Abstract
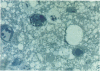
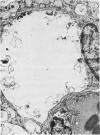
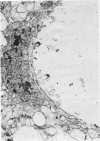
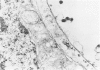
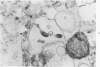
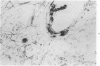
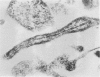
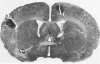
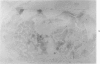
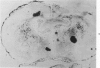
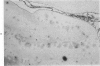
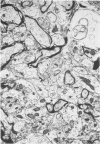
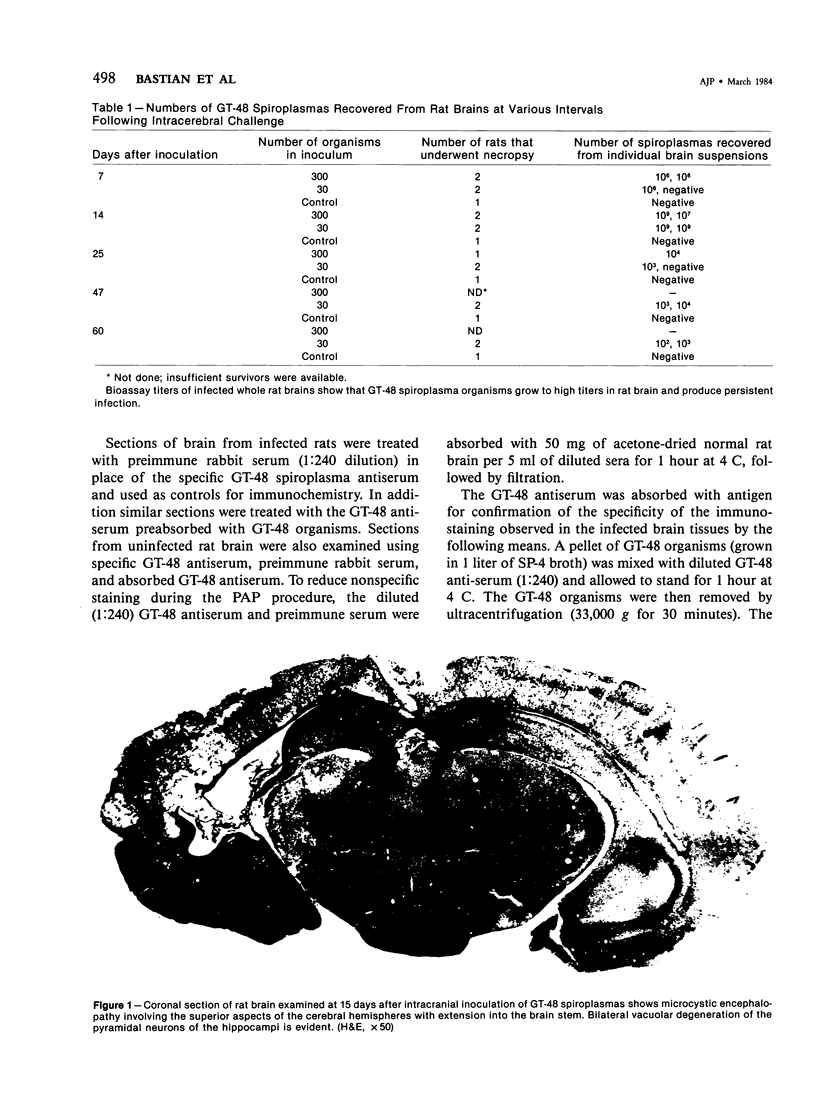
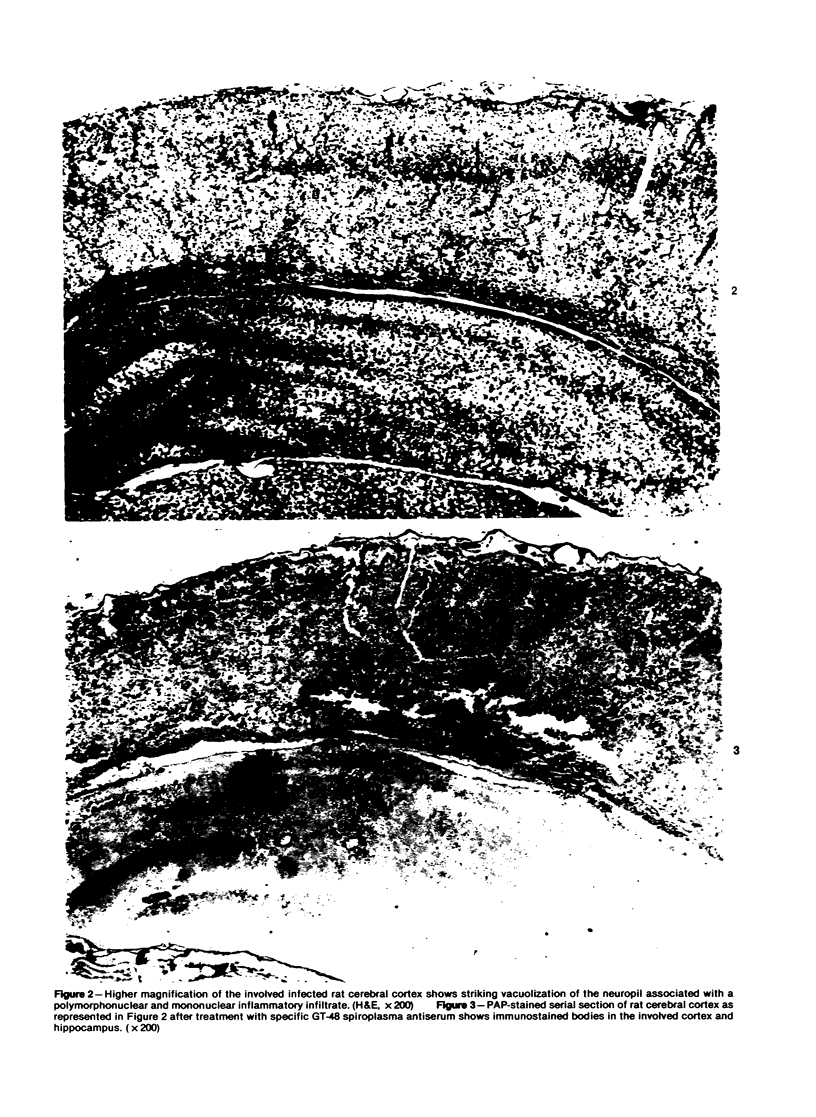
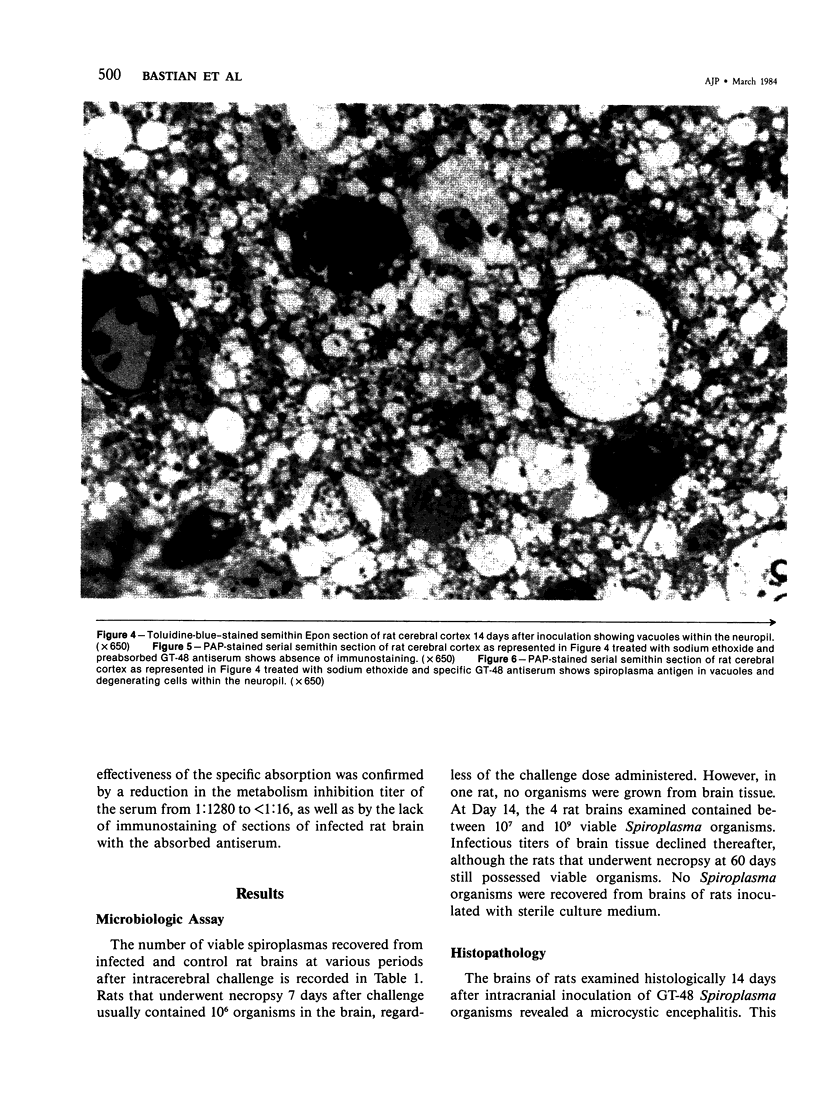
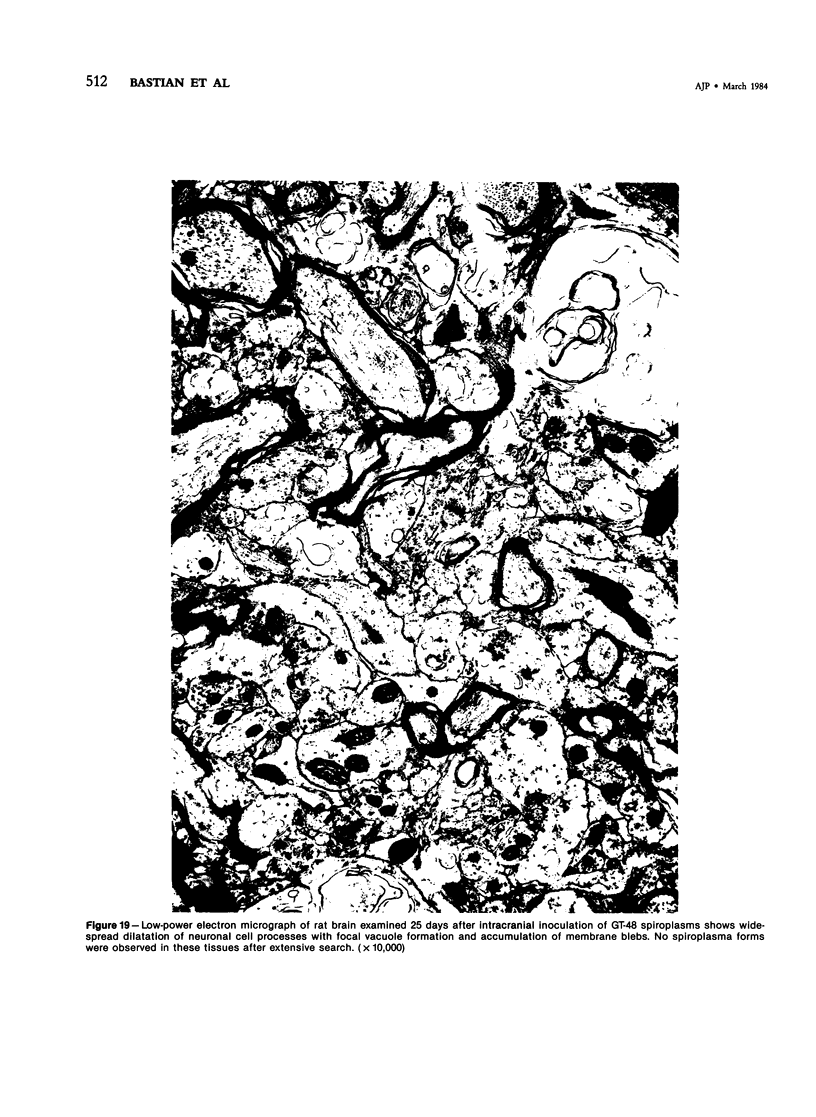
This study was designed to demonstrate the neuropathology of persistent spiroplasma infection in the rat brain. GT-48 spiroplasmas were inoculated intracranially into a series of suckling Sprague-Dawley rats. Their brains were evaluated at specific time intervals by microbiologic assay and by morphologic studies including histology, electron microscopy, and immunocytochemistry. The spiroplasmas were observed in the tissues by electron microscopy at peak infection 14 days after intracranial inoculation. At that time they were seen in vacuoles and neuronal processes within the neuropil as filamentous or bleb-like forms. A single tight spiral was identified that closely resembled the spiroplasma-like inclusions previously reported in Creutzfeldt-Jakob disease. The spiroplasmas were shown to spread rapidly throughout the brain tissues presumably by intraneuronal transport. In specimens examined at 25 days after intracranial inoculation and beyond, organisms were localized to gray matter without inflammatory response. The spiroplasmas could not be identified by electron microscopy in the rat brain tissue at late stages of infection. This study has shown an unusual adaptation of spiroplasma infection to the mammalian host brain tissues.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian F. O., Hart M. N., Cancilla P. A. Additional evidence of spiroplasma in Creutzfeldt-Jakob disease. Lancet. 1981 Mar 21;1(8221):660–660. doi: 10.1016/s0140-6736(81)91571-3. [DOI] [PubMed] [Google Scholar]
- Bastian F. O., Jennings R. A. Creutzfeldt-Jakob disease: procedures for handling diagnostic and research materials. Infect Control. 1984 Jan;5(1):48–50. doi: 10.1017/s0195941700058823. [DOI] [PubMed] [Google Scholar]
- Bastian F. O. Spiroplasma-like inclusions in Creutzfeldt-Jakob disease. Arch Pathol Lab Med. 1979 Dec;103(13):665–669. [PubMed] [Google Scholar]
- CLARK H. F. SUCKLING MOUSE CATARACT AGENT. J Infect Dis. 1964 Dec;114:476–487. doi: 10.1093/infdis/114.5.476. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Karson D. T. Suckling mouse cataract agent (SMCA) in mice. I. Factors affecting the incidence of cataracts and growth of virus in several strains of mice. J Immunol. 1968 Oct;101(4):776–781. [PubMed] [Google Scholar]
- Clark H. F. The suckling mouse cataract agent (SMCA). A slow mycoplasma-like agent? Prog Med Virol. 1974;18(0):307–322. [PubMed] [Google Scholar]
- Elizan T. S., Fabiyi A., Clark H. F. Suckling mouse cataract agent (SMCA)-induced hydrocephalus and chronic brain infection in newborn rats. Proc Soc Exp Biol Med. 1972 Jan;139(1):51–55. doi: 10.3181/00379727-139-36074. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Gray A., Francis R. J., Scholtz C. L. Spiroplasma and Creutzfeldt-Jakob disease. Lancet. 1980 Jul 19;2(8186):152–152. doi: 10.1016/s0140-6736(80)90041-0. [DOI] [PubMed] [Google Scholar]
- Merz P. A., Somerville R. A., Wisniewski H. M., Iqbal K. Abnormal fibrils from scrapie-infected brain. Acta Neuropathol. 1981;54(1):63–74. doi: 10.1007/BF00691333. [DOI] [PubMed] [Google Scholar]
- Reyes J. M., Hoenig E. M. Intracellular spiral inclusions in cerebral cell processes in Creutzfeldt-Jakob disease. J Neuropathol Exp Neurol. 1981 Jan;40(1):1–8. [PubMed] [Google Scholar]
- Schwartz J., Elizan T. S. Further characterization of suckling mouse cataract agent (SMCA): a slow, persistent infection of the nervous system. Proc Soc Exp Biol Med. 1972 Nov;141(2):699–704. doi: 10.3181/00379727-141-36855. [DOI] [PubMed] [Google Scholar]
- Stanek G., Hirschl A., Laber G. Sensitivity of various spiroplasma strains against ethanol, formalin, glutaraldehyde, and phenol. Zentralbl Bakteriol Mikrobiol Hyg B. 1981 Dec;174(4):348–354. [PubMed] [Google Scholar]
- Townsend R., Burgess J., Plaskitt K. A. Morphology and ultrastructure of helical and nonhelical strains of Spiroplasma citri. J Bacteriol. 1980 Jun;142(3):973–981. doi: 10.1128/jb.142.3.973-981.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Bastian F. O., Rose D. L. Localization and persistence of spiroplasmas in an experimental brain infection in suckling rats. Ann Microbiol (Paris) 1984 Jan-Feb;135A(1):111–117. doi: 10.1016/s0769-2609(84)80066-6. [DOI] [PubMed] [Google Scholar]
- Tully J. G. Interaction of spiroplasmas with plant, arthropod, and animal hosts. Rev Infect Dis. 1982 May-Jun;4 (Suppl):S193–S199. doi: 10.1093/clinids/4.supplement_1.s193. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Williamson D. L., Clark H. F. Suckling mouse cataract agent is a helical wall-free prokaryote (spiroplasma) pathogenic for vertebrates. Nature. 1976 Jan 15;259(5539):117–120. doi: 10.1038/259117a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. L. Unusual fibrils from the spirochete-like sex ratio organism. J Bacteriol. 1974 Feb;117(2):904–906. doi: 10.1128/jb.117.2.904-906.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]