Abstract
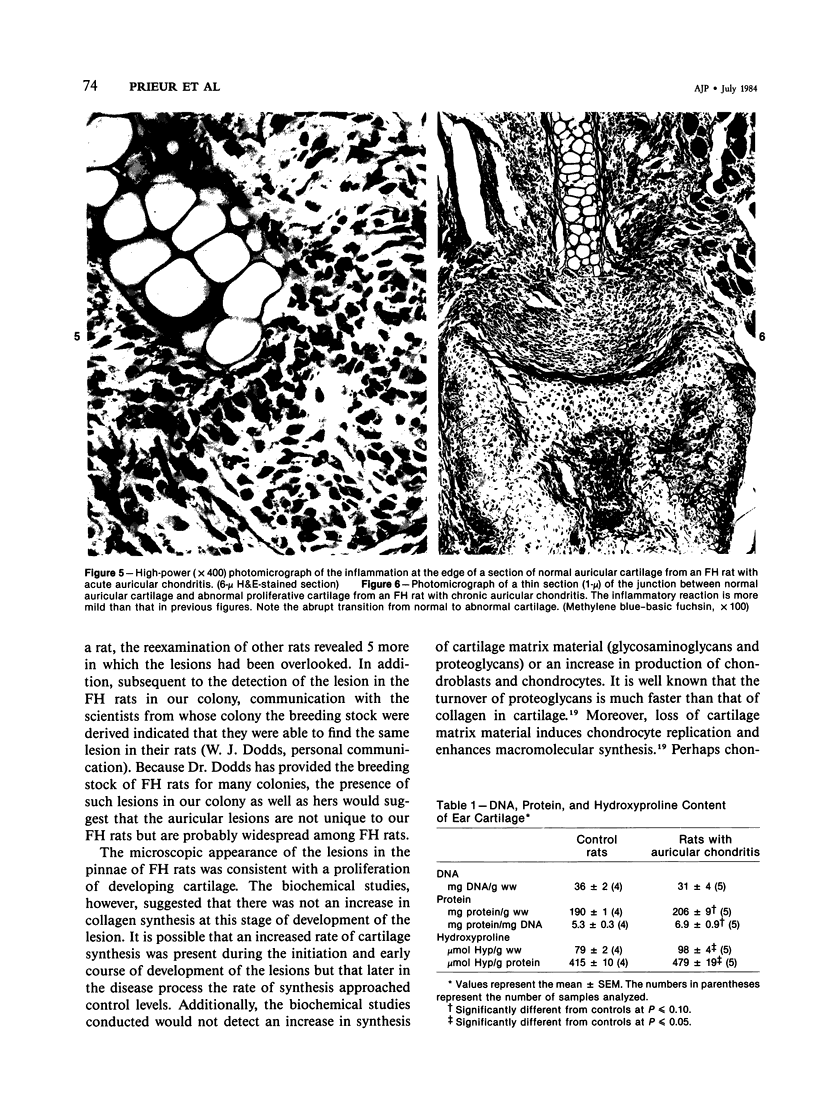
A spontaneous apparently unique auricular chondritis in the pinna of fawn-hooded rats is described. The chondritis was bilateral, with adult onset, and resulted in a marked thickening of the auricular cartilage. Microscopically, islands of proliferative cartilage were present, and at the margins between the normal cartilage and the thickened abnormal cartilage a marked cellular inflammatory response was present. The condition was familial in rats of the fawn-hooded strain and appeared to be unrelated to the platelet storage pool deficiency in this strain of rats. Biochemically no increased synthesis of pinna cartilage was detected. No histopathologic lesions were detected in other cartilaginous tissues of affected rats. The lesions in the pinna bore a striking resemblance to those induced in rats by immunization with Type II collagen. The spontaneous condition described herein may, therefore, represent a unique model of relapsing polychondritis of human beings, a disease with auricular chondritis associated with antibodies to Type II collagen.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett H. S., Wyrick A. D., Lee S. W., McNeil J. H. Science and art in preparing tissues embedded in plastic for light microscopy, with special reference to glycol methacrylate, glass knives and simple stains. Stain Technol. 1976 Mar;51(2):71–97. doi: 10.3109/10520297609116677. [DOI] [PubMed] [Google Scholar]
- Clague R. B., Morgan K., Shaw M. J., Holt P. J. Native type II collagen-induced arthritis in the rat. II. Relationship between the humoral immune response to native type II collagen and arthritis. J Rheumatol. 1980 Nov-Dec;7(6):775–782. [PubMed] [Google Scholar]
- Clayton R. N., Hoffenberg R. Relapsing polychondritis: an autoimmune disease? Br Med J. 1978 Oct 7;2(6143):999–1000. doi: 10.1136/bmj.2.6143.999-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counts D. F., Knighten P., Hegreberg G. Biochemical changes in the skin of mink with Ehlers-Danlos syndrome: increased collagen biosynthesis in the dermis of affected mink. J Invest Dermatol. 1977 Dec;69(6):521–526. doi: 10.1111/1523-1747.ep12687965. [DOI] [PubMed] [Google Scholar]
- Courtenay J. S., Dallman M. J., Dayan A. D., Martin A., Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980 Feb 14;283(5748):666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- Cremer M. A., Pitcock J. A., Stuart J. M., Kang A. H., Townes A. S. Auricular chondritis in rats. An experimental model of relapsing polychondritis induced with type II collagen. J Exp Med. 1981 Aug 1;154(2):535–540. doi: 10.1084/jem.154.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutroneo K. R., Counts D. F. Anti-inflammatory steroids and collagen metabolism: glucocorticoid-mediated alterations of prolyl hydroxylase activity and collagen synthesis. Mol Pharmacol. 1975 Sep;11(5):632–639. [PubMed] [Google Scholar]
- Foidart J. M., Abe S., Martin G. R., Zizic T. M., Barnett E. V., Lawley T. J., Katz S. I. Antibodies to type II collagen in relapsing polychondritis. N Engl J Med. 1978 Nov 30;299(22):1203–1207. doi: 10.1056/NEJM197811302992202. [DOI] [PubMed] [Google Scholar]
- Hughes R. A., Berry C. L., Seifert M., Lessof M. H. Relapsing polychondritis. Three cases with a clinico-pathological study and literature review. Q J Med. 1972 Jul;41(163):363–380. [PubMed] [Google Scholar]
- Kelleher J. C., Sullivan J. G., Baibak G. J., Dean R. K. The wrestler's ear. Plast Reconstr Surg. 1967 Dec;40(6):540–546. doi: 10.1097/00006534-196740060-00005. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Karnovsky M. J. Focal glomerular sclerosis in the fawn-hooded rat. Am J Pathol. 1978 Sep;92(3):637–652. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaVail M. M. Fawn-hooded rats, the fawn mutation and interaction of pink-eyed and red-eyed dilution genes. J Hered. 1981 Jul-Aug;72(4):286–287. doi: 10.1093/oxfordjournals.jhered.a109500. [DOI] [PubMed] [Google Scholar]
- McCune W. J., Schiller A. L., Dynesius-Trentham R. A., Trentham D. E. Type II collagen-induced auricular chondritis. Arthritis Rheum. 1982 Mar;25(3):266–273. doi: 10.1002/art.1780250304. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Isolation and characterization of the cyanogen bromide peptides from the l(II) chain of chick cartilage collagen. Biochemistry. 1971 Aug 3;10(16):3030–3035. doi: 10.1021/bi00792a007. [DOI] [PubMed] [Google Scholar]
- Morgan K., Clague R. B., Shaw M. J., Holt P. J. Native type II collagen-induced arthritis in the rat. I. Incidence and humoral response to collagen. Ann Rheum Dis. 1980 Jun;39(3):285–290. doi: 10.1136/ard.39.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray T. F., DeBarrows B. R., Prieur D. J., Meyers K. M. [3H]-imipramine binding sites in fawn-hooded rats. Neuropharmacology. 1983 Jun;22(6):781–784. doi: 10.1016/0028-3908(83)90103-x. [DOI] [PubMed] [Google Scholar]
- Pandya N. J. Experimental production of "cauliflower ear" in rabbits. Plast Reconstr Surg. 1973 Nov;52(5):534–537. doi: 10.1097/00006534-197311000-00010. [DOI] [PubMed] [Google Scholar]
- Raymond S. L., Dodds W. J. Characterization of the fawn-hooded rat as a model for hemostatic studies. Thromb Diath Haemorrh. 1975 Apr 30;33(2):361–369. [PubMed] [Google Scholar]
- Rudofsky U. H., Magro A. M. Spontaneous hypertension in fawn-hooded rats. Lab Anim Sci. 1982 Aug;32(4):389–391. [PubMed] [Google Scholar]
- Starkey J. R., Prieur D. J., Ristow S. S. Natural killer cell activity in fawn-hooded rats. Experientia. 1983 Mar 15;39(3):308–310. doi: 10.1007/BF01955321. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Kang A. H., Townes A. S. Collagen-induced arthritis in rats. Evaluation of early immunologic events. Arthritis Rheum. 1979 Dec;22(12):1344–1351. doi: 10.1002/art.1780221205. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Townes A. S., Kang A. H. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982 Jan 1;155(1):1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart J. M., Townes A. S., Kang A. H. The role of collagen autoimmunity in animal models and human diseases. J Invest Dermatol. 1982 Jul;79 (Suppl 1):121s–127s. doi: 10.1111/1523-1747.ep12545958. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., St Louis P. J. Passive and active calcium fluxes across plasma membranes. Prog Biophys Mol Biol. 1980;35(3):135–195. doi: 10.1016/0079-6107(80)90005-x. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., McCune W. J., Susman P., David J. R. Autoimmunity to collagen in adjuvant arthritis of rats. J Clin Invest. 1980 Nov;66(5):1109–1117. doi: 10.1172/JCI109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H., David J. R. Humoral and cellular sensitivity to collagen in type II collagen-induced arthritis in rats. J Clin Invest. 1978 Jan;61(1):89–96. doi: 10.1172/JCI108929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp B., Weiss H. J. Decreased ATP, ADP and serotonin in young platelets of fawn-hooded rats with storage pool disease. Thromb Diath Haemorrh. 1974 Dec 31;32(2-3):670–677. [PubMed] [Google Scholar]
- Tschopp T. B., Zucker M. B. Hereditary defect in platelet function in rats. Blood. 1972 Aug;40(2):217–226. [PubMed] [Google Scholar]









