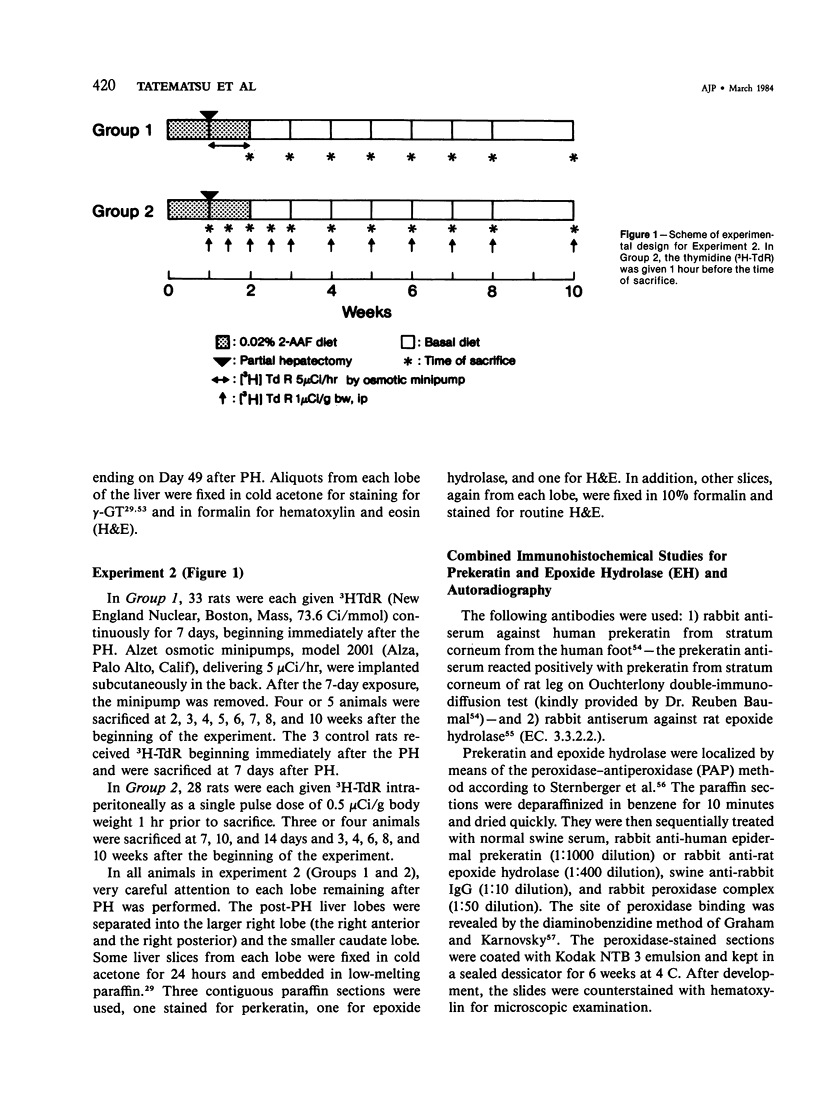
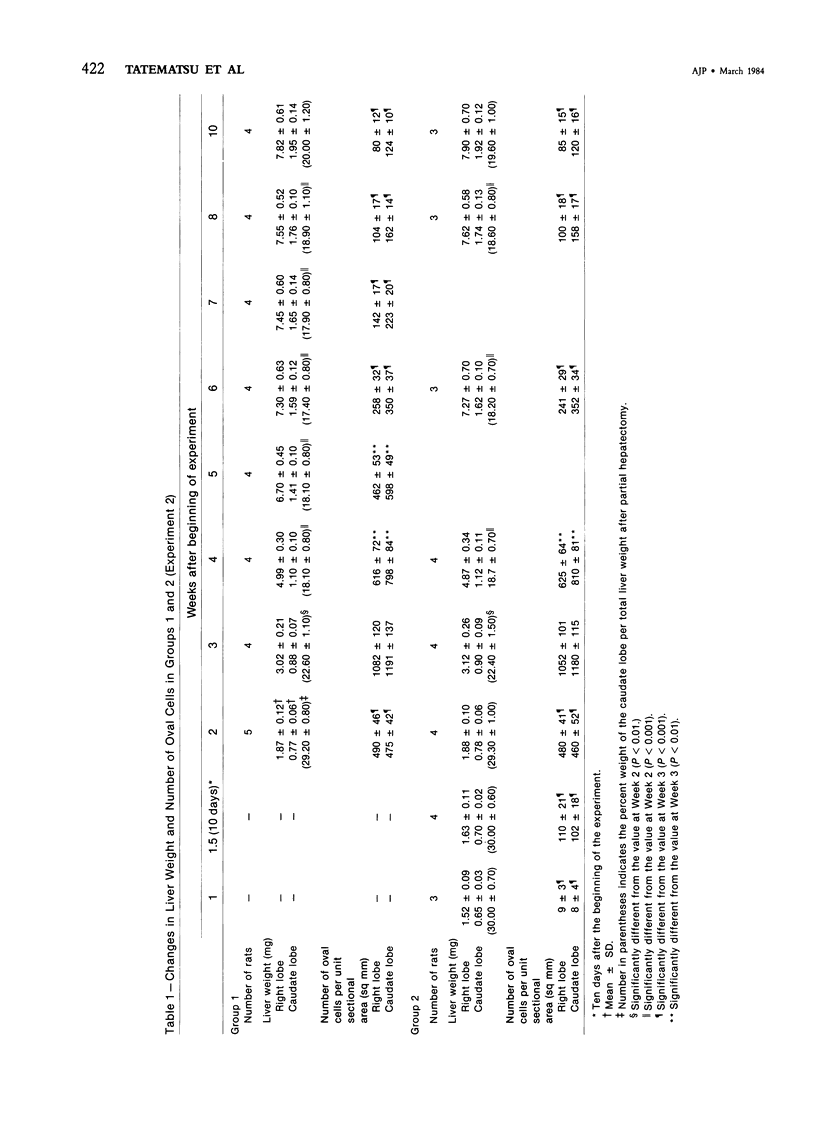
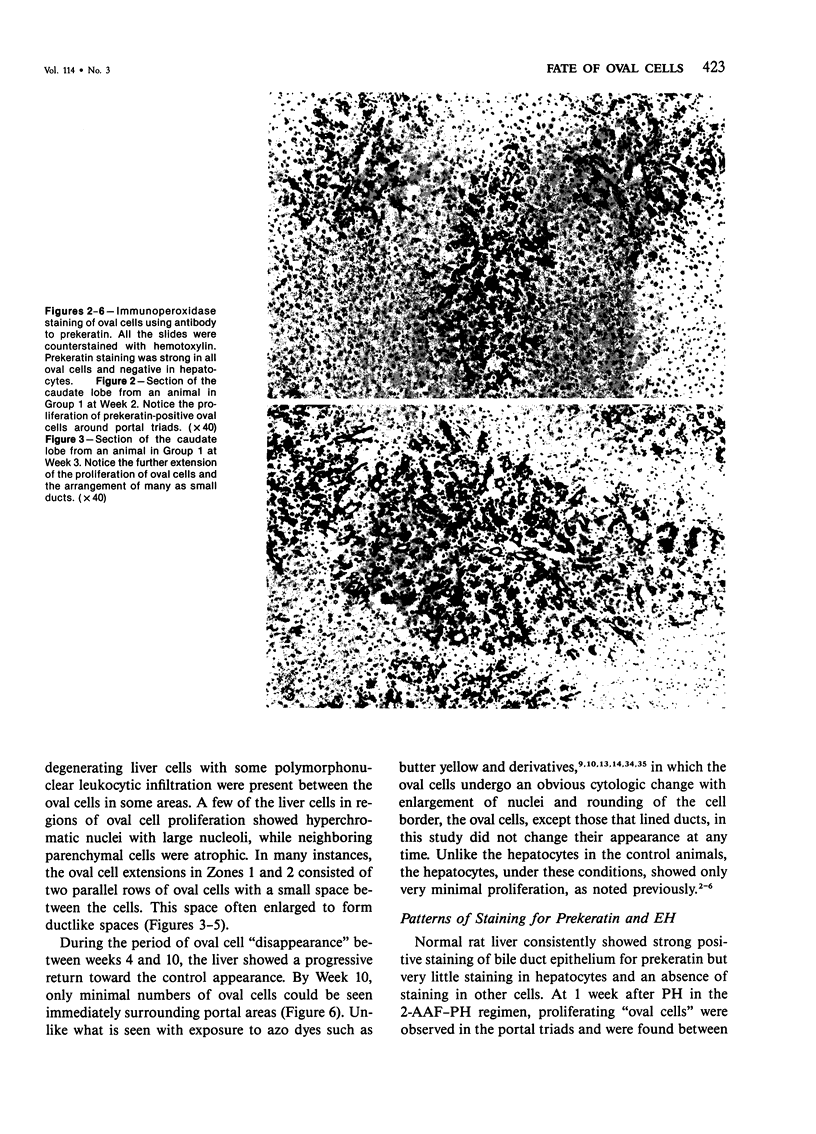
Abstract
The kinetics of oval cell proliferation in the liver and their fate were studied by combined autoradiography and immunohistochemical staining for epidermal prekeratin and epoxide hydrolase (EH). The oval cell proliferation was induced in rats by exposure to dietary 2-acetylaminofluorene (2-AAF) for 2 weeks with the midway performance of partial hepatectomy (PH). The labeling with 3H-thymidine [3H-TdR] was done in different groups of rats by two procedures: continuous exposure for 1 week with the aid of a minipump and brief exposure by the administration of a single dose. The livers of groups of animals were examined from 1 to 10 weeks after PH. Oval cells and duct epithelium showed positive staining for prekeratin and negative for EH, whereas hepatocytes showed the reverse pattern of staining. A critical finding was the observation that the exposure to the 2-AAF inhibited virtually completely the labeling of hepatocytes with [3H]-TdR in the caudate lobe and incompletely in the right lobe without interfering with the labeling of the oval cells in either lobe. This made it possible to study the fate of the oval cells vis-à-vis hepatocytes. This qualitative-quantitative study of oval cells and hepatocytes clearly indicates that oval cells under these experimental conditions do not become hepatocytes within 10 weeks. Over 80% of oval cells disappear within this period, and the remainder persist as such. These results indicate that under one set of experimental conditions related to hepatocarcino-genesis in the rat, no evidence for the conversion of oval cells to hepatocytes was obtained.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auerbach R., Auerbach W. Regional differences in the growth of normal and neoplastic cells. Science. 1982 Jan 8;215(4529):127–134. doi: 10.1126/science.7053564. [DOI] [PubMed] [Google Scholar]
- Bentley P., Waechter F., Oesch F., Stäubli W. Immunochemical localization of epoxide hydratase in rat liver: effects of 2-acetylaminofluorene. Biochem Biophys Res Commun. 1979 Dec 14;91(3):1101–1108. doi: 10.1016/0006-291x(79)91994-6. [DOI] [PubMed] [Google Scholar]
- Cameron R., Kellen J., Kolin A., Malkin A., Farber E. Gamma-glutamyltransferase in putative premalignant liver cell populations during hepatocarcinogenesis. Cancer Res. 1978 Mar;38(3):823–829. [PubMed] [Google Scholar]
- Dempo K., Chisaka N., Yoshida Y., Kaneko A., Onoé T. Immunofluorescent study on alpha-fetoprotein-producing cells in the early stage of 3'-methyl-4-dimethylaminoazobenzene carcinogenesis. Cancer Res. 1975 May;35(5):1282–1287. [PubMed] [Google Scholar]
- Enomoto K., Farber E. Kinetics of phenotypic maturation of remodeling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982 Jun;42(6):2330–2335. [PubMed] [Google Scholar]
- Enomoto K., Ying T. S., Griffin M. J., Farber E. Immunohistochemical study of epoxide hydrolase during experimental liver carcinogenesis. Cancer Res. 1981 Sep;41(9 Pt 1):3281–3287. [PubMed] [Google Scholar]
- Epstein S., Ito N., Merkow L., Farber E. Cellular analysis of liver carcinogenesis: the induction of large hyperplastic nodules in the liver with 2-fluorenylacetamide or ethionine and some aspects of their morphology and glycogen metabolism. Cancer Res. 1967 Sep;27(9):1702–1711. [PubMed] [Google Scholar]
- FARBER E. ETHIONINE CARCINOGENESIS. Adv Cancer Res. 1963;7:383–474. doi: 10.1016/s0065-230x(08)60986-0. [DOI] [PubMed] [Google Scholar]
- FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956 Feb;16(2):142–148. [PubMed] [Google Scholar]
- Farber E., Cameron R. The sequential analysis of cancer development. Adv Cancer Res. 1980;31:125–226. doi: 10.1016/s0065-230x(08)60658-2. [DOI] [PubMed] [Google Scholar]
- Farber E. Chemicals, evolution, and cancer development: Rous-Whipple Award Lecture. Am J Pathol. 1982 Sep;108(3):270–275. [PMC free article] [PubMed] [Google Scholar]
- GILLMAN J., GILBERT C., SPENCE I. Some factors regulating the structural integrity of the intrahepatic bile ducts with special reference to primary carcinoma of the liver and vitamin A. Cancer. 1954 Nov;7(6):1119–1154. doi: 10.1002/1097-0142(195411)7:6<1109::aid-cncr2820070605>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- GRISHAM J. W., HARTROFT W. S. Morphologic identification by electron microscopy of "oval" cells in experimental hepatic degeneration. Lab Invest. 1961 Mar-Apr;10:317–332. [PubMed] [Google Scholar]
- GRISHAM J. W., PORTA E. A. ORIGIN AND FATE OF PROLIFERATED HEPATIC DUCTAL CELLS IN THE RAT: ELECTRON MICROSCOPIC AND AUTORADIOGRAPHIC STUDIES. Exp Mol Pathol. 1964 Jun;86:242–261. doi: 10.1016/0014-4800(64)90057-7. [DOI] [PubMed] [Google Scholar]
- Ghoshal A. K., Mullen B., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis. Regeneration of liver after carbon tetrachloride-induced liver necrosis when hepatocyte proliferation is inhibited by 2-acetylaminofluorene. Lab Invest. 1983 Feb;48(2):224–230. [PubMed] [Google Scholar]
- Goldfarb S. A morphological and histochemical study of carcinogenesis of the liver in rats fed 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1973 May;33(5):1119–1128. [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Guillouzo A., Weber A., Le Provost E., Rissel M., Schapira F. Cell types involved in the expression of foetal aldolases during rat azo-dye hepatocarcinogenesis. J Cell Sci. 1981 Jun;49:249–260. doi: 10.1242/jcs.49.1.249. [DOI] [PubMed] [Google Scholar]
- Inaoka Y. Significance of the so-called oval cell proliferation during azo-dye hepatocarcinogenesis. Gan. 1967 Aug;58(4):355–366. [PubMed] [Google Scholar]
- Iwasaki T., Dempo K., Kaneko A., Onoe T. Fluctuation of various cell populations and their characteristics during azo-dye carcinogenesis. Gan. 1972 Feb;63(1):21–30. [PubMed] [Google Scholar]
- Kahn H. J., Hanna W., Yeger H., Baumal R. Immunohistochemical localization of prekeratin filaments in benign and malignant cells in effusions. Comparison with intermediate filament distribution by electron microscopy. Am J Pathol. 1982 Nov;109(2):206–214. [PMC free article] [PubMed] [Google Scholar]
- Kitagawa T., Yokochi T., Sugano H. -Fetoprotein and hepatocarcinogenesis in rats fed 3'-methyl-4-(dimethylamino)azobenzene or N-2-fluorenylacetamide. Int J Cancer. 1972 Sep 15;10(2):368–381. doi: 10.1002/ijc.2910100219. [DOI] [PubMed] [Google Scholar]
- Kuhlmann W. D. Localization of alpha1-fetoprotein and DNA-synthesis in liver cell populations during experimental hepatocarcinogenesis in rats. Int J Cancer. 1978 Mar 15;21(3):368–380. doi: 10.1002/ijc.2910210319. [DOI] [PubMed] [Google Scholar]
- LEDUC E. H. Cell modulation in liver pathology. J Histochem Cytochem. 1959 Jul;7(4):253–255. doi: 10.1177/7.4.253. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Medline A., Farber E. Sequential analysis of hepatic carcinogenesis: a comparative study of the ultrastructure of preneoplastic, malignant, prenatal, postnatal, and regenerating liver. Lab Invest. 1979 Jul;41(1):22–35. [PubMed] [Google Scholar]
- Ogawa K., Minase T., Onhoe T. Demonstration of glucose 6-phosphatase activity in the oval cells of rat liver and the significance of the oval cells in azo dye carcinogenesis. Cancer Res. 1974 Dec;34(12):3379–3386. [PubMed] [Google Scholar]
- Ogawa K., Solt D. B., Farber E. Phenotypic diversity as an early property of putative preneoplastic hepatocyte populations in liver carcinogenesis. Cancer Res. 1980 Mar;40(3):725–733. [PubMed] [Google Scholar]
- Onda H. Immunohistological studies on alpha1-fetoprotein and alpha1-acid glycoprotein during azo dye hepatocarcinogenesis in rats. Gan. 1976 Apr;67(2):253–262. [PubMed] [Google Scholar]
- Onoé T., Kaneko A., Dempo K., Ogawa K., Minase T. Alpha-Fetoprotein and early histological changes of hepatic tissue in DAB-hepatocarcinogenesis. Ann N Y Acad Sci. 1975 Aug 22;259:168–180. doi: 10.1111/j.1749-6632.1975.tb25412.x. [DOI] [PubMed] [Google Scholar]
- POPPER H., KENT G., STEIN R. Ductular cell reaction in the liver in hepatic injury. J Mt Sinai Hosp N Y. 1957 Sep-Oct;24(5):551–556. [PubMed] [Google Scholar]
- POPPER H., STERNBERG S. S., OSER B. L., OSER M. The carcinogenic effect of aramite in rats. A study of hepatic nodules. Cancer. 1960 Sep-Oct;13:1035–1046. doi: 10.1002/1097-0142(196009/10)13:5<1035::aid-cncr2820130526>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- PRICE J. M., HARMAN J. W., MILLER E. C., MILLER J. A. Progressive microscopic alterations in the livers of rats fed the hepatic carcinogens 3'-methyl-4-dimethylaminoazobenzene and 4'-fluoro-4-dimethylaminoazobenzene. Cancer Res. 1952 Mar;12(3):192–200. [PubMed] [Google Scholar]
- Pitot H. C., Barsness L., Goldsworthy T., Kitagawa T. Biochemical characterisation of stages of hepatocarcinogenesis after a single dose of diethylnitrosamine. Nature. 1978 Feb 2;271(5644):456–458. doi: 10.1038/271456a0. [DOI] [PubMed] [Google Scholar]
- Pugh T. D., Goldfarb S. Quantitative histochemical and autoradiographic studies of hepatocarcinogenesis in rats fed 2-acetylaminofluorene followed by phenobarbital. Cancer Res. 1978 Dec;38(12):4450–4457. [PubMed] [Google Scholar]
- RUBIN E., HUTTERER F., DANON G., POPPER H. Deoxyribonucleic acid turnover in a heterogeneous population. Life span of hepatic ductular cells. Lab Invest. 1963 Jun;12:657–662. [PubMed] [Google Scholar]
- RUBIN E. THE ORIGIN AND FATE OF PROLIFERATED BILE DUCTULAR CELLS. Exp Mol Pathol. 1964 Jun;86:279–286. doi: 10.1016/0014-4800(64)90059-0. [DOI] [PubMed] [Google Scholar]
- Rutenburg A. M., Kim H., Fischbein J. W., Hanker J. S., Wasserkrug H. L., Seligman A. M. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969 Aug;17(8):517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- SCHAFFNER F., POPPER H. Electron microscopic studies of normal and proliferated bile ductules. Am J Pathol. 1961 Apr;38:393–410. [PMC free article] [PubMed] [Google Scholar]
- STEINER J. W., CARRUTHERS J. S. Electron microscopy of hyperplastic ductular cells in alpha-naphthyl isothiocyanateinduced cirrhosis. Lab Invest. 1963 Apr;12:471–498. [PubMed] [Google Scholar]
- Sell S. Distribution of alpha-fetoprotein- and albumin-containing cells in the livers of Fischer rats fed four cycles of N-2-fluorenylacetamide. Cancer Res. 1978 Sep;38(9):3107–3113. [PubMed] [Google Scholar]
- Sell S., Leffert H. L. An evaluation of cellular lineages in the pathogenesis of experimental hepatocellular carcinoma. Hepatology. 1982 Jan-Feb;2(1):77–86. doi: 10.1002/hep.1840020113. [DOI] [PubMed] [Google Scholar]
- Sell S., Osborn K., Leffert H. L. Autoradiography of "oval cells" appearing rapidly in the livers of rats fed N-2-fluorenylacetamide in a choline devoid diet. Carcinogenesis. 1981;2(1):7–14. doi: 10.1093/carcin/2.1.7. [DOI] [PubMed] [Google Scholar]
- Shinozuka H., Lombardi B., Sell S., Iammarino R. M. Early histological and functional alterations of ethionine liver carcinogenesis in rats fed a choline-deficient diet. Cancer Res. 1978 Apr;38(4):1092–1098. [PubMed] [Google Scholar]
- Shinozuka H., Sells M. A., Katyal S. L., Sell S., Lombardi B. Effects of a choline-devoid diet on the emergence of gamma-glutamyltranspeptidase-positive foci in the liver of carcinogen-treated rats. Cancer Res. 1979 Jul;39(7 Pt 1):2515–2521. [PubMed] [Google Scholar]
- Solt D. B., Cayama E., Tsuda H., Enomoto K., Lee G., Farber E. Promotion of liver cancer development by brief exposure to dietary 2-acetylaminofluorene plus partial hepatectomy or carbon tetrachloride. Cancer Res. 1983 Jan;43(1):188–191. [PubMed] [Google Scholar]
- Solt D. B., Medline A., Farber E. Rapid emergence of carcinogen-induced hyperplastic lesions in a new model for the sequential analysis of liver carcinogenesis. Am J Pathol. 1977 Sep;88(3):595–618. [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Tchipysheva T. A., Guelstein V. I., Bannikov G. A. alpha-fetoprotein-containing cells in the early stages of liver carcinogenesis induced by 3'-methyl-4-dimethyl-aminoazobenzene and 2-acetylaminofluorene. Int J Cancer. 1977 Sep 15;20(3):388–393. doi: 10.1002/ijc.2910200310. [DOI] [PubMed] [Google Scholar]
- Tsuda H., Lee G., Farber E. Induction of resistant hepatocytes as a new principle for a possible short-term in vivo test for carcinogens. Cancer Res. 1980 Apr;40(4):1157–1164. [PubMed] [Google Scholar]
- WILSON J. W., LEDUC E. H. Role of cholangioles in restoration of the liver of the mouse after dietary injury. J Pathol Bacteriol. 1958 Oct;76(2):441–449. doi: 10.1002/path.1700760213. [DOI] [PubMed] [Google Scholar]
- Watabe H. Early appearance of embryonic -globulin in rat serum during carcinogenesis with 4-dimethylaminoazobenzene. Cancer Res. 1971 Sep;31(9):1192–1194. [PubMed] [Google Scholar]