Abstract
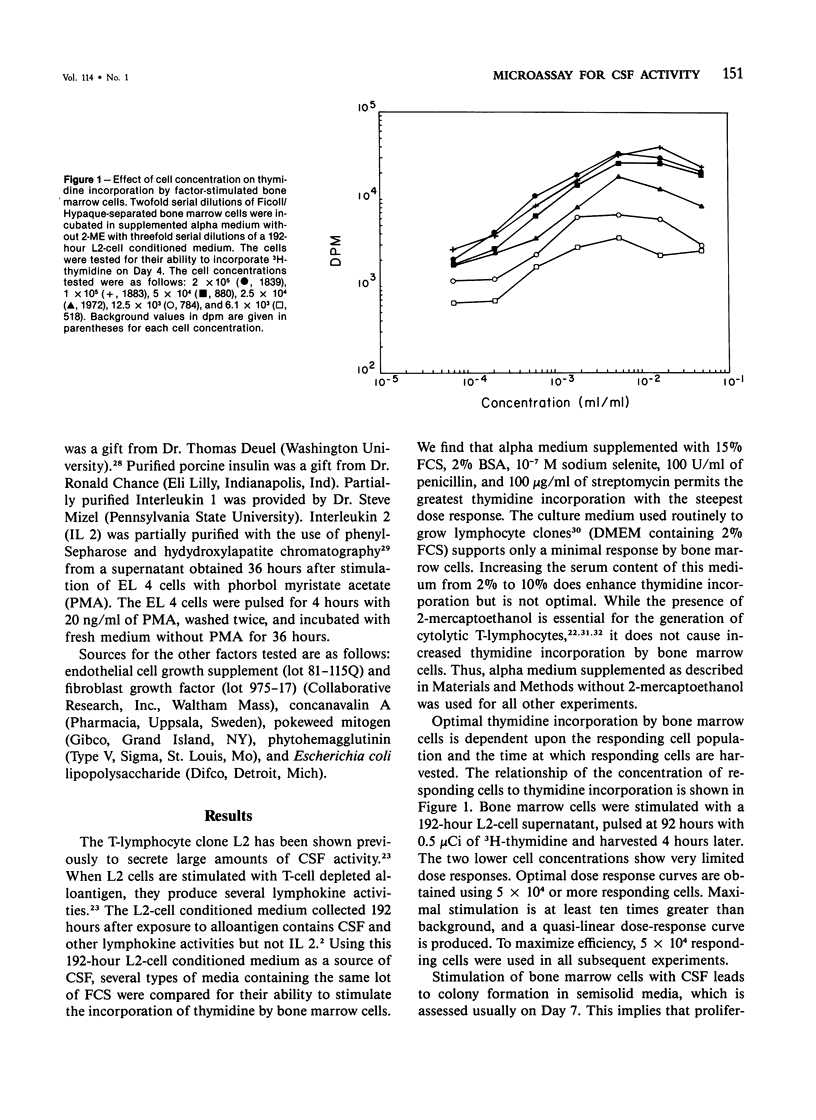
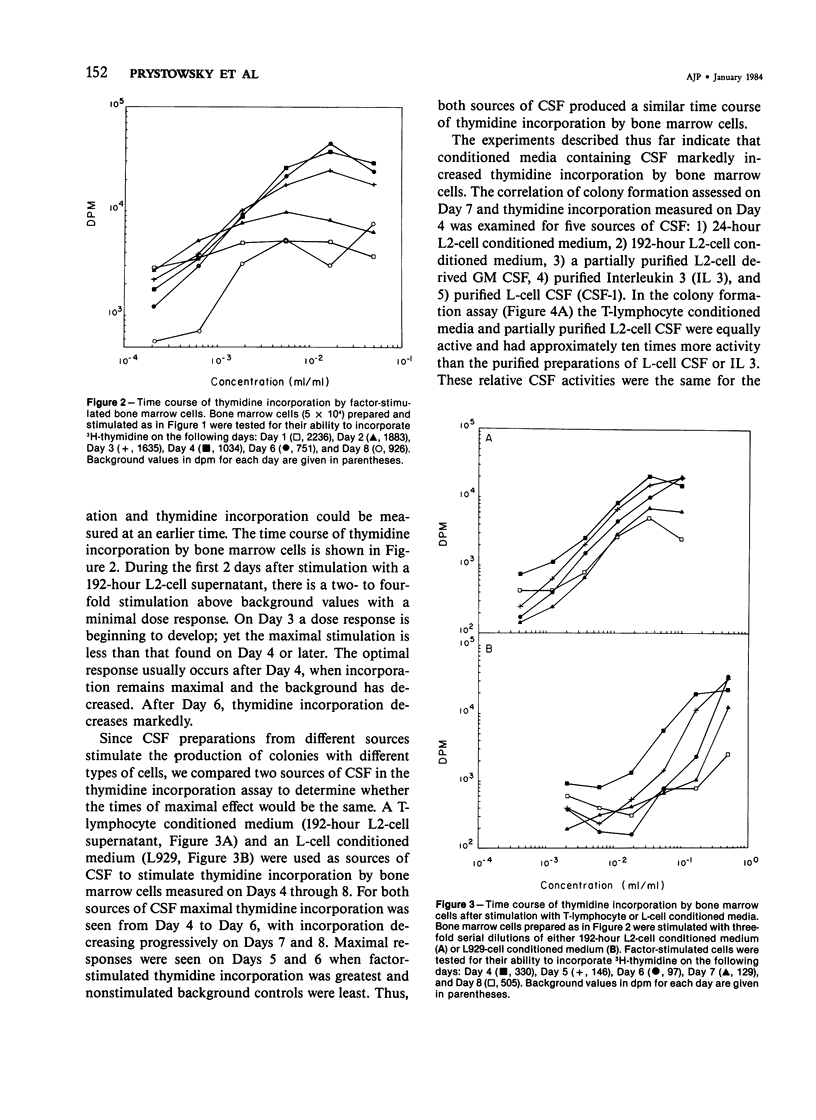
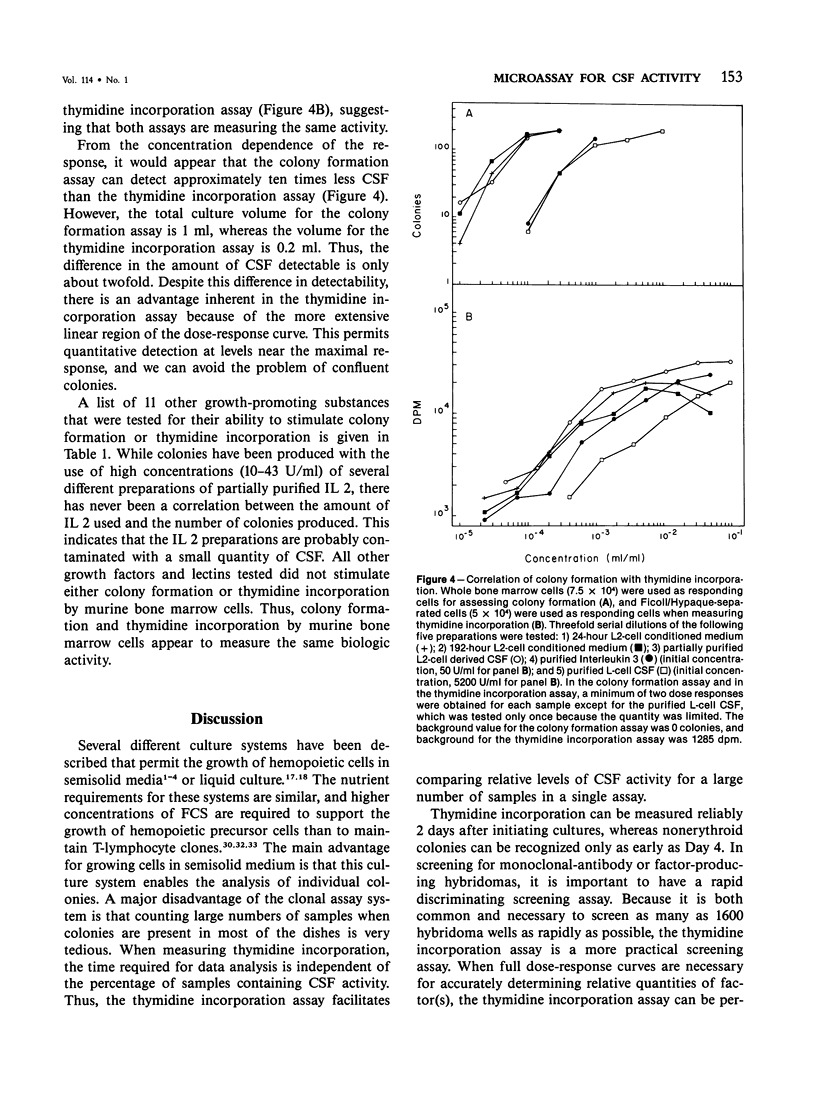
A variety of growth factors and lectins were tested; only colony-stimulating factors CSF-1, Interleukin 3, and a T-lymphocyte GM CSF induced colony formation in semisolid medium and stimulated thymidine incorporation in liquid culture. All other growth factors and lectins were inactive in both assays. Factor-stimulated thymidine incorporation was detectable 24 hours after stimulation and reached maximal levels 4-6 days after stimulation. A convenient microassay for measuring CSF activity has been developed, enabling a large number of samples to be screened qualitatively in 2 days and permitting CSF activity to be measured quantitatively in 4-5 days. This microassay can supplement the clonal-cell assay method and be especially useful as an initial screening assay for CSF activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin P. E., McCulloch E. A., Till J. E. Stimulation of uptake of tritiated thymidine into mouse marrow cells in culture by a factor from L-cell conditioned medium. J Cell Physiol. 1972 Apr;79(2):181–188. doi: 10.1002/jcp.1040790204. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood. 1980 Dec;56(6):947–958. [PubMed] [Google Scholar]
- Castro-Malaspina H., Gay R. E., Resnick G., Kapoor N., Meyers P., Chiarieri D., McKenzie S., Broxmeyer H. E., Moore M. A. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980 Aug;56(2):289–301. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching L. M., Muraoka S., Miller R. G. Differentiation of T cells from immature precursors in murine T cell colonies. J Immunol. 1981 Nov;127(5):2156–2163. [PubMed] [Google Scholar]
- Das S. K., Stanley E. R., Guilbert L. J., Forman L. W. Human colony-stimulating factor (CSF-1) radioimmunoassay: resolution of three subclasses of human colony-stimulating factors. Blood. 1981 Sep;58(3):630–641. [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Ely J. M., Prystowsky M. B., Eisenberg L., Quintans J., Goldwasser E., Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. V. Differential kinetics of IL 2, CSF, and BCSF release by a cloned T amplifier cell and its variant. J Immunol. 1981 Dec;127(6):2345–2349. [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. I. Interactions between cloned amplifier and cytolytic T cell lines. J Exp Med. 1980 Apr 1;151(4):876–895. doi: 10.1084/jem.151.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. T-cell lines which cooperate in generation of specific cytolytic activity. Nature. 1979 Mar 8;278(5700):171–173. doi: 10.1038/278171a0. [DOI] [PubMed] [Google Scholar]
- Glasebrook A. L., Quintans J., Eisenberg L., Fitch F. W. Alloreactive cloned T cell lines. II. Polyclonal stimulation of B cells by a cloned helper T cell line. J Immunol. 1981 Jan;126(1):240–244. [PubMed] [Google Scholar]
- Glasebrook A. L., Sarmiento M., Loken M. R., Dialynas D. P., Quintans J., Eisenberg L., Lutz C. T., Wilde D., Fitch F. W. Murine T lymphocyte clones with distinct immunological functions. Immunol Rev. 1981;54:225–266. doi: 10.1111/j.1600-065x.1981.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Harris J. W., MacDonald H. R., Engers H. D., Fitch F. W., Cerottini J. Increased cytolytic T lymphocyte activity induced by 2-mercaptoethanol in mixed leukocyte cultures: kinetics and possible mechanisms of action. J Immunol. 1976 Apr;116(4):1071–1077. [PubMed] [Google Scholar]
- Hilfiker M. L., Moore R. N., Farrar J. J. Biologic properties of chromatographically separated murine thymoma-derived Interleukin 2 and colony-stimulating factor. J Immunol. 1981 Nov;127(5):1983–1987. [PubMed] [Google Scholar]
- Horak H., Turner A. R., Shaw A. R., Yau O. W. Stimulation of [3H]thymidine uptake in mouse marrow by granulocyte-macrophage colony stimulating factor from mouse lung conditioned medium. J Immunol Methods. 1983 Jan 28;56(2):253–260. doi: 10.1016/0022-1759(83)90417-9. [DOI] [PubMed] [Google Scholar]
- Humphries R. K., Eaves A. C., Eaves C. J. Self-renewal of hemopoietic stem cells during mixed colony formation in vitro. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3629–3633. doi: 10.1073/pnas.78.6.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Lusis A. J., Koeffler H. P. Action of granulocyte-macrophage colony-stimulating factors: studies using a human leukemia cell line. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5346–5350. doi: 10.1073/pnas.77.9.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R., Odartchenko N., Sordat B. Growth of mouse megakaryocyte colonies in vitro. Proc Natl Acad Sci U S A. 1975 May;72(5):1744–1748. doi: 10.1073/pnas.72.5.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Parker J., Chester H. M., Kincade P. W. Formation of eosinophilic-like granulocytic colonies by mouse bone marrow cells in vitro. J Cell Physiol. 1974 Oct;84(2):275–289. doi: 10.1002/jcp.1040840214. [DOI] [PubMed] [Google Scholar]
- Metcalf D. Studies on colony formation in vitro by mouse bone marrow cells. II. Action of colony stimulating factor. J Cell Physiol. 1970 Aug;76(1):89–99. doi: 10.1002/jcp.1040760113. [DOI] [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Johnson G. R., Burgess A. W. Separation of functionally distinct human granulocyte-macrophage colony-stimulating factors. Blood. 1979 Sep;54(3):614–627. [PubMed] [Google Scholar]
- Ogawa M., Parmley R. T., Bank H. L., Spicer S. S. Human marrow erythropoiesis in culture. I. Characterization of methylcellulose colony assay. Blood. 1976 Sep;48(3):407–417. [PubMed] [Google Scholar]
- Prystowsky M. B., Ely J. M., Beller D. I., Eisenberg L., Goldman J., Goldman M., Goldwasser E., Ihle J., Quintans J., Remold H. Alloreactive cloned T cell lines. VI. Multiple lymphokine activities secreted by helper and cytolytic cloned T lymphocytes. J Immunol. 1982 Dec;129(6):2337–2344. [PubMed] [Google Scholar]
- Prystowsky M. B., Ely J., Vogel S. N., Goldwasser E., Fitch F. W. Biochemical enrichment of lymphokines secreted by a cloned helper T lymphocyte. Fed Proc. 1983 Jul;42(10):2757–2761. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Stanley E. R., Hansen G., Woodcock J., Metcalf D. Colony stimulating factor and the regulation of granulopoiesis and macrophage production. Fed Proc. 1975 Dec;34(13):2272–2278. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stanley E. R., Metcalf D., Maritz J. S., Yeo G. F. Standardized bioassay for bone marrow colony stimulating factor in human urine: levels in normal man. J Lab Clin Med. 1972 Apr;79(4):657–668. [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Tushinski R. J., Oliver I. T., Guilbert L. J., Tynan P. W., Warner J. R., Stanley E. R. Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell. 1982 Jan;28(1):71–81. doi: 10.1016/0092-8674(82)90376-2. [DOI] [PubMed] [Google Scholar]



