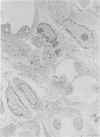
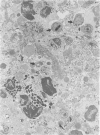
Abstract
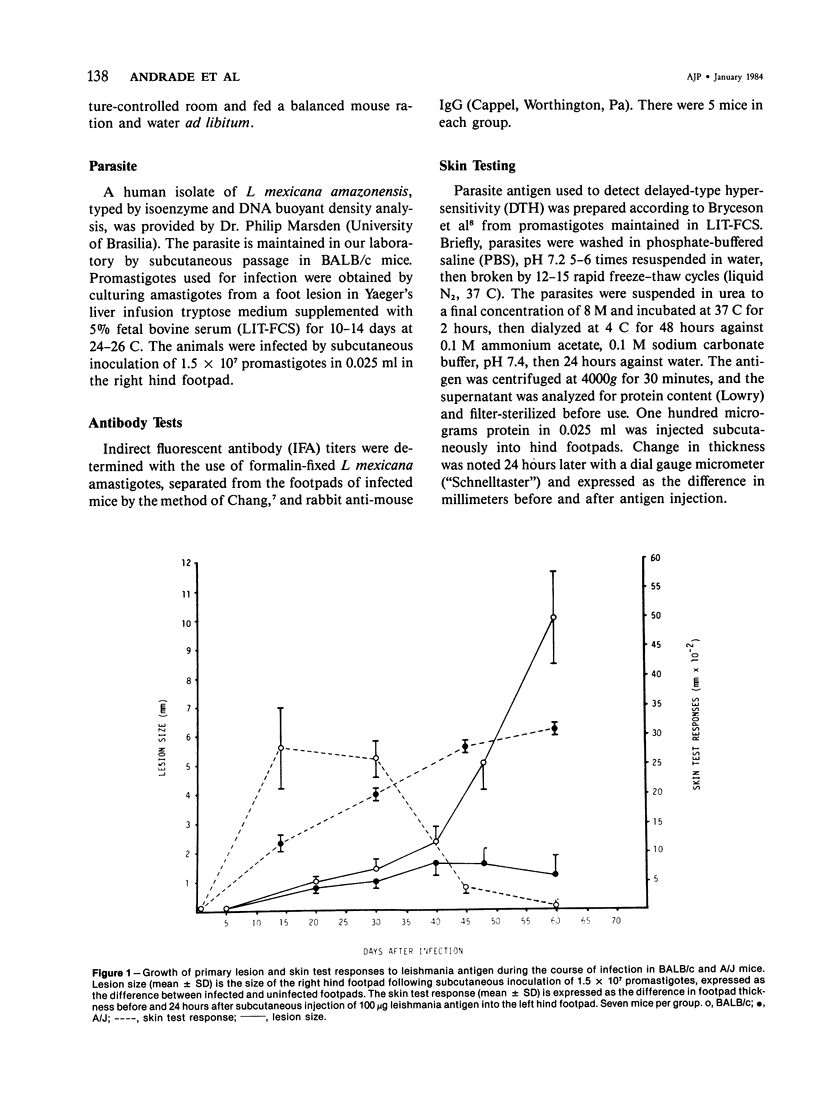
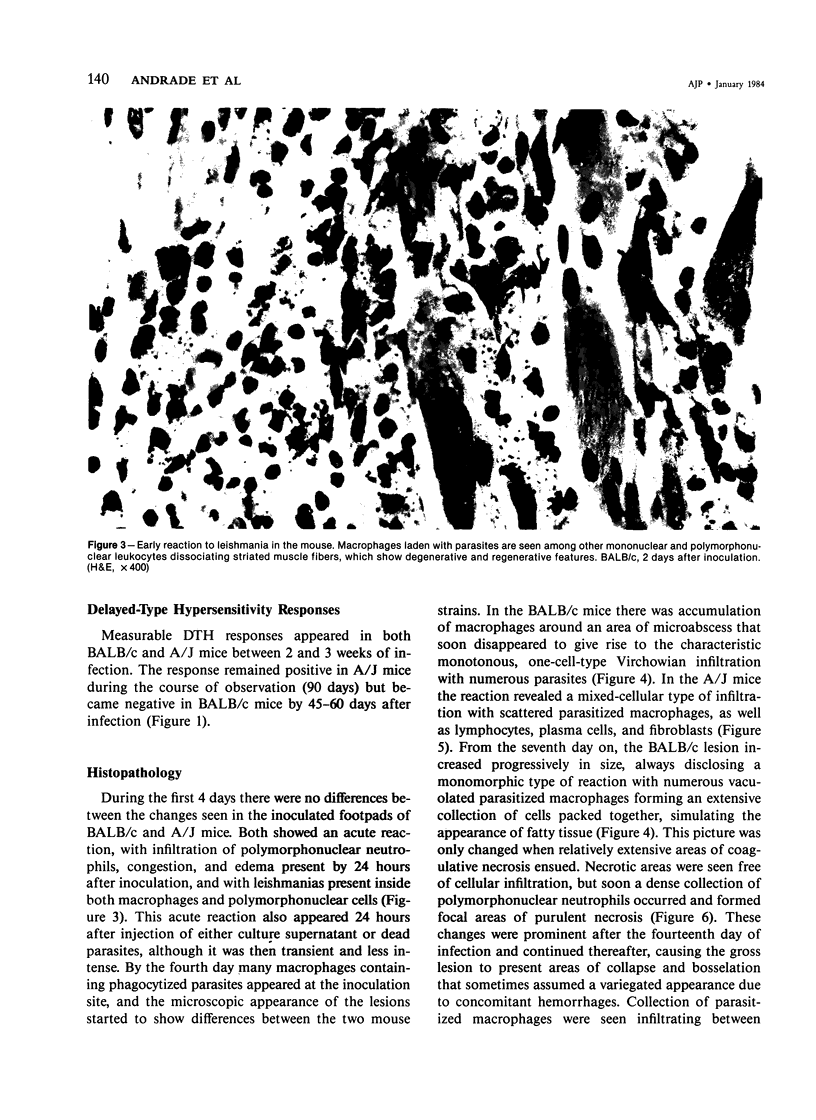
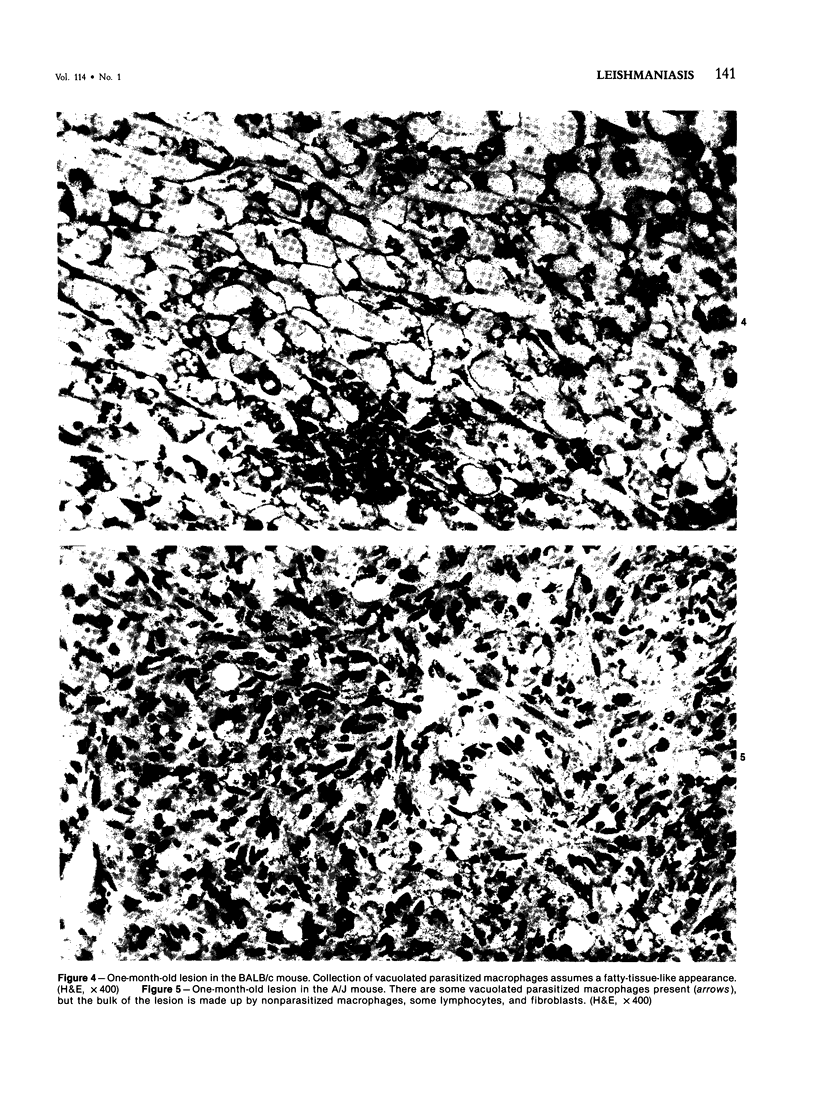
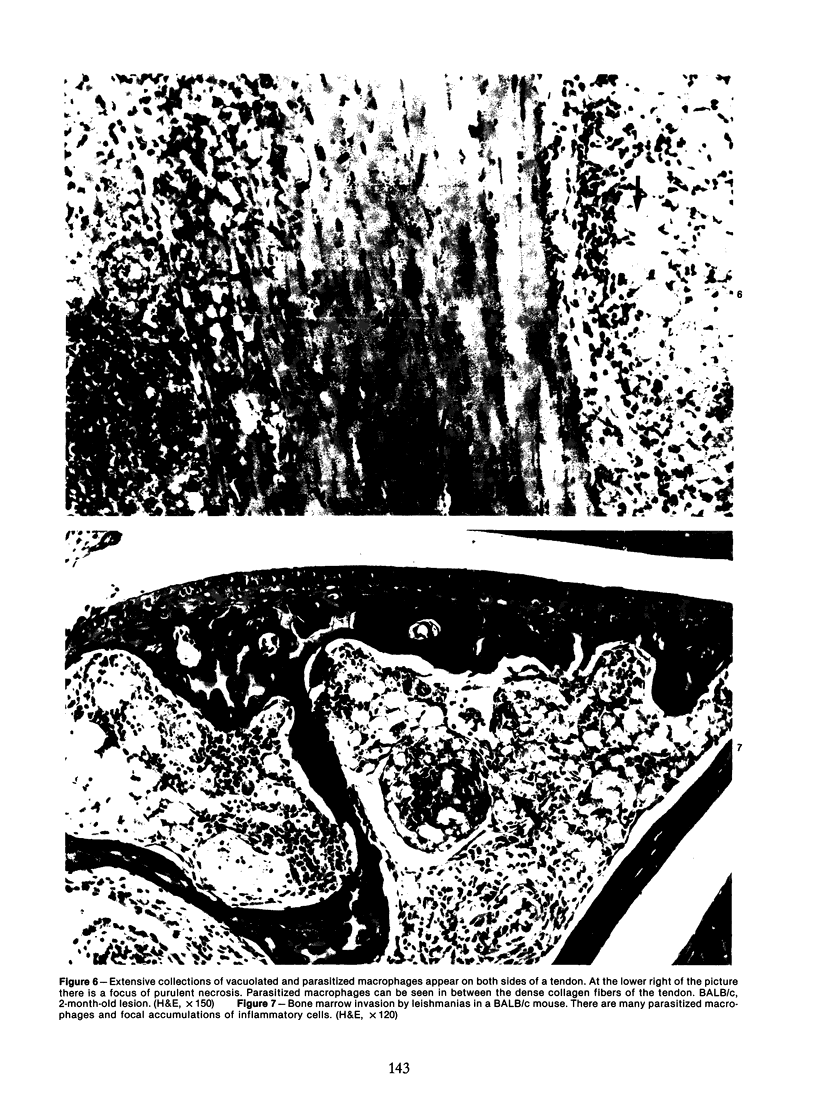
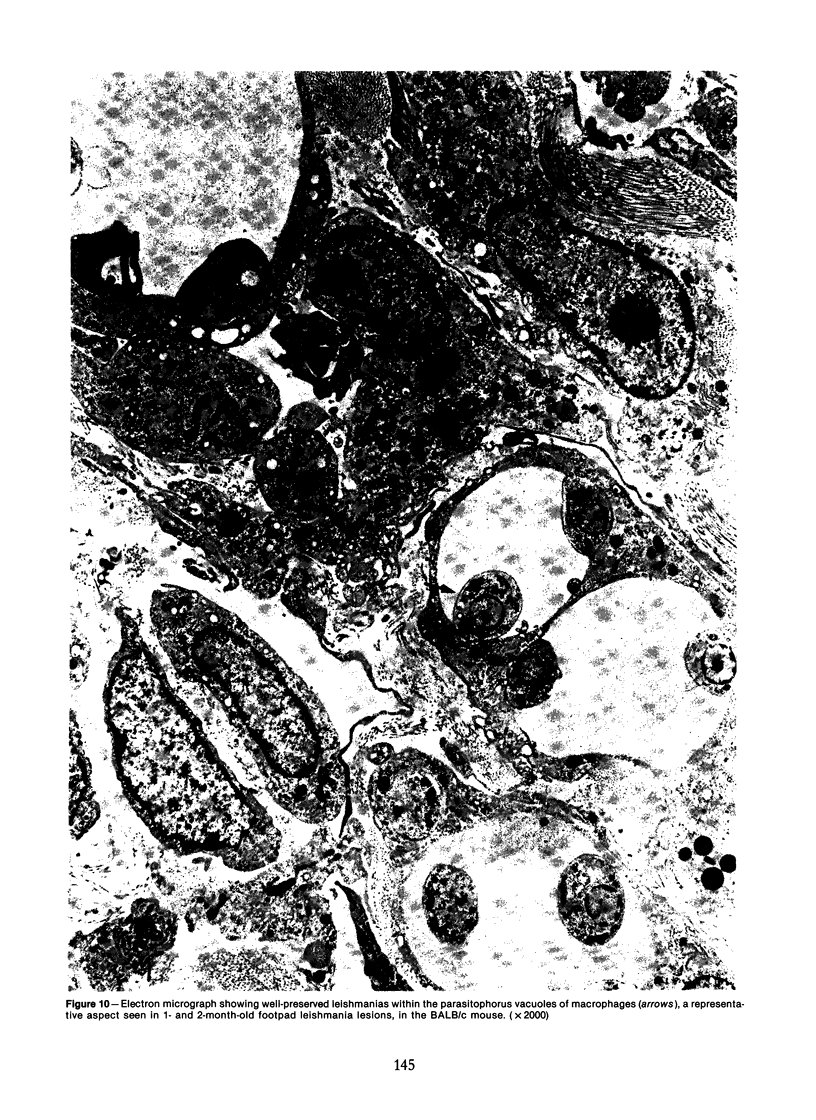
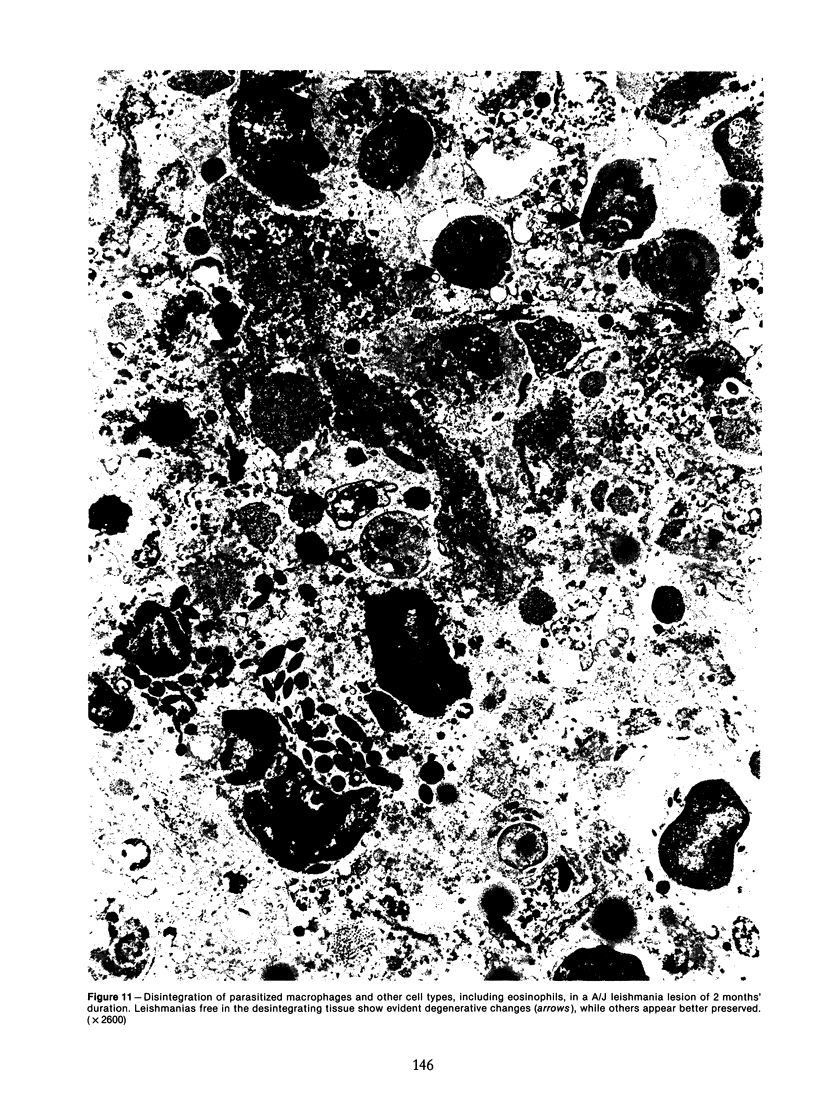
Relatively susceptible BALB/c and relatively resistant A/J mice were infected subcutaneously in the right hind footpad with promastigotes of Leishmania mexicana amazonensis. A large localized lesion developed within 2 months after infection in the BALB/c mice, while A/J mice exhibited a small discrete fibrotic nodule. Sequential immunologic and histologic examination demonstrated that BALB/c mice developed a nodular foam-cell type of lesion and progressive depression of a delayed-type hypersensitivity (DTH) response to leishmania antigen, while the A/J mice had a mixed cellular fibrosing and encapsulating reaction and developed and maintained positive DTH responses to leishmania antigen. Anti-leishmania antibody responses were positive at similar levels in both strains. The lesions in BALB/c mice were found in bone marrow, tendon, skin appendages, and regional lymph nodes, with a tendency toward cutaneous metastases. Lesions in A/J mice remained localized. Fibrosis, focal fibrinoid necrosis, and lymphocytic and macrophagic infiltration were the outstanding features. Light and transmission electron microscopic studies indicated that no outstanding destruction of leishmanias seemed to occur within macrophages of either mouse strain. In the more resistant A/J mice, however, parasitized macrophages were frequently necrotic, and degenerating leishmanias were often seen free in the interstitial tissue. These observations help the interpretation of the histologic features, as well as the pathogenesis, of cutaneous and mucocutaneous leishmaniasis in man.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barral A., Petersen E. A., Sacks D. L., Neva F. A. Late metastatic Leishmaniasis in the mouse. A model for mucocutaneous disease. Am J Trop Med Hyg. 1983 Mar;32(2):277–285. doi: 10.4269/ajtmh.1983.32.277. [DOI] [PubMed] [Google Scholar]
- Behforouz N., Rezai H. R., Gettner S. Application of immunofluorescence to detection of antibody in Leishmania infections. Ann Trop Med Parasitol. 1976 Sep;70(3):293–301. doi: 10.1080/00034983.1976.11687125. [DOI] [PubMed] [Google Scholar]
- Behin R., Mauel J., Sordat B. Leishmania tropica: pathogenicity and in vitro macrophage function in strains of inbred mice. Exp Parasitol. 1979 Aug;48(1):81–91. doi: 10.1016/0014-4894(79)90057-2. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Dwyer D. M. Expression of Leishmania antigen on the surface membrane of infected human macrophages in vitro. Clin Exp Immunol. 1981 May;44(2):342–348. [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Dwyer D. M., Wyler D. J. Multiplication of Leishmania in human macrophages in vitro. Infect Immun. 1979 Oct;26(1):375–379. doi: 10.1128/iai.26.1.375-379.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt A. L., Andrade Z. A. Aspectos imunopatológicos na leishmaniose cutâneo-mucosa. Hospital (Rio J) 1967 Apr;71(4):975–984. [PubMed] [Google Scholar]
- Bradley D. J., Kirkley J. Variation in susceptibility of mouse strains to Leishmania donovani infection. Trans R Soc Trop Med Hyg. 1972;66(4):527–528. [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Wolstencroft R. A., Dumonde D. C. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin Exp Immunol. 1970 Sep;7(3):301–341. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. 3. Immunological studies. IV. Pathogenesis of diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1970;64(3):380–393. doi: 10.1016/0035-9203(70)90174-4. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. I. The clinical and histological features of the disease. Trans R Soc Trop Med Hyg. 1969;63(6):708–737. doi: 10.1016/0035-9203(69)90116-3. [DOI] [PubMed] [Google Scholar]
- Buchanan R. D., Fine D. P., Colley D. G. Schistosoma mansoni infection in mice depleted of thymus-dependent lymphocytes. II. Pathology and altered pathogenesis. Am J Pathol. 1973 May;71(2):207–218. [PMC free article] [PubMed] [Google Scholar]
- Chang K. P. Human cutaneous lieshmania in a mouse macrophage line: propagation and isolation of intracellular parasites. Science. 1980 Sep 12;209(4462):1240–1242. doi: 10.1126/science.7403880. [DOI] [PubMed] [Google Scholar]
- DESTOMBES P. [Application of the concept of "polar classification" to cutaneous leishmaniasis]. Bull Soc Pathol Exot Filiales. 1960 Mar-Apr;53:299–301. [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Genetically determined susceptibility to Leishmania tropica infection is expressed by haematopoietic donor cells in mouse radiation chimaeras. Nature. 1980 Nov 13;288(5787):161–162. doi: 10.1038/288161a0. [DOI] [PubMed] [Google Scholar]
- Howard J. G., Hale C., Liew F. Y. Immunological regulation of experimental cutaneous leishmaniasis. III. Nature and significance of specific suppression of cell-mediated immunity in mice highly susceptible to Leishmania tropica. J Exp Med. 1980 Sep 1;152(3):594–607. doi: 10.1084/jem.152.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. H., Peters W. The resistance of intracellular Leishmania parasites to digestion by lysosomal enzymes. Ann Trop Med Parasitol. 1977 Sep;71(3):295–312. doi: 10.1080/00034983.1977.11687192. [DOI] [PubMed] [Google Scholar]
- Moriearty P. L., Bittencourt A. L., Pereira C., Teixeira R., Barreto E., Guimarães N. A. Borderline cutaneous leishmaniasis. Clinical, immunological and histological differences from mucocutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo. 1978 Jan-Feb;20(1):15–21. [PubMed] [Google Scholar]
- Nasseri M., Modabber F. Z. Generalized infection and lack of delayed hypersensitivity in BALB/c mice infected with Leishmania tropica major. Infect Immun. 1979 Nov;26(2):611–614. doi: 10.1128/iai.26.2.611-614.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Arredondo B., González M. Comparative study of American cutaneous leishmaniasis and diffuse cutaneous leishmaniasis in two strains of inbred mice. Infect Immun. 1978 Nov;22(2):301–307. doi: 10.1128/iai.22.2.301-307.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez H., Labrador F., Torrealba J. W. Variations in the response of five strains of mice to Leishmania mexicana. Int J Parasitol. 1979 Feb;9(1):27–32. doi: 10.1016/0020-7519(79)90062-6. [DOI] [PubMed] [Google Scholar]
- Ridley D. S. A histological classification of cutaneous leishmaniasis and its geographical expression. Trans R Soc Trop Med Hyg. 1980;74(4):515–521. doi: 10.1016/0035-9203(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Marsden P. D., Cuba C. C., Barreto A. C. A histological classification of mucocutaneous leishmaniasis in Brazil and its clinical evaluation. Trans R Soc Trop Med Hyg. 1980;74(4):508–514. doi: 10.1016/0035-9203(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Scott P. A., Farrell J. P. Experimental cutaneous leishmaniasis: disseminated leishmaniasis in genetically susceptible and resistant mice. Am J Trop Med Hyg. 1982 Mar;31(2):230–238. doi: 10.4269/ajtmh.1982.31.230. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Wyler D. J., Wahl S. M., Wahl L. M. Hepatic fibrosis in schistosomiasis: egg granulomas secrete fibroblast stimulating factor in vitro. Science. 1978 Oct 27;202(4366):438–440. doi: 10.1126/science.705337. [DOI] [PubMed] [Google Scholar]