Abstract
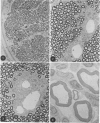
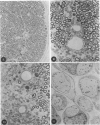
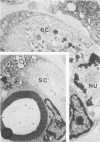
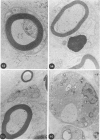
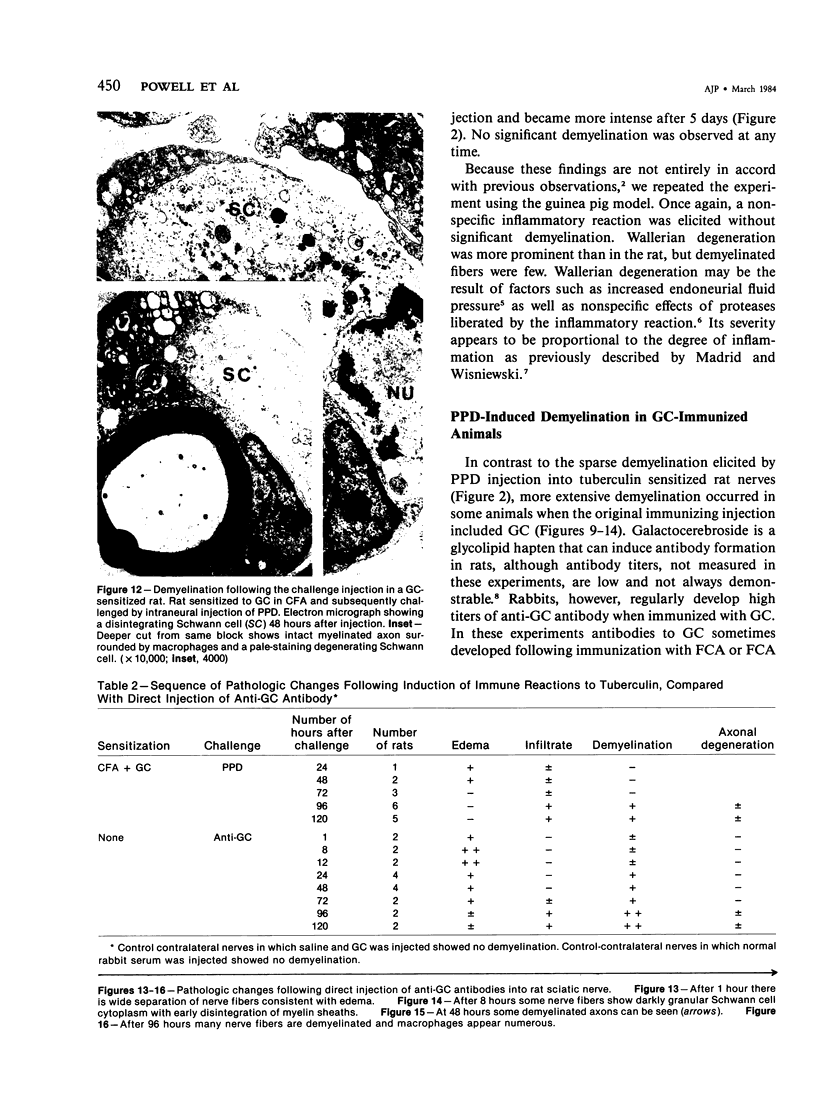
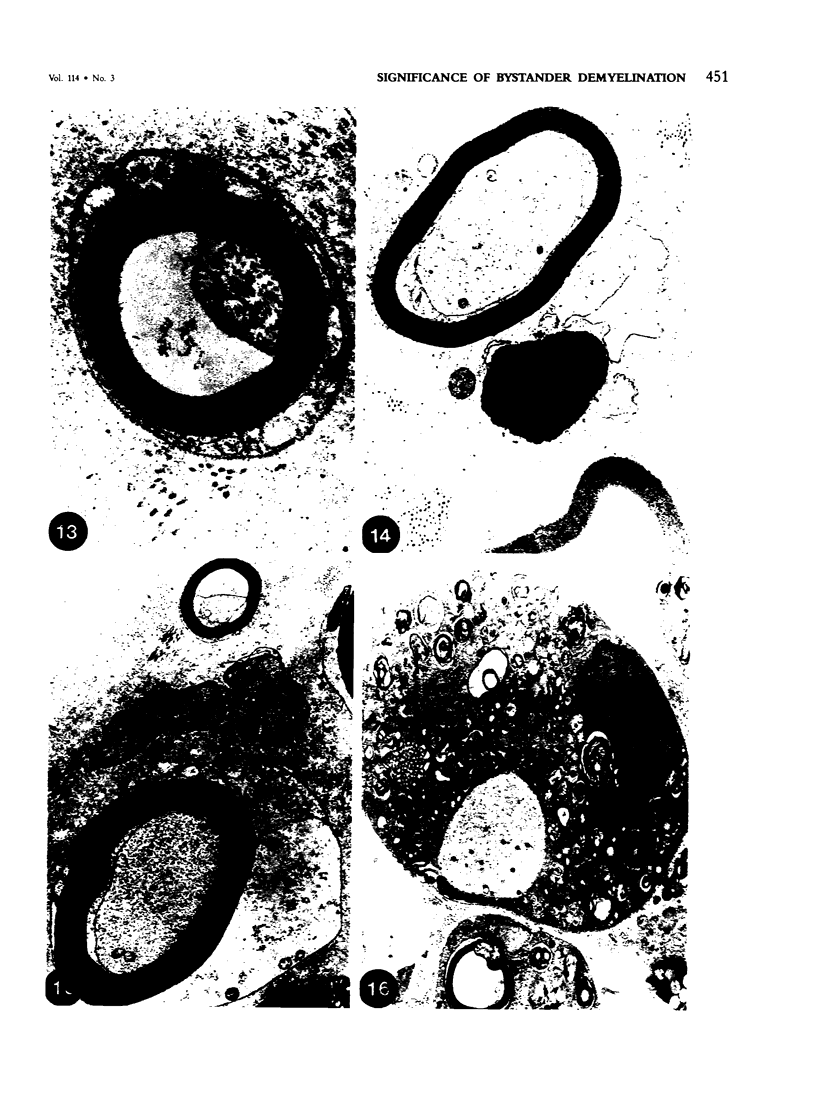
In an experiment designed to evaluate bystander demyelination in peripheral nerve, immune reactions to tuberculin and albumin failed to induce demyelination, except in animals previously sensitized to a myelin component. The peripheral nerves of tuberculin-sensitized rats and guinea pigs and rabbits were examined at intervals from 8 to 144 hours after intraneural challenge with purified protein derivative (PPD). The results were compared with the immune reaction produced by endoneurial injection of bovine serum albumin (BSA) in rats sensitized to BSA in Freund's complete antigen (FCA). Tuberculin-sensitized rats responded to endoneurial PPD injection by developing perivascular lymphocytic infiltrates which were most severe 5 days after injection. Albumin-sensitized rats responded within hours of BSA injection, showing severe endoneurial edema due to vasculitis with extravasation of polymorphonuclear leukocytes, red cells, and fibrin. The reaction tapered off within 2 days. No significant demyelination occurred in either group. However, demyelination was elicited when the hapten galactocerebroside (GC) was included in the sensitizing inoculum. Schwann-cell necrosis was visible 2 days after endoneurial challenge injection, and demyelination became extensive 5 days after injection. The findings were compared with those after direct injection of anti-GC-antibody, which produced demyelination by lysis of Schwann cells. In rabbits the occurrence of demyelination correlated with the presence of circulating antibodies to GC.
Full text
PDF

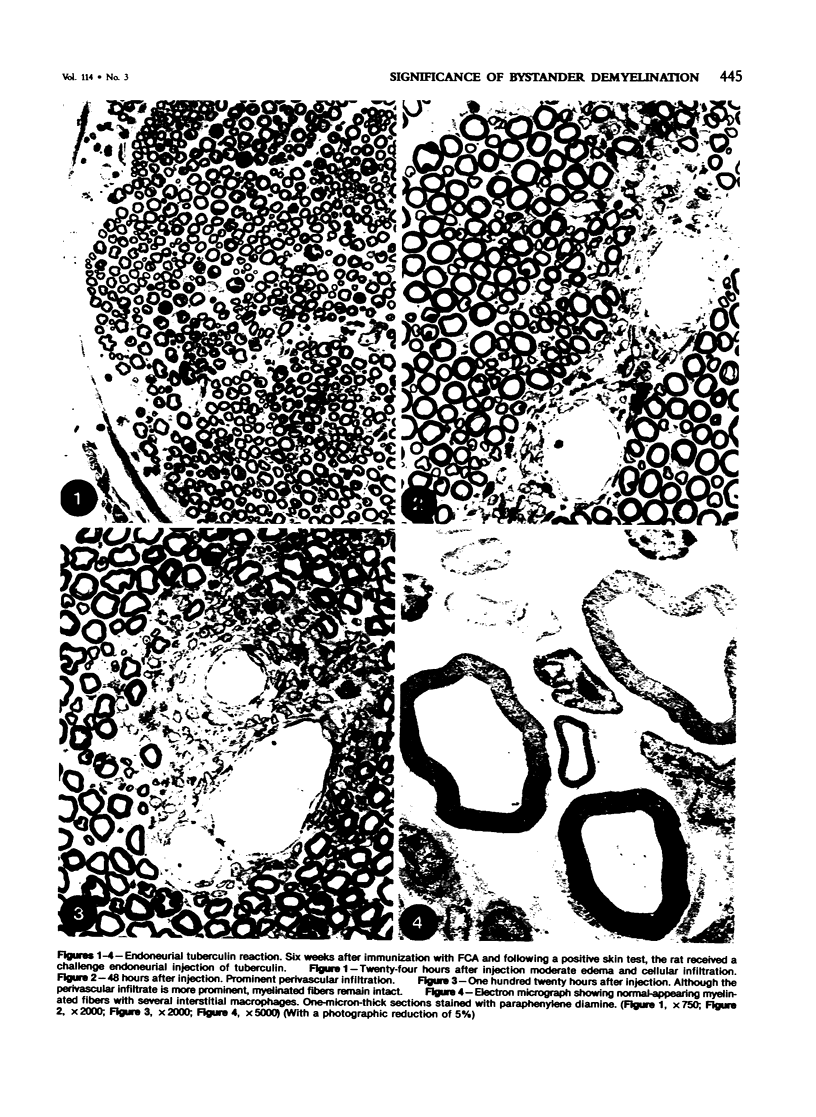

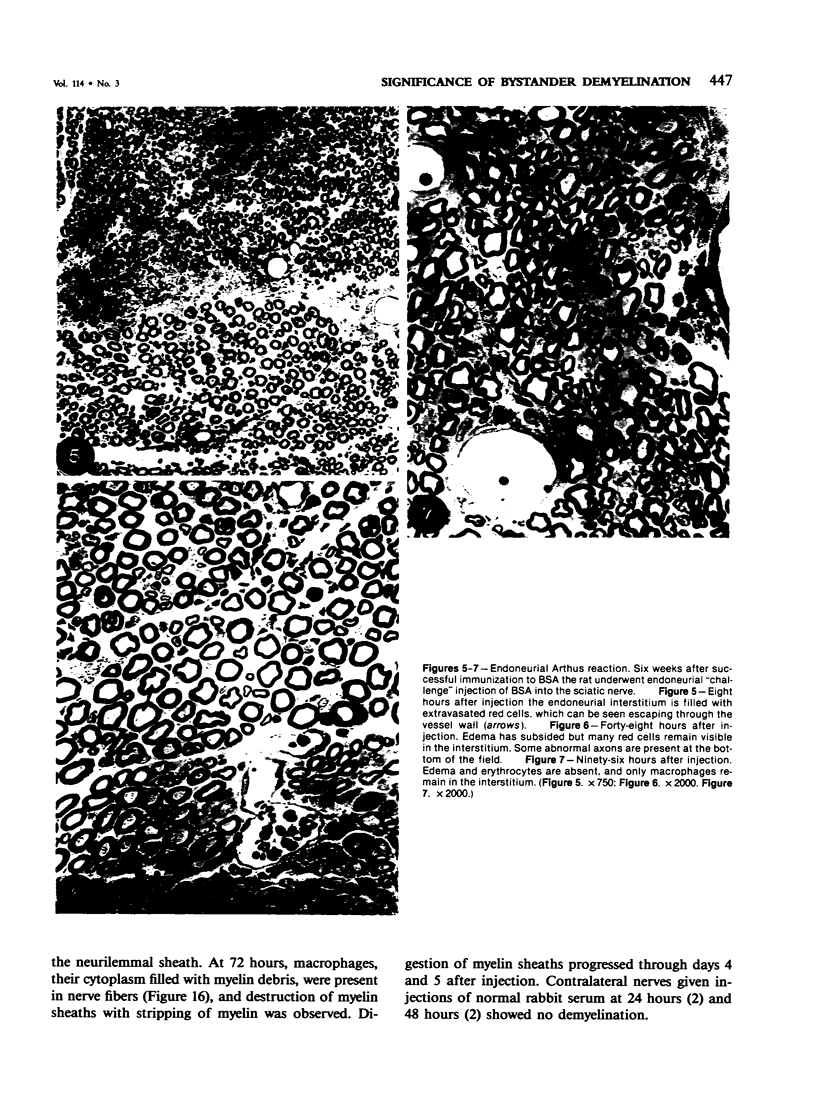
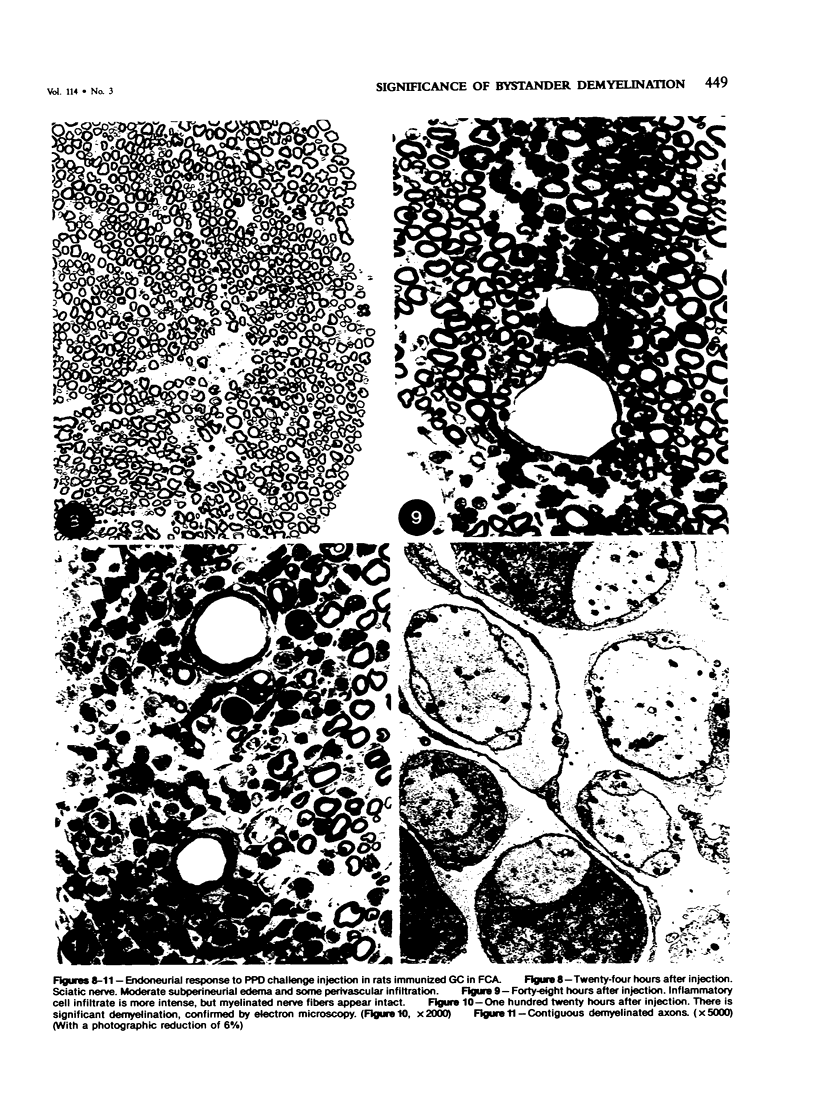

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arstila A. U., Riekkinen P. J., Rinne U. K., Pelliniemi T. T., Nevalainen T. Guillan-Barré syndrome. Neurochemical and ultrastructural study. Eur Neurol. 1971;5(5):257–269. doi: 10.1159/000114078. [DOI] [PubMed] [Google Scholar]
- Carpenter S. An ultrastructural study of an acute fatal case of the Guillain-Barré syndrome. J Neurol Sci. 1972 Feb;15(2):125–140. doi: 10.1016/0022-510x(72)90001-9. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A., Leibowitz S. The effect of homologous and heterologous carriers on the immunogenicity of the galactocerebroside hapten. Immunology. 1974 Dec;27(6):1117–1126. [PMC free article] [PubMed] [Google Scholar]
- Hart M. N., Hanks D. T., Mackay R. Ultrastructural observations in Guillain-Barre syndrome. Arch Pathol. 1972 Jun;93(6):552–555. [PubMed] [Google Scholar]
- Hughes R. A., Stedronska J. The susceptibility of rat strains to experimental allergic encephalomyelitis. Immunology. 1973 May;24(5):879–884. [PMC free article] [PubMed] [Google Scholar]
- Lampert P. W. Mechanism of demyelination in experimental allergic neuritis. Electron microscopic studies. Lab Invest. 1969 Feb;20(2):127–138. [PubMed] [Google Scholar]
- Lampert P., Garrett R., Powell H. Demyelination in allergic and Marek's disease virus induced neuritis. Comparative electron microscopic studies. Acta Neuropathol. 1977 Oct 10;40(2):103–110. doi: 10.1007/BF00688697. [DOI] [PubMed] [Google Scholar]
- Madrid R. E., Wiśniewski H. M. Axonal degeneration in demyelinating disorders. J Neurocytol. 1977 Feb;6(1):103–117. doi: 10.1007/BF01175417. [DOI] [PubMed] [Google Scholar]
- Powell H. C., Braheny S. L., Myers R. R., Rodriguez M., Lampert P. W. Early changes in experimental allergic neuritis. Lab Invest. 1983 Mar;48(3):332–338. [PubMed] [Google Scholar]
- Prineas J. W. Acute idiopathic polyneuritis. An electron microscope study. Lab Invest. 1972 Feb;26(2):133–147. [PubMed] [Google Scholar]
- Ridley A., Rainbird S. An immunofluorescence study of the passive arthus reaction in rat sciatic nerve. Neuropathol Appl Neurobiol. 1978 May-Jun;4(3):225–234. doi: 10.1111/j.1365-2990.1978.tb00539.x. [DOI] [PubMed] [Google Scholar]
- Saida K., Saida T., Brown M. J., Silberberg D. H. In vivo demyelination induced by intraneural injection of anti-galactocerebroside serum: a morphologic study. Am J Pathol. 1979 Apr;95(1):99–116. [PMC free article] [PubMed] [Google Scholar]
- Stoner G. L., Brosnan C. F., Wisniewski H. M., Bloom B. R. Studies on demyelination by activated lymphocytes in the rabbit eye. I. Effects of a mononuclear cell infiltrate induced by products of activated lymphocytes. J Immunol. 1977 Jun;118(6):2094–2102. [PubMed] [Google Scholar]
- Wisniewski H. M., Bloom B. R. Primary demyelination as a nonspecific consequence of a cell-mediated immune reaction. J Exp Med. 1975 Feb 1;141(2):346–359. doi: 10.1084/jem.141.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiśniewski H., Prineas J., Raine C. S. An ultrastructural study of experimental demyelination and remyelination. I. Acute experimental allergic encephalomyelitis in the peripheral nervous system. Lab Invest. 1969 Aug;21(2):105–118. [PubMed] [Google Scholar]
- Wiśniewski H., Terry R. D., Whitaker J. N., Cook S. D., Dowling P. C. Landry-Guillain-Barré syndrome. A primary demyelinating disease. Arch Neurol. 1969 Sep;21(3):269–276. doi: 10.1001/archneur.1969.00480150059008. [DOI] [PubMed] [Google Scholar]