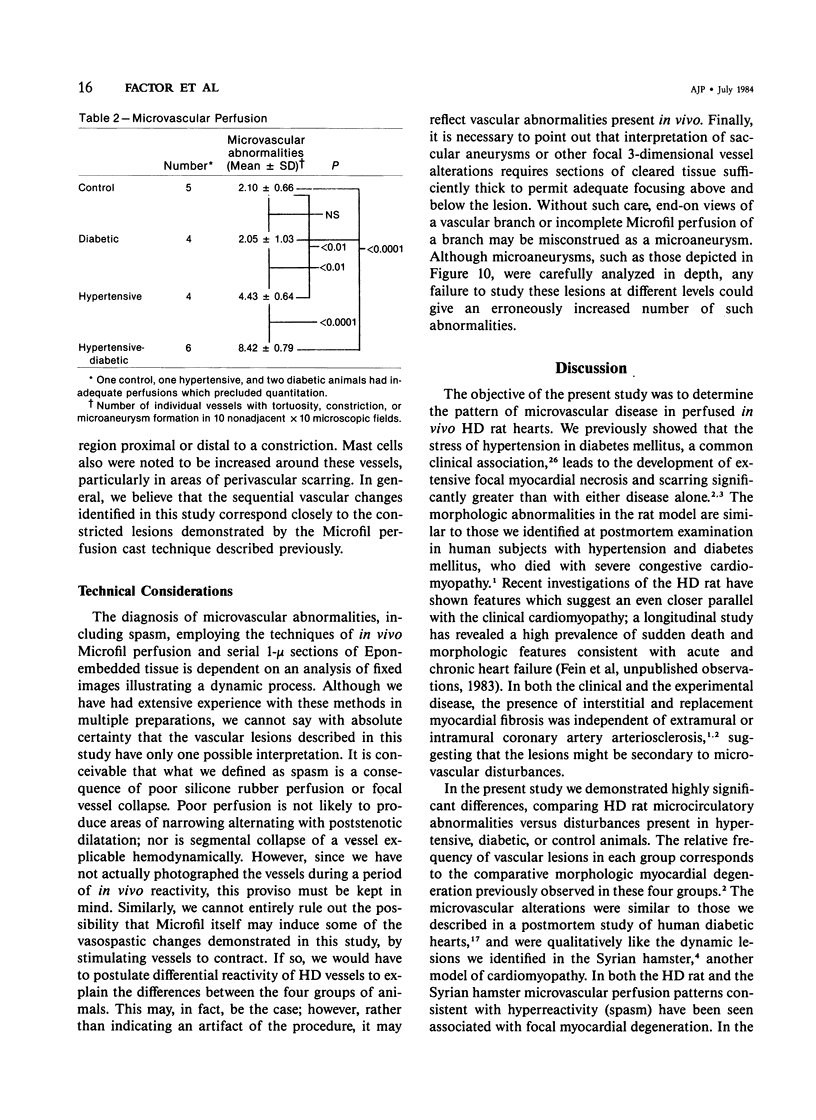
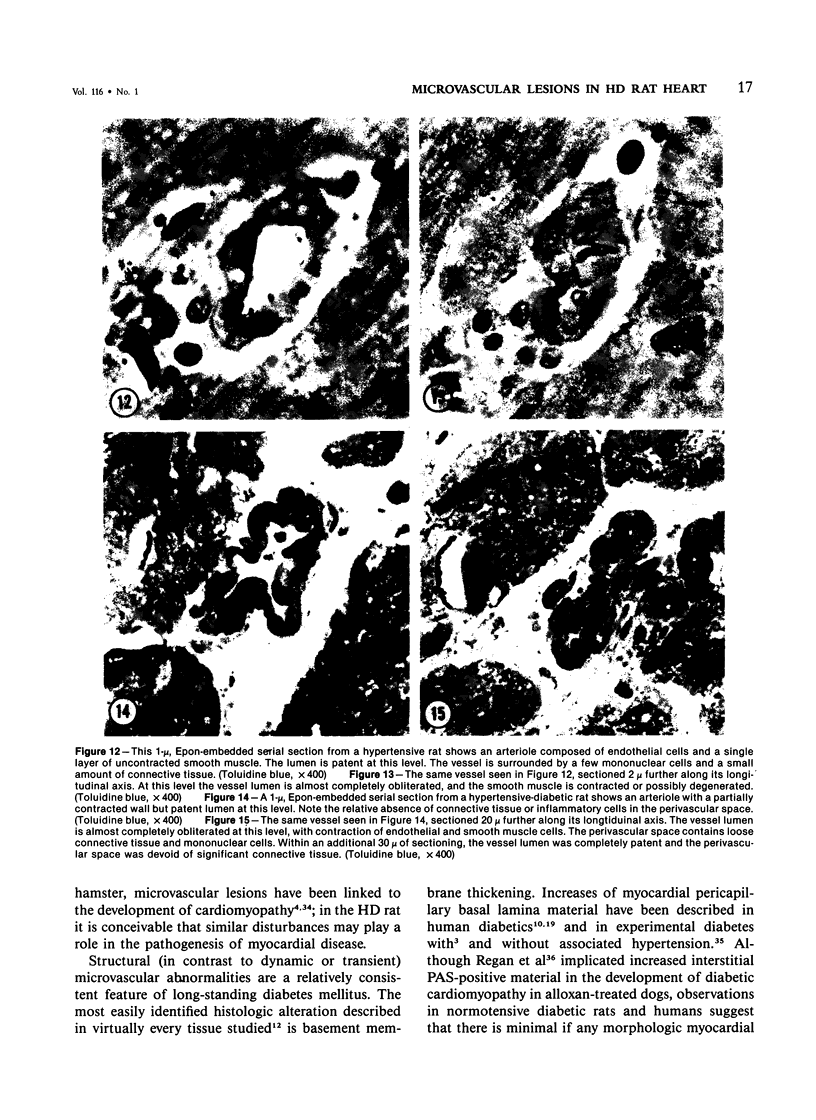
The authors have continued their investigation of the hypertensive-diabetic (HD) rat by evaluating changes in the myocardial microvasculature in this model. Perfusion of HD animals in vivo with a silicone rubber solution revealed numerous areas of microvascular tortuosity, focal constrictions, and microaneurysm formation. These alterations were present to a lesser extent in normoglycemic hypertensive (H) rats, and were distinctly rare in normotensive diabetic rats and unaffected control animals. Quantitation of these vascular lesions revealed highly significant differences between HD animals and the other three groups, with hypertensive rats intermediate between HD rats and diabetic control rats. Areas of pronounced arteriolar constriction were also identified in the HD and H animals with the use of serial sections of Epon-embedded myocardium. It is believed that these lesions represent dynamic changes in the microcirculation, which may cause segmental reperfusion injury to the myocardium, leading to focal replacement fibrosis. Interstitial scarring may result from increased leakiness of small vessels exacerbated by the combined disease. The authors propose that the additive effects of hypertension and diabetes mellitus on the myocardial microcirculation may be a primary cause of cardiomyopathy in this model of human disease.
Full text
PDF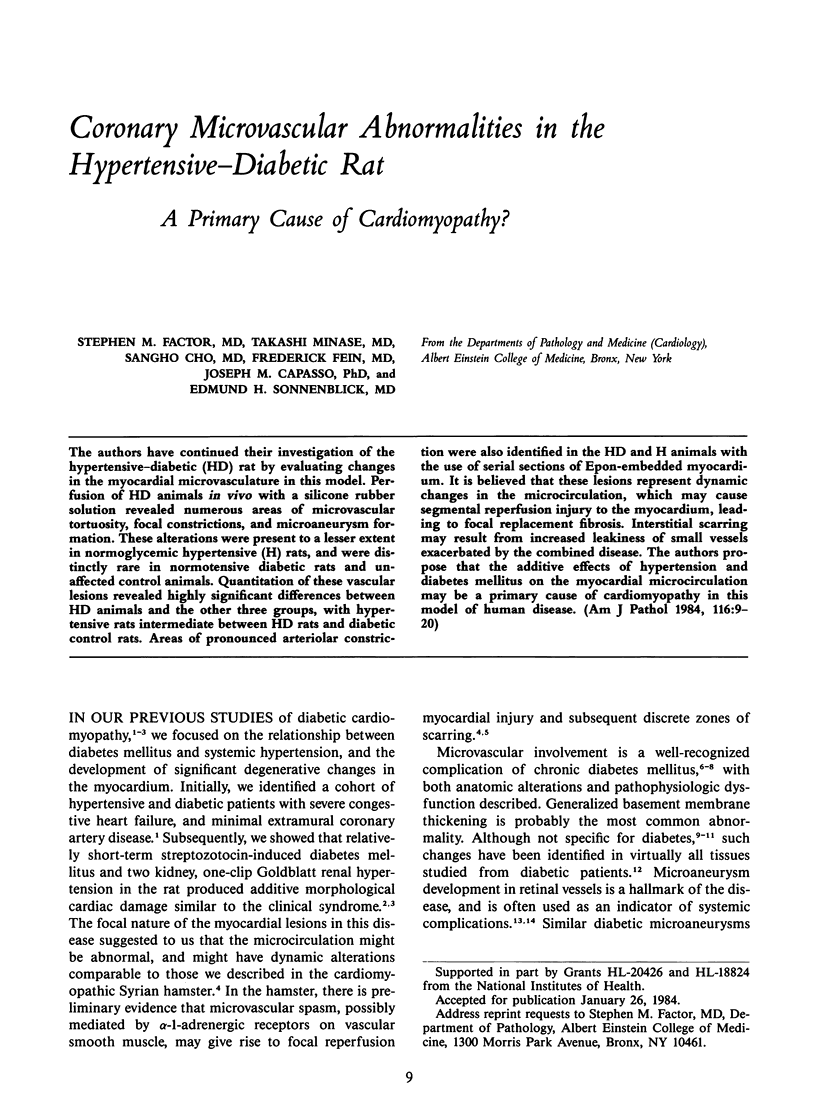








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BYROM F. B. The pathogenesis of hypertensive encephalopathy and its relation to the malignant phase of hypertension; experimental evidence from the hypertensive rat. Lancet. 1954 Jul 31;267(6831):201–211. doi: 10.1016/s0140-6736(54)91821-8. [DOI] [PubMed] [Google Scholar]
- Bhan R. D., Giacomelli F., Wiener J. Adrenoreceptor blockade in angiotensin-induced hypertension: effect on rat coronary arteries and myocardium. Am J Pathol. 1982 Jul;108(1):60–71. [PMC free article] [PubMed] [Google Scholar]
- Bloodworth J. M., Jr A re-evaluation of diabetic glomerulosclerosis 50 years after the discovery of insulin. Hum Pathol. 1978 Jul;9(4):439–453. doi: 10.1016/s0046-8177(78)80029-x. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G., Hankins K. D. Early arteriolar and capillary changes in streptozotocin-induced diabetic rats and intraperitoneal hyperglycaemic rats. Diabetologia. 1982 May;22(5):344–348. doi: 10.1007/BF00253579. [DOI] [PubMed] [Google Scholar]
- Bohlen H. G., Niggl B. A. Arteriolar anatomical and functional abnormalities in juvenile mice with genetic or streptozotocin-induced diabetes mellitus. Circ Res. 1979 Sep;45(3):390–396. doi: 10.1161/01.res.45.3.390. [DOI] [PubMed] [Google Scholar]
- COGAN D. G., TOUSSAINT D., KUWABARA T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 1961 Sep;66:366–378. doi: 10.1001/archopht.1961.00960010368014. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R. Diabetes and hypertensive vascular disease. Mechanisms and treatment. Am J Cardiol. 1973 Sep 20;32(4):592–606. doi: 10.1016/s0002-9149(73)80051-7. [DOI] [PubMed] [Google Scholar]
- Eng C., Cho S., Factor S. M., Sonnenblick E. H., Kirk E. S. Myocardial micronecrosis produced by microsphere embolization. Role of an alpha-adrenergic tonic influence on the coronary microcirculation. Circ Res. 1984 Jan;54(1):74–82. doi: 10.1161/01.res.54.1.74. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Bhan R., Minase T., Wolinsky H., Sonnenblick E. H. Hypertensive-diabetic cardiomyopathy in the rat: an experimental model of human disease. Am J Pathol. 1981 Feb;102(2):219–228. [PMC free article] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Bhan R., Wolinsky H., Sonnenblick E. H. Hypertensive diabetic cardiomyopathy in the rat: ultrastructural features. Virchows Arch A Pathol Anat Histopathol. 1983;398(3):305–317. doi: 10.1007/BF00583587. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Cho S., Dominitz R., Sonnenblick E. H. Microvascular spasm in the cardiomyopathic Syrian hamster: a preventable cause of focal myocardial necrosis. Circulation. 1982 Aug;66(2):342–354. doi: 10.1161/01.cir.66.2.342. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Minase T., Sonnenblick E. H. Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am Heart J. 1980 Apr;99(4):446–458. doi: 10.1016/0002-8703(80)90379-8. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Okun E. M., Minase T. Capillary microaneurysms in the human diabetic heart. N Engl J Med. 1980 Feb 14;302(7):384–388. doi: 10.1056/NEJM198002143020706. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Okun E. M., Minase T., Kirk E. S. The microcirculation of the human heart: end-capillary loops with discrete perfusion fields. Circulation. 1982 Dec;66(6):1241–1248. doi: 10.1161/01.cir.66.6.1241. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Sonnenblick E. H. Hypothesis: is congestive cardiomyopathy caused by a hyperreactive myocardial microcirculation (microvascular spasm)? Am J Cardiol. 1982 Nov;50(5):1149–1152. doi: 10.1016/0002-9149(82)90435-0. [DOI] [PubMed] [Google Scholar]
- Fischer V. W., Barner H. B., LaRose L. S. Quadriceps and myocardial capillary basal laminae. Their comparison in diabetic patients. Arch Pathol Lab Med. 1982 Jul;106(7):336–341. [PubMed] [Google Scholar]
- Fischer V. W., Leskiw M. L., Barner H. B. Myocardial structure and capillary basal laminar thickness in experimentally diabetic rats. Exp Mol Pathol. 1981 Oct;35(2):244–256. doi: 10.1016/0014-4800(81)90064-2. [DOI] [PubMed] [Google Scholar]
- GIESE J. ACUTE HYPERTENSIVE VASCULAR DISEASE. 2. STUDIES ON VASCULAR REACTION PATTERNS AND PERMEABILITY CHANGES BY MEANS OF VITAL MICROSCOPY AND COLLOIDAL TRACER TECHNIQUE. Acta Pathol Microbiol Scand. 1964;62:497–515. doi: 10.1111/apm.1964.62.4.497. [DOI] [PubMed] [Google Scholar]
- Hill G. S., Heptinstall R. H. Steroid-induced hypertension in the rat. A microangiographic and histologic study on the pathogenesis of hypertensive vascular and glomerular lesions. Am J Pathol. 1968 Jan;52(1):1–40. [PMC free article] [PubMed] [Google Scholar]
- Jordan S. W., Perley M. J. Microangiopathy in diabetes mellitus and aging. Arch Pathol. 1972 Mar;93(3):261–265. [PubMed] [Google Scholar]
- Joyner W. L., Mayhan W. G., Johnson R. L., Phares C. K. Microvascular alterations develop in Syrian hamsters after the induction of diabetes mellitus by streptozotocin. Diabetes. 1981 Feb;30(2):93–100. doi: 10.2337/diab.30.2.93. [DOI] [PubMed] [Google Scholar]
- Knowler W. C., Bennett P. H., Ballintine E. J. Increased incidence of retinopathy in diabetics with elevated blood pressure. A six-year follow-up study in Pima Indians. N Engl J Med. 1980 Mar 20;302(12):645–650. doi: 10.1056/NEJM198003203021201. [DOI] [PubMed] [Google Scholar]
- McMillan D. E. Deterioration of the microcirculation in diabetes. Diabetes. 1975 Oct;24(10):944–957. doi: 10.2337/diab.24.10.944. [DOI] [PubMed] [Google Scholar]
- McMillan D. E. Diabetic angiopathy--its lessons in vascular physiology. Am Heart J. 1978 Sep;96(3):401–406. doi: 10.1016/0002-8703(78)90053-4. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. Long-term antihypertensive treatment (over six years) inhibiting the progression of diabetic nephropathy. Acta Endocrinol Suppl (Copenh) 1981;242:31–32. [PubMed] [Google Scholar]
- Mueller S. M., Mueller T. M., Ertel P. J. Sympathetic and vascular dysfunction in early experimental juvenile diabetes mellitus. Am J Physiol. 1982 Aug;243(2):H139–H144. doi: 10.1152/ajpheart.1982.243.2.H139. [DOI] [PubMed] [Google Scholar]
- Nakamoto Y., Takazakura E., Hayakawa H., Kawai K., Dohi K., Fujioka M., Kida H., Hattori N., Takeuchi J. Intrarenal microaneurysms in diabetic nephropathy. Lab Invest. 1980 Apr;42(4):433–439. [PubMed] [Google Scholar]
- Okun E. M., Factor S. M., Kirk E. S. End-capillary loops in the heart: an explanation for discrete myocardial infarctions without border zones. Science. 1979 Nov 2;206(4418):565–567. doi: 10.1126/science.493960. [DOI] [PubMed] [Google Scholar]
- P ARVING H. H., Rasmussen S. M. Transcapillary escape rate of albumin and plasma volume in short- and long-term juvenile diabetics. Scand J Clin Lab Invest. 1973 Aug;32(1):81–87. doi: 10.3109/00365517309082454. [DOI] [PubMed] [Google Scholar]
- Parving H. H., Andersen A. R., Smidt U. M., Oxenbøll B., Edsberg B., Christiansen J. S. Diabetic nephropathy and arterial hypertension. Diabetologia. 1983 Jan;24(1):10–12. doi: 10.1007/BF00275939. [DOI] [PubMed] [Google Scholar]
- Pfaffman M. A., Hilman R., Darby A. Contractile and relaxing activity of arterial smooth muscle from streptozotocin-diabetic rats. Res Commun Chem Pathol Pharmacol. 1980 Nov;30(2):283–299. [PubMed] [Google Scholar]
- Prewitt R. L., Chen I. I., Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am J Physiol. 1982 Aug;243(2):H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- Rand L. I. Recent advances in diabetic retinopathy. Am J Med. 1981 Mar;70(3):595–602. doi: 10.1016/0002-9343(81)90581-7. [DOI] [PubMed] [Google Scholar]
- Silver M. D., Huckell V. F., Lorber M. Basement membranes of small cardiac vessels in patients with diabetes and myxoedema: preliminary observations. Pathology. 1977 Jul;9(3):213–220. doi: 10.3109/00313027709084812. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Kilo C. Basement-membrane thickening and diabetic microangiopathy. Diabetes. 1976;25(2 Suppl):925–927. [PubMed] [Google Scholar]
- Williamson J. R., Kilo C. Vascular complications in diabetes mellitus. N Engl J Med. 1980 Feb 14;302(7):399–400. doi: 10.1056/NEJM198002143020710. [DOI] [PubMed] [Google Scholar]
- Wolinsky H. Effects of hypertension and its reversal on the thoracic aorta of male and female rats. Morphological and chemical studies. Circ Res. 1971 Jun;28(6):622–637. doi: 10.1161/01.res.28.6.622. [DOI] [PubMed] [Google Scholar]