Abstract
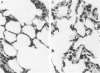
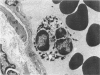
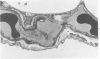
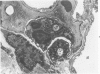
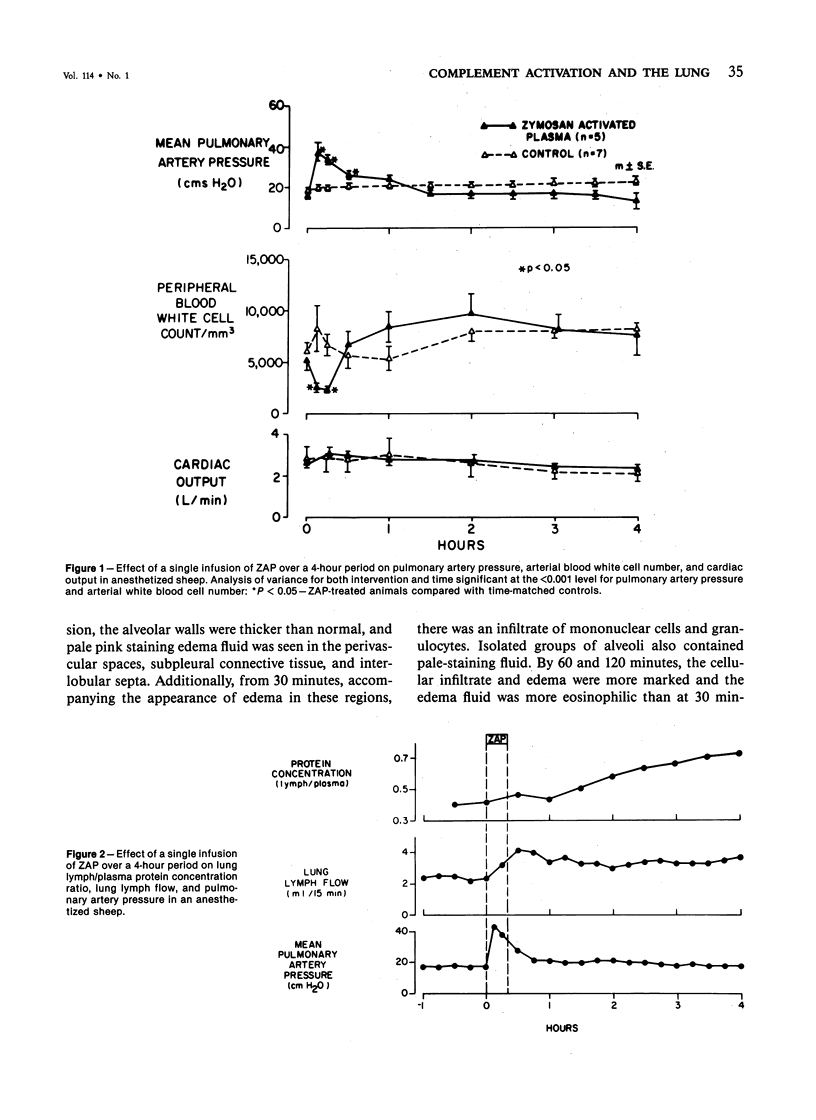
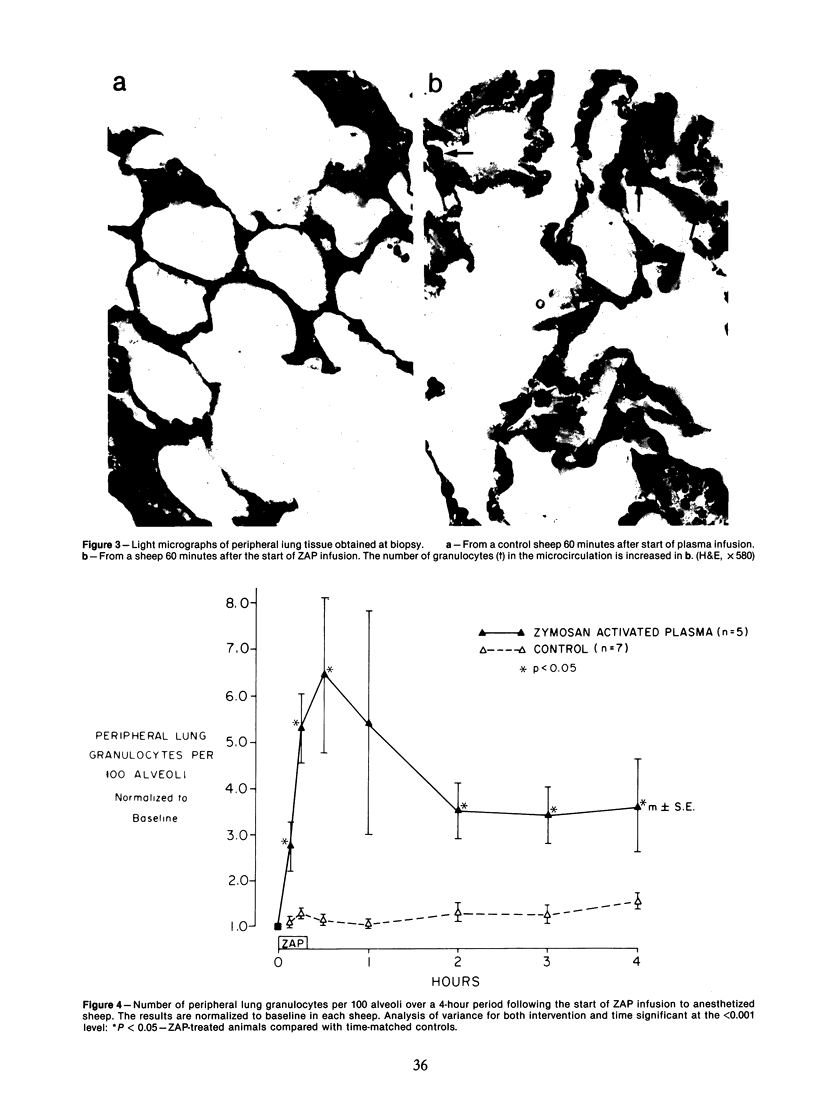
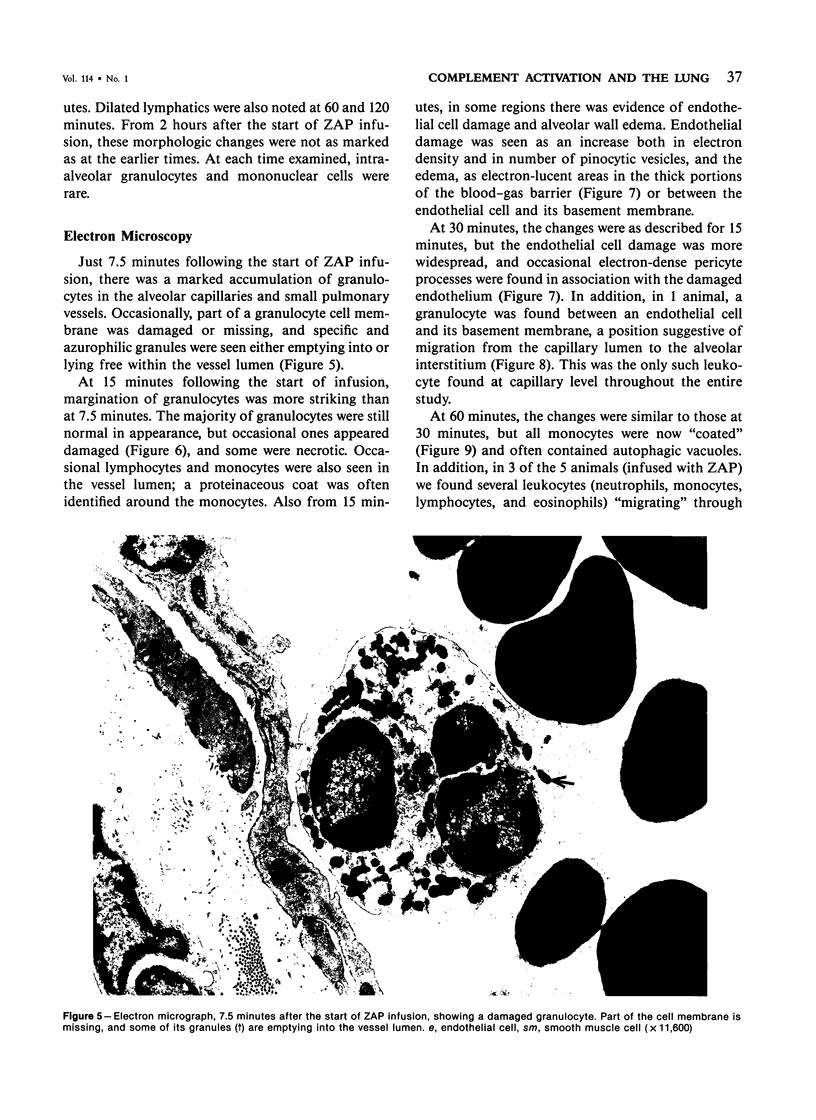
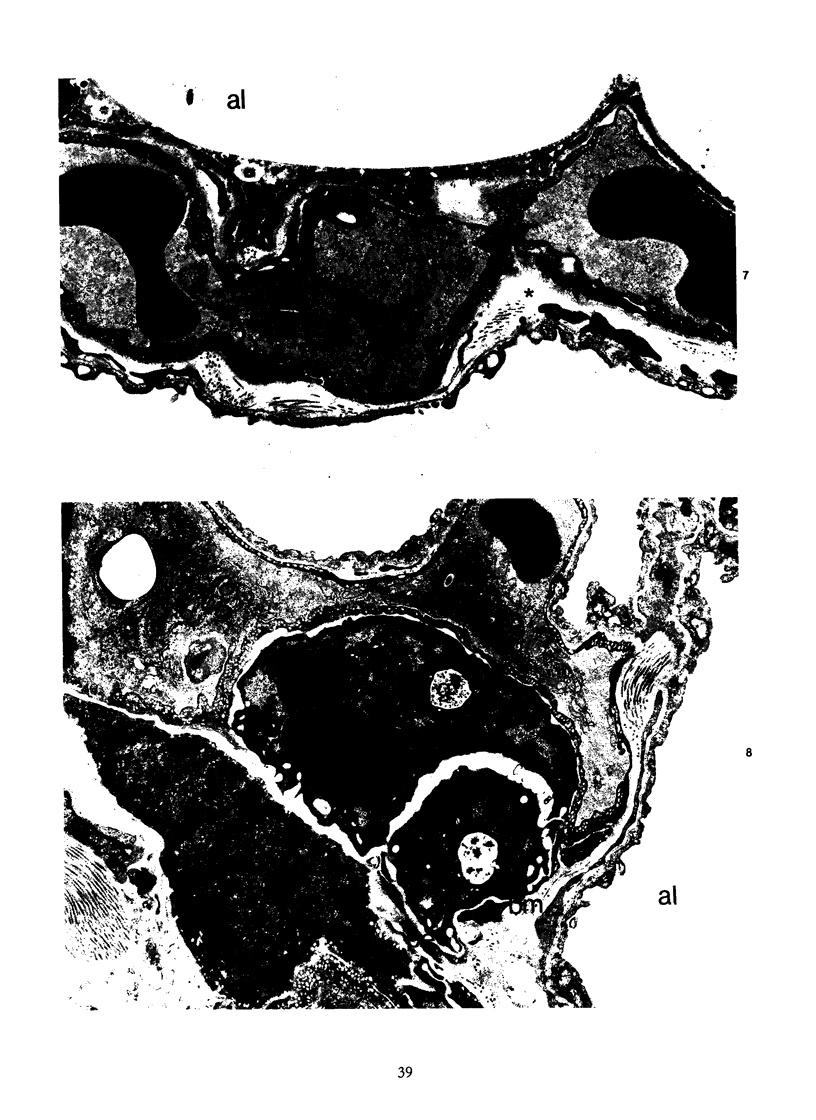
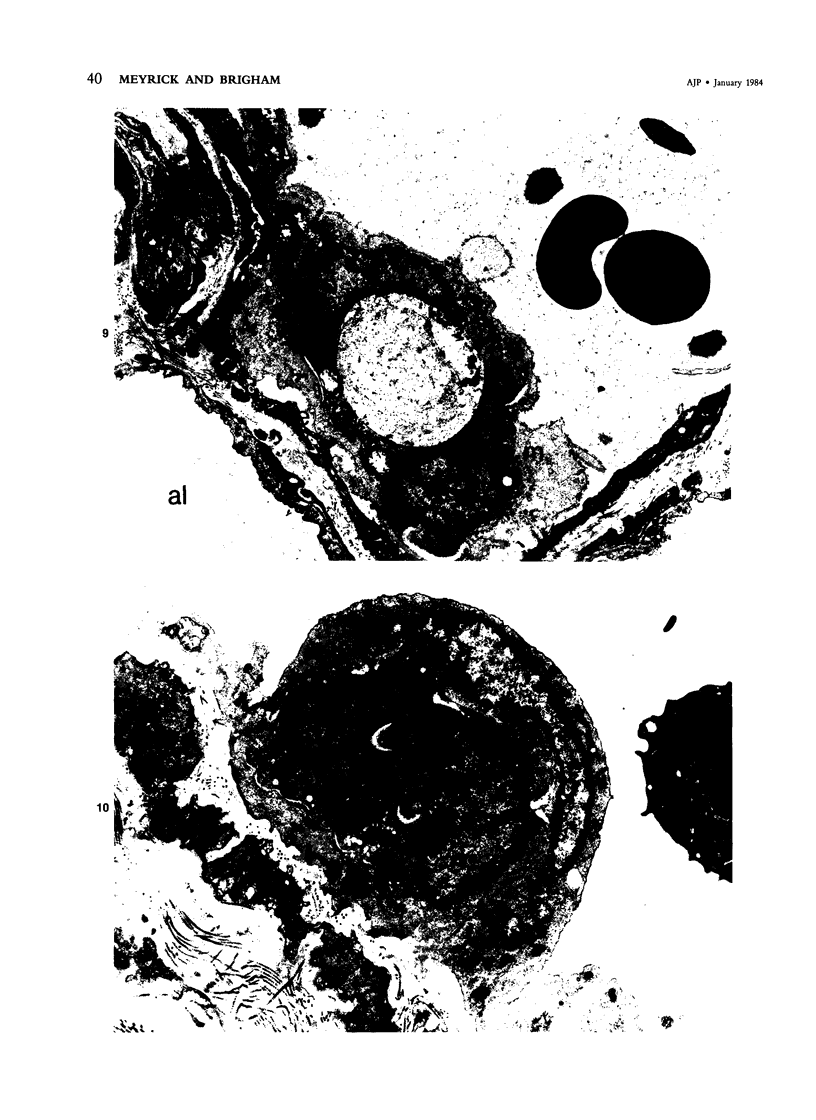
Activation of the complement cascade is one of the mechanisms through which endotoxin may cause acute lung damage. The structural and functional changes following infusion of complement-activated plasma are described. In five anesthetized open-chest sheep, the authors monitored pulmonary and systemic artery pressures for 1 hour before and for 4 hours following the start of zymosan-activated plasma (ZAP) infusion (2 ml/min over a 20-minute period). Cardiac output, blood gases, and the number of circulating white cells were also measured. In addition, we took lung biopsy tissue at baseline, 7.5, 15, 30, 60, 120, 180, and 240 minutes following the start of infusion. Lung lymph flow and protein concentration were also monitored in 2 sheep. Following ZAP infusion there was an early phase of leukopenia and marked pulmonary hypertension, followed by a phase characterized by a modest increase in the flow of protein-rich lung lymph. By light microscopy pulmonary sequestration of granulocytes was evident just 7.5 minutes following the start of ZAP infusion. Peripheral lung granulocytes increased threefold above control values by 7.5 minutes, increasing to sevenfold by 30 minutes. Electron-microscopic studies showed that some of the granulocytes were disrupted, and specific and azurophilic granules were seen in the lumen. By 15 minutes endothelial damage was apparent, and intravascular monocytes were surrounded by a proteinaceous coat. Edema accumulation and an infiltration of inflammatory cells in the lungs' connective tissue regions increased to 2 hours. From 2 hours, lung injury was less marked, and the number of peripheral lung granulocytes, fewer. Sequestration of granulocytes occurred with the onset of pulmonary hypertension and leukopenia, and was most marked when lung injury was most severe. Transient endothelial damage and edema preceded the physiologic changes interpreted as an increase in pulmonary vascular permeability. Although pulmonary sequestration of granulocytes was at least as great as that with endotoxemia, unlike endotoxemia, ZAP caused only transient endothelial injury and modest changes in vascular permeability.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balis J. U., Rappaport E. S., Gerber L., Fareed J., Buddingh F., Messmore H. L. A primate model for prolonged endotoxin shock. Blood-vascular reactions and effects of glucocorticoid treatment. Lab Invest. 1978 Apr;38(4):511–523. [PubMed] [Google Scholar]
- Bohs C. T., Fish J. C., Miller T. H., Traber D. L. Pulmonary vascular response to endotoxin in normal and lymphocyte depleted sheep. Circ Shock. 1979;6(1):13–21. [PubMed] [Google Scholar]
- Brigham K. L., Bowers R., Haynes J. Increased sheep lung vascular permeability caused by Escherichia coli endotoxin. Circ Res. 1979 Aug;45(2):292–297. doi: 10.1161/01.res.45.2.292. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain S. W., Martin B. A., Musclow C. E., Cooper J. D. Pulmonary leukostasis and its relationship to pulmonary dysfunction in sheep and rabbits. Circ Res. 1980 Feb;46(2):175–180. doi: 10.1161/01.res.46.2.175. [DOI] [PubMed] [Google Scholar]
- Frölich J. C., Ogletree M., Peskar B. A., Brigham K. L. Pulmonary hypertension correlated to pulmonary thromboxane synthesis. Adv Prostaglandin Thromboxane Res. 1980;7:745–750. [PubMed] [Google Scholar]
- Gordon L. I., Douglas S. D., Kay N. E., Yamada O., Osserman E. F., Jacob H. S. Modulation of neutrophil function by lysozyme. Potential negative feedback system of inflammation. J Clin Invest. 1979 Jul;64(1):226–232. doi: 10.1172/JCI109443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heflin A. C., Jr, Brigham K. L. Prevention by granulocyte depletion of increased vascular permeability of sheep lung following endotoxemia. J Clin Invest. 1981 Nov;68(5):1253–1260. doi: 10.1172/JCI110371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Larsen G. L., Webster R. O., Mitchell B. C., Goins A. J., Henson J. E. Pulmonary microvascular alterations and injury induced by complement fragments: synergistic effect of complement activation, neutrophil sequestration, and prostaglandins. Ann N Y Acad Sci. 1982;384:287–300. doi: 10.1111/j.1749-6632.1982.tb21379.x. [DOI] [PubMed] [Google Scholar]
- Henson P. M., McCarthy K., Larsen G. L., Webster R. O., Giclas P. C., Dreisin R. B., King T. E., Shaw J. O. Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol. 1979 Oct;97(1):93–110. [PMC free article] [PubMed] [Google Scholar]
- Horn R. G., Collins R. D. Fragmentation of granulocytes in pulmonary capillaries during development of the generalized Shwartzman reaction. Lab Invest. 1968 Nov;19(5):451–459. [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of rabbit C5ades arg: probable role of polymorphonuclear leucocyte lysosomes. Clin Exp Immunol. 1980 Sep;41(3):512–520. [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Movat K. W., Movat H. Z. Enhanced vascular permeability and haemorrhage-inducing activity of zymosan-activated plasma. Clin Exp Immunol. 1980 Sep;41(3):505–511. [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., McCarthy K., Webster R. O., Henson J., Henson P. M. A differential effect of C5a and C5a des Arg in the induction of pulmonary inflammation. Am J Pathol. 1980 Jul;100(1):179–192. [PMC free article] [PubMed] [Google Scholar]
- LeQUIRE V. S. The augmentation of the thermogenic effects of pyrogens by homologous plasma in rabbits. J Infect Dis. 1951 Mar-Apr;88(2):194–206. doi: 10.1093/infdis/88.2.194. [DOI] [PubMed] [Google Scholar]
- McDonald J. W., Ali M., Morgan E., Townsend E. R., Cooper J. D. Thromboxane synthesis by sources other than platelets in association with complement-induced pulmonary leukostasis and pulmonary hypertension in sheep. Circ Res. 1983 Jan;52(1):1–6. doi: 10.1161/01.res.52.1.1. [DOI] [PubMed] [Google Scholar]
- Meyrick B., Brigham K. L. Acute effects of Escherichia coli endotoxin on the pulmonary microcirculation of anesthetized sheep structure:function relationships. Lab Invest. 1983 Apr;48(4):458–470. [PubMed] [Google Scholar]
- Morrison D. C., Ulevitch R. J. The effects of bacterial endotoxins on host mediation systems. A review. Am J Pathol. 1978 Nov;93(2):526–618. [PMC free article] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. O., Henson P. M. Pulmonary intravascular sequestration of activated neutrophils: failure to induce light-microscopic evidence of lung injury in rabbits. Am J Pathol. 1982 Jul;108(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Snapper J. R., Hutchison A. A., Ogletree M. L., Brigham K. L. Effects of cyclooxygenase inhibitors on the alterations in lung mechanics caused by endotoxemia in the unanesthetized sheep. J Clin Invest. 1983 Jul;72(1):63–76. doi: 10.1172/JCI110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub N. C., Bland R. D., Brigham K. L., Demling R., Erdmann A. J., 3rd, Woolverton W. C. Preparation of chronic lung lymph fistulas in sheep. J Surg Res. 1975 Nov;19(5):315–320. doi: 10.1016/0022-4804(75)90056-6. [DOI] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Webster R. O., Larsen G. L., Mitchell B. C., Goins A. J., Henson P. M. Absence of inflammatory lung injury in rabbits challenged intravascularly with complement-derived chemotactic factors. Am Rev Respir Dis. 1982 Mar;125(3):335–340. doi: 10.1164/arrd.1982.125.3.335. [DOI] [PubMed] [Google Scholar]