Abstract
The proteasome plays a fundamental role in processes essential for cell viability. A loss in proteasome function has been associated with aging, as well as a number of age-related diseases. Defining the mechanism(s) behind this loss in function will add important information regarding the molecular basis for aging. In the current study, we performed an age-based comparison of proteasome function and composition of subunits and regulatory proteins in the neural retina and retinal pigment epithelium (RPE) in Fischer 344 rats. In the RPE, there was no age-dependent difference in activity, subunit composition, or content of proteasome regulators, PA28 and PA700. In contrast, the aged neural retina demonstrated a significant reduction in the chymotrypsin-like activity and decreased degradation of both casein and casein modified by 4-hydroxynonenal. This loss in function could not be explained by differences in subunit composition, content of PA28 and PA700, or reversible modification of cysteine residues. To begin investigating the molecular basis for the age-associated decrement in proteasome function, we modified the cysteine residues in proteasome from young rats with the sulfhydryl reactive chemical N-ethylmaleimide. We observed inhibition of the chymotrypsin-like activity and decreased degradation of casein that was comparable to that seen in aged retinas. Thus, chemical modification of cysteine provides an in vitro method that partially recapitulates aging proteasome. Further studies are required to confirm irreversible modification of functionally significant cysteine as a potential mechanism behind the age-related loss in proteasome function.
Keywords: Proteasome, retina, retinal pigment epithelium, aging, chymotrypsin-like activity
INTRODUCTION
The proteasome plays a fundamental role in many processes essential for cell viability, such as cell cycle regulation, control of signal transduction and gene expression, and the degradation of oxidized and misfolded proteins (Voges et al., 1999). The proteasome exists as different oligomeric assemblies, including the 20S, 26S, and immunoproteasome. These different subtypes exhibit some specificity in function and are defined by the regulatory complex associated with the catalytic core and the composition of the catalytic subunits (Coux et al., 1996).
The 20S catalytic core is composed of four stacked rings of seven subunits each. The two outer rings contain the constitutive α subunits that interact with the regulatory complexes. The inner two rings contain the β subunits. Three of the β subunits (β1, β2, β5) contain the catalytic sites that perform distinct proteolytic activities (Craiu et al., 1997; Lee and Goldberg, 1996; Rock et al., 1994). These activities are classified as caspase-like (β1), trypsin-like (β2), and chymotrypsin-like (β5) as defined by cleavage after acidic, basic, or hydrophobic amino acids, respectively. The ATP-independent degradation of proteins by the 20S core proteasome has been suggested as the primary mechanism for degrading oxidized proteins following an oxidative insult (Davies, 2001; Reinheckel et al., 1998).
Following an inflammatory stimulus, such as exposure to tumor necrosis factor α or interferon γ, the constitutive catalytic subunits β1, β2, and β5 can be replaced in nascent proteasomes by the inducible subunits LMP2 (β1i), MECL-1 (β2i), and LMP7 (β5i), respectively (Hallermalm et al., 2001; Kloetzel et al., 1999; Nelson et al., 2000). The core containing the inducible subunits associates with the proteasome regulatory complex, PA28, to form the immunoproteasome, which generates antigenic peptides suitable for presentation by MHC class 1 molecules.
Association of the regulatory complex PA700 with the 20S catalytic core forms the 26S proteasome (Voges et al., 1999). The 26S proteasome requires ATP for activation and is responsible for the degradation of many ubiqutinated (Hochstrasser, 1996), and some non-ubiquitinated proteins (Kisselev et al., 1999). The dynamic assembly of proteasome subtypes is essential for allowing the cell to accommodate fluctuations in environmental conditions. Thus, an age-dependent change in relative abundance of components for each subtype could alter proteasome’s ability to perform specific functions that subsequently could have an impact on cell viability.
In previously published work, we showed a significant decrease in the chymotrypsin-like activity in the neural retina from aged F344 x Brown Norway F1 hybrid rats (Louie et al., 2002). In the current study, we have extended the investigation of proteasome function to albino F344 rats, an alternative rodent model of aging, to determine if the loss in function was a bona fide aging effect or simply due to a genetic anomaly associated with a specific rat strain. In addition, we have included an analysis of the retinal pigment epithelium (RPE) to fully characterize age-related changes to retinal proteasome. The neural retina and RPE are anatomically and functionally distinct regions of the retina (Gordon and Bazan, 1997). The neural retina is composed of seven different cell types that are responsible for transmitting the signals for vision. The RPE is a monolayer of cells that form the blood-retina barrier. It’s function is to provide nutrients to the neural retina and phagocytose the spent tips of rod outer segments. Our results show region-specific differences in how aging affects proteasome function; inhibition of the chymotrypsin-like activity and decrease degradation of a model substrate was observed only in the neural retina. Additionally, in vitro chemical modification of cysteine residues recapitulated the effect of aging on proteasome function, suggesting cysteine modification as a potential mechanism for the age-related loss in proteasome function.
MATERIALS AND METHODS
Description of the Animal Model
Fisher 344 rats were obtained from the aging rodent colony of the University of Minnesota maintained by the Minneapolis Veterans Administration. Rats were housed under pathogen-free conditions with 12 hour cycles of light and dark. Food and water were provided ad libitum. An animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota and followed the guidelines established by the National Institutes of Health. Ages of rats included in this study and the corresponding percent survival for each age are 7–12 months (young mature adult, ~99% survival), 13–16 months (middle age, ~98% survival), 22–24 months (old, ~58% survival), and 26–28 months (very old, ~27% survival) (Turturro et al., 1999).
Preparation of retinal homogenates
Each retinal sample contained the paired neural retinas from a single rat that were processed as outlined previously (Louie et al., 2002). The supernatant containing soluble retinal proteins from the final step of processing was retained and aliquots were stored at −80°C. Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay reagents (Pierce, Rockford, IL). Bovine serum albumin was used as the standard protein.
Enrichment of retinal homogenates for Proteasome
Retinal homogenates were enriched for proteasome by centrifugation at 100,000 x g at 4 °C for 16 hours. Pellets were resuspended in 50 mM Tris (pH 7.8). Preparations enriched in proteasome were used for comparing the degradation of modified and unmodified casein.
Preparation of RPE lysates
Following removal of the neural retina, RPE cells were harvested from the eyecups by gentle disruption of the cell layer with a soft bristled paintbrush. Cells were collected in 0.3M sucrose, 20mM Tris-acetate (pH 7.2) and pelleted at 1200 x g for 30 minutes at 4 °C. The cell pellet was resuspended in 50 mM Tris (pH 7.8), 2% 3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate (CHAPS). The resuspended cells were transferred to cryotubes and incubated on ice for 45 minutes. Each sample was then subjected to two cycle of freeze/thaw with liquid nitrogen followed by homogenization with 10–20 passes in a glass homogenizer with a Teflon pestle. Cellular debris was then collected by centrifugation at 600 x g for 15 minutes at 4 °C. The supernatant was retained and the protein concentration determined using the BCA protein assay. The RPE from one rat was used for each preparation.
Western immuoblotting
Retinal and RPE proteins were electrophoretically separated on 13% SDS-polyacrylamide gels (Laemmli, 1970), transferred to PVDF membranes, and semi-quantitatively analyzed by Western immunoblotting as previously described (Louie et al., 2002). Membranes were incubated for 16 hours at 4°C with one of the following primary polyclonal antibodies: C2/α6 subunit of the 20S proteasome, LMP2, LMP7, 20SX(β5), 20SY (β1), α-subunit of PA28 (Affinity Bioreagents, Golden, CO), and S4 subunit of PA700 (Biomol, Plymouth Meeting, PA). Monoclonal antibodies used in this study include: α7 subunit of the 20S proteasome (Biomol, Plymouth Meeting, PA) and HSP90 (StressGen). All primary antibodies were diluted 1:1000. Immune reactions were quantified by densitometric analysis using Sigma Scan Pro 4.0. The immune reaction of a reference sample, run on each blot, was used to normalize sample reactions and allowed for comparison between blots. Results from densitometry are reported as the immunoreaction density relative to the mean value from young rats.
Hydrolysis of fluorogenic peptides
The fluorogenic peptides LLE-AMC (200 μM), LLVY-AMC (75 μM) (EMD Biosciences, San Diego, CA), and VGR-AMC (150 μM) (Biomol, Plymouth Meeting, PA), were used to measure the caspase-like, chymotrypsin-like and trypsin-like activities, respectively, as outlined (Louie et al., 2002). Assays were done in the presence or absence of the proteasome inhibitor MG132 (200 μM). (Preliminary experiments comparing activities with either MG132 or the more selective inhibitor lactacystin produced similar results. MG132 was selected for our experiments due to the significantly greater cost associated with lactacystin.) The buffer for all degradation experiments (peptides and protein) contained 50 mM Tris (pH 7.5), 5 mM MgCl2, 20 mM KCl and 0.5 mM ATP. In select experiments, chymotrypsin-like activity was measured in the absence and presence of 5 mM dithiotreitol (DTT).
Degradation of BODIPY TR-X Casein
Retinal homogenates enriched for proteasome (0.5 μg) were pre-incubated in the absence and presence of 200 μM MG132 for 30 minutes, followed by the addition of 1 μg BODIPY TR-X dye-labeled casein (EnzChek Protease Assay Kit, Invitrogen, Carlsbad, CA). Experiments were performed at 37ºC in microtiter plates using a CytoFluor 4000 Multiwell Plate Reader (ex=590 nm, em=645 nm).
Proteasome inhibition by N-ethylmaleimide (NEM)
Retinal homogenates were incubated with varying concentrations of NEM (0–200 μM) for 20 minutes at 37°C. Following NEM incubation, LLVY-AMC was added to the reaction mixture to monitor the chymotrypsin-like activity. To test the degradation of fluorogenic peptides (LLE-AMC and VGR-AMC), and BODIPY-Casein, samples were pre-incubated in 2 μM NEM using the same experimental procedure listed above.
Modification of BODIPY Casein by 4-hydroxynonenal
BODIPY casein was incubated with 4-hydroxynonenal (HNE)(Cayman Chemical) in a buffer containing 0.1 M sodium bicarbonate (pH 8.3) for one hour at 37°C. The molar ratio of HNE to casein used in these experiments was 200:1. These conditions were chosen based on preliminary experiments comparing HNE-immune reactions on Western blots for casein modified at molar ratios of 100, 200, 500, and 1000 to 1 (HNE:casein). Our results showed 200:1 produced a strong HNE immune reaction in a single band corresponding with the molecular mass of casein. Additionally, no high molecular mass aggregates were observed when casein was incubated at the 200:1 molar ratio. The HNE reaction was quenched by dialysis of the BODIPY casein in phosphate buffered saline (pH 7.2) overnight at 4°C. HNE modification was confirmed by Western immunoblotting using an antibody that recognizes 1:1 amino acid- HNE Michael adducts (1:1000) (Alpha Diagnostics, San Antonio, TX). HNE-modified BSA was used on blots as a positive control.
Degradation of HNE-modified BODIPY TR-X Dye-labeled Casein
Retinal homogenates enriched for proteasome (1.0 μg) were pre-incubated in the absence and presence of 200 μM MG132 followed by the addition of 10.0 μg HNE-modified BODIPY casein.
Statistical analysis
Differences between age groups were tested for statistical significance using a Student’s t-test analysis or ANOVA with a Tukey post-hoc test using Origin version 7.5. The level of significance was set at P < 0.05. Data are reported as mean ± SEM for all groups.
RESULTS
Measurement of Proteasome Activity
Activity of the three catalytic sites was monitored using the fluorogenic peptide substrates LLE-AMC, VGR-AMC, and LLVY-AMC to measure the caspase-like, trypsin-like, and chymotrypsin-like activities, respectively. To distinguish proteolysis by the proteasome from other co-purifying proteases, all assays were performed in the absence and presence of the proteasome inhibitor, MG132. In retinal homogenates, there was no age-dependent change in the caspase- and trypsin-like activities (Figure 1A). However, there was an ~70% decrease in the chymotrypsin-like activity of old and very old rats compared with young rats. To further define the kinetics of chymotrypsin-like inhibition, we extended our analysis to a middle aged group (13–16 months). Activity in middle aged rats was ~40% lower than young adult rats, suggesting a gradual age-dependent inhibition of the chymotrypsin-like activity. It should be noted that this reduction in activity is not statistically different than activity in young rats, but rather demonstrates a trend toward the significantly lower activity in old and very old rats.
Figure 1.
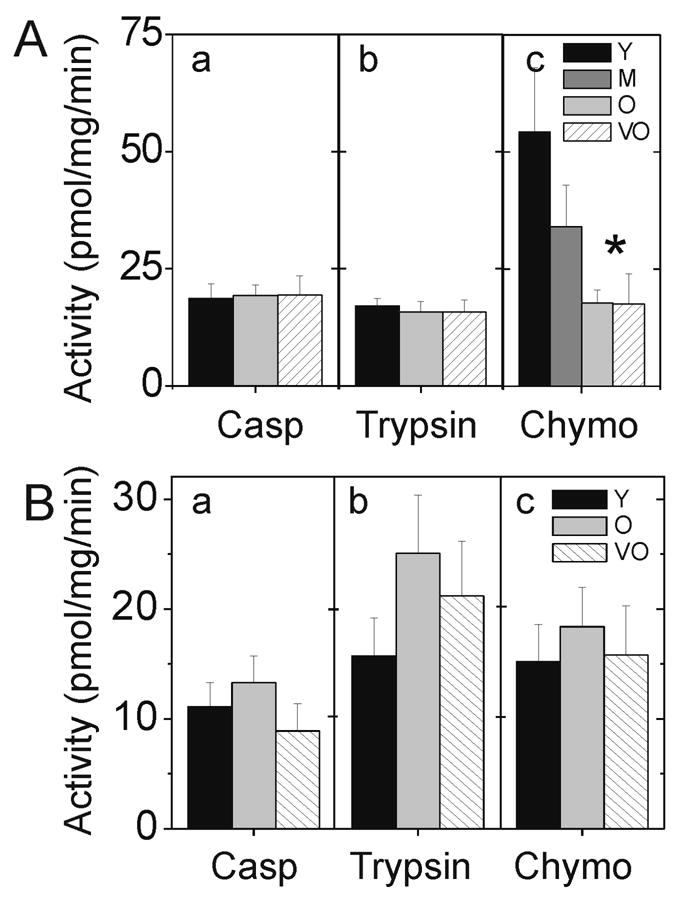
Proteasomal catalytic activity in (A) retina and (B) RPE. Hydrolysis of the fluorogenic peptides (a) LLE-AMC, (b) VGR-AMC, and (c) LLVY-AMC measure the caspase-like (Casp), trypsin-like, and chymotrypsin-like (Chymo) activities, respectively. Comparison of age groups for retinal chymotrypsin-like activity by ANOVA * p=0.02, statistically different between young and both old and very old. Comparison of age groups by ANOVA (for other activities) showed no statistical differences; all p>0.89 for retina and all p>0.38 for RPE. n= 8 – 13 per age group. Values are mean ± SEM.
In examining proteasome function in RPE lysates, no age-dependent difference in peptide cleavage was observed (Figure 1B). Taken together, our results show both tissue-specific and catalytic site-specific differences in how aging affects retinal proteasome activity.
Analysis of Proteasome Content and Subunit Composition
To provide a more complete characterization of retinal proteasome, we measured proteasome content by Western immunoblotting using antibodies directed against specific proteasomal subunits. Total proteasome content was determined from the immune reactions of the α6 or α7 subunits of the 20S core. The α-subunits are part of the constitutive complex and therefore, provide a valid estimate of the total content (Ferrington et al., 2005; Husom et al., 2004). Densitometric analysis of the immunoreaction revealed no age-dependent change in proteasome content in both the retina (Figure 2A) and RPE (Figure 2B).
Figure 2.
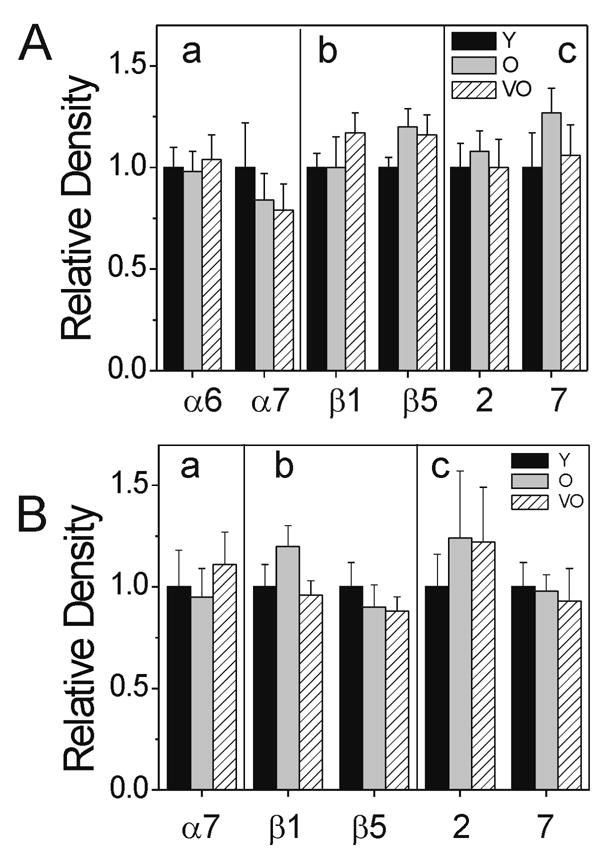
Proteasome subunit content measured by Western immunoblot in (A) retina and (B) RPE. Relative density is the immune reaction normalized to young rats. (a) Total proteasome content was determined using antibodies to either the α6 or α7 subunits of the 20S core; n= 7–14. β-subunit composition was determined using antibodies to the constitutive subunits β5 and β1 (b), or to the inducible subunits LMP2 (2) and LMP7 (7) (c). Relative density of the β-subunits is reported as a ratio of their values normalized to the α6 or α7 immunoreaction for each sample. n= 6–23 per age group. Values are mean ± SEM. Comparison of age groups by ANOVA showed no statistical differences; all p>0.15 for retina and all p>0.39 for RPE.
Since the presence of the inducible β-subunits can alter the activity of each catalytic site (Dahlmann et al., 2001; Ehring et al., 1996; Nelson et al., 2000), we examined the content of the constitutive β-subunits (β1and β5) and their inducible counterparts (LMP2 and LMP7) to seek a potential explanation for the age-dependent inhibition of the chymotrypsin-like activity in the retina. Comparison of the immune reactions showed there was no change in content of the βsubunits in either retina (Figure 2A) or RPE (Figure 2B), suggesting that the loss in chymotrypsin-like activity observed in aged retina is not due to altered composition of proteasome catalytic subunit.
Content of Regulatory Complexes
Association of the regulatory complexes, PA28 and PA700, with the 20S catalytic core has been shown to increase proteasome activity (DeMartino et al., 1996; Dubiel et al., 1992; Ferrington et al., 2005; Reidlinger et al., 1997). Using antibodies that recognize the α-subunit of PA28 and the S4 subunit of PA700, we evaluated the content of these proteasome activators in the retina and RPE. Densitometric analysis of the immune reactions showed no statistically significant age-dependent change in content for either PA28 or PA700 (Figure 3). Of note, there is a trend (p=0.19) for a decline in PA28 in aged retina that may be biologically important. Never the less, our data suggest the age-dependent loss in chymotrypsin-like activity is not due to significant changes in content of these proteasome activators.
Figure 3.
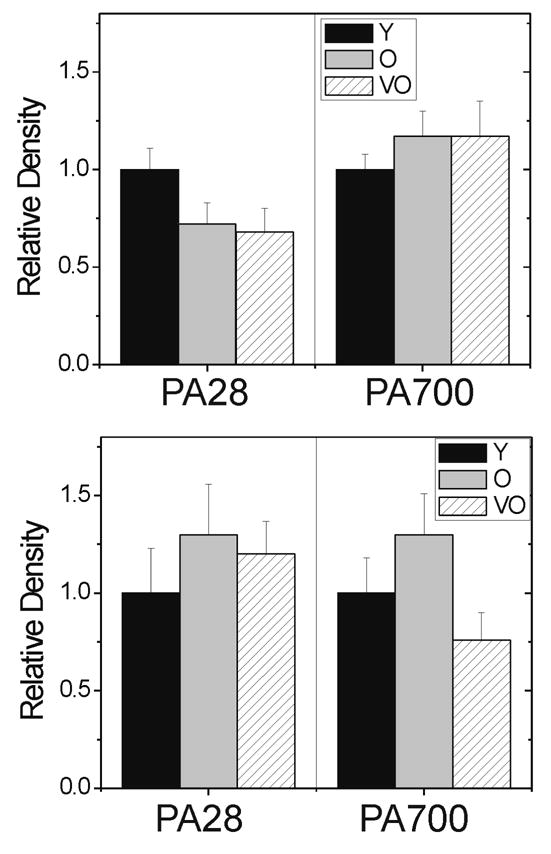
Content of PA28 and PA700 measured by Western immunoblot in the (A) retina and (B) RPE. Relative density is the immune reaction normalized to young rats. (a) PA28 content was determined from the antibody reaction to the PA28 α-subunit. (b) PA700 content was determined from the antibody reaction to the S4 subunit. n=5–7 per age group. Values are mean ± SEM. Comparison of age groups by ANOVA showed no statistical differences; all p>0.19 for PA28 and all p>0.13 for PA700.
Inhibition of Proteasome Activity with N-ethylmaleimide
Previous studies have shown oxidation or modification of a reactive cysteine residue can inhibit the chymotrypsin-like activity (Andersson et al., 1999; Demasi et al., 2003). To determine if there were age-dependent differences in the extent of reversible cysteine modification, we measured the chymotrypsin-like activity in the absence and presence of DTT. Our results showed DTT induced a slight increase in the rate of peptide hydrolysis for both young (38 ± 19%, n=4) and old (14 ± 8%, n=5) retina (p=0.23), suggesting similar degrees of cysteine oxidation. Therefore, the inhibition of the chymotrypsin-like activity was not due to differences in reversible cysteine modification but rather, could be due to irreversible cysteine modification. To test this idea, we used N-ethylmaleimide (NEM), which covalently modifies the sulfhydryl of cysteine, as a way to mimic aging through this specific chemical modification. While this chemical is not present in the cell, it is a useful means of specifically targeting cysteine residues. Incubation of retinal homogenates from young rats with increasing concentrations of NEM resulted in an exponential decrease in chymotrypsin-like activity (Figure 4). At 2 μM NEM (Figure 4, inset) chymotrypsin-like activity was inhibited ~65%, which approximated the inhibition observed in aged retina. Under these same conditions, the trypsin-like activity was not affected (data not shown). However, 2 μM NEM inhibited the caspase-like activity ~80%. These results suggest there are functionally significant cysteine residues that can be modified by NEM and inhibit both the caspase- and chymotrypsin-like activities. Thus, this reagent partially recapitulates the in vivo loss of chymotrypsin-like activity observed in aged retina. The irreversible modification of cysteine by NEM provides an in vitro method that allows parallel comparison of proteasomes aged in vivo with this in vitro model of aging. These comparisons are designed to explore the possible involvement of cysteine oxidation as a mechanism behind the selective loss of chymotrypsin-like activity in aged retina.
Figure 4.
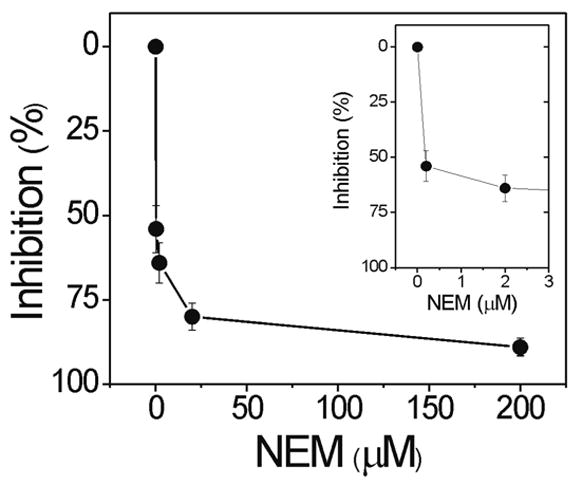
Inhibition of chymotrypsin-like activity with N-ethylmaleimide (NEM). Chymotrypsin-like activity in retinal homogenates from young rats was determined from hydrolysis of the fluorogenic peptide LLVY-AMC in the absence and presence of increasing concentrations of NEM. Inset is zoom of graph showing 2 μM NEM inhibited activity by ~ 65%. Data are mean ± SE from two separate experiments.
Degradation of Protein Substrates
To determine if the loss in chymotrypsin-like activity affects protein degradation, we used BODIPY TR-X labeled casein as a model protein substrate. This unstructured protein has been shown to be a good substrate for both the 20S and 26S proteasome (Reidlinger et al., 1997). Comparing the rates of degradation, we found a significant ~40% decrease in the rate of casein degradation for proteasome modified by 2 μM NEM (Figure 5). In addition, rates of casein degradation by aged proteasome were comparable to the slower casein degradation by NEM-modified proteasomes.
Figure 5.
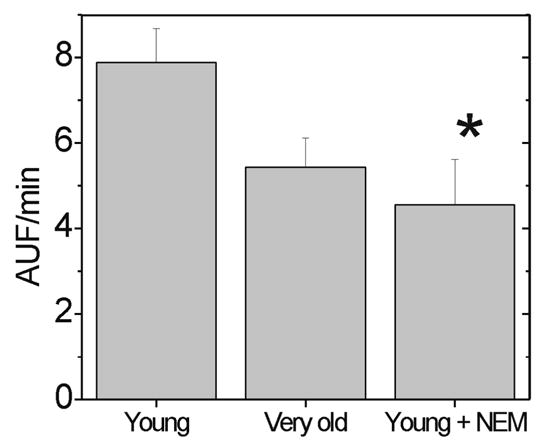
Protein Degradation with Aging and Cysteine Modification. BODIPY-Casein degradation was measured in proteasome-enriched retinal homogenates from young (n=8), very old (n=7), and young following incubation with 2 μM NEM (n=5). Comparison of groups by ANOVA *p= 0.026, statistically different between young in the absence and presence of NEM.
We also compared proteasomal degradation of an oxidized substrate to determine if there were age-dependent differences. For these experiments, we modified casein with 4-hydroxynonenal (HNE), a product of lipid peroxidation (Figure 6A). Comparing the rates of degradation, we found a significant decrease in degradation of HNE-modified casein by proteasome in aged retinas (Figure 6B).
Figure 6.
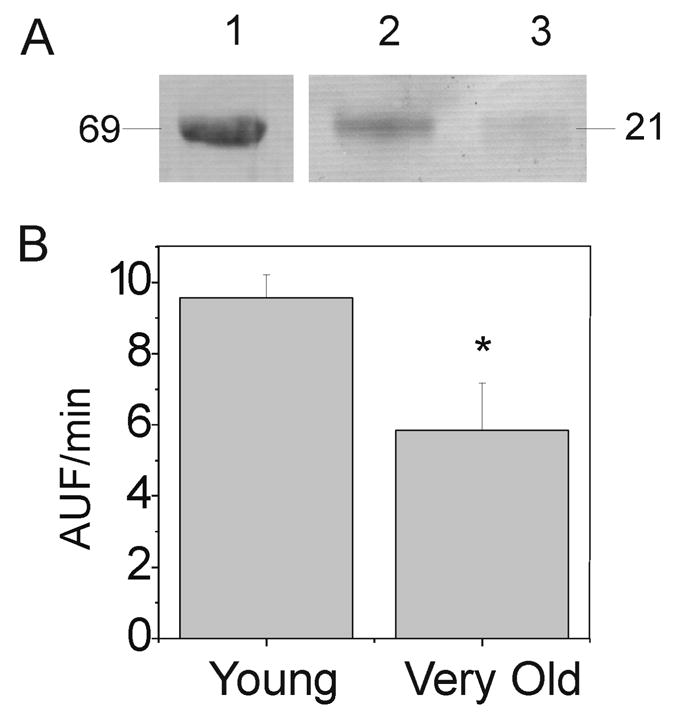
Degradation of Oxidized BODIPY-casein. (A)Western immunoblot of HNE modified proteins. Lane 1, HNE-modified BSA; Lane 2, HNE-modified BODIPY- Casein; Lane 3, Unmodified BODIPY- Casein. Apparent molecular mass (kDa) of BSA and casein are indicated. (B) Degradation of HNE-modified BODIPY-casein by proteasome-enriched retinal homogenates from young (n=5), and very old (n=5) rats. Statistically different by t-test between ages, *p=0.036.
Under our assay conditions, the 20S proteasome is the main species that is actively degrading protein (Louie et al., 2002). Recent evidence has shown that the presence of HSP90 is essential for the degradation of oxidized protein substrates by the 20S proteasome (Whittier et al., 2004). To determine if lower HSP90 content could help explain the slower degradation of HNE-modified casein, we measured HSP90 content by Western immunoblotting. Comparison of the immune reaction in proteasome-enriched preparations from young and aged retina showed HSP90 content was not different; densities were 1.0 ± 0.11 and 1.02 ± 0.12 for preparations from young (n=3) and old (n=4) rats, respectively.
DISCUSSION
Altered Proteasome function with aging
In a previous publication, we reported a significant decrease in the chymotrypsin-like activity from the neural retina of aged F344 x Brown Norway (F344BN) F1 hybrid rats (Louie et al., 2002). In the current study, we have extended the investigation to F344 rats, an alternative albino rat model of aging. In addition, we have included an analysis of the RPE to fully characterize age-related changes to the proteasome in anatomically distinct regions of the retina. For the RPE, no age-related change in activity, subunit composition, or proteasome activators was observed. However, in the aged neural retina, we confirmed our initial findings showing the chymotrypsin-like activity is preferentially inhibited. Thus, the loss in chymotrypsin-like activity is not simply due to a genetic anomaly associated with a specific rat strain.
A discrepancy in results from the two experimental systems was that old F344BN rats showed a ~50% reduction in proteasome content that was not observed in the aged albino rats. One possible explanation is that the greater propensity for light damage experienced by albino rats (Weisse, 1995) would stimulate proteasome expression to compensate for the cumulative effect of light damage. A second consideration is the chronological difference between the aged rats; the age of the oldest F344 rats was 28 months whereas the oldest F344BN rats were 36 months. While these ages correspond to approximately the same point on the lifespan curve for each strain, it is possible that the loss in proteasome content is due to the effect of environmental stresses that require greater than 28 months to become evident.
One of the limitations of estimating subunit composition from Western immunoblots of retinal homogenates is that this method does not distinguish unassembled subunits from subunits that are part of the functional complex. It is possible that the assembly of subunits into functional complexes is altered with aging. However, since peptide hydrolysis occurs only within the functional catalytic core, the observed unchanging trypsin- and caspase-like activities support the finding that there were no significant changes in total proteasome content with aging. A second limitation is that our method does not differentiate proteasomes in different cellular compartments. As a result, age-associated changes in subcellular localization would not be detected using our method. Altering the subcellular content of proteasomes could impact on specific functions, such as the degradation of misfolded proteins by proteasomes docked outside the endoplasmic reticulum.
Our results highlight two important points to consider regarding the effect of aging on proteasome function. First, while the general consensus has been that proteasome content and function declines with age (reviewed in Carrard et al., 2002; Gaczynska et al., 2001), this phenomenon is not universal as effects appear to be tissue-specific. For example, in contrast with the general decline in content reported in many tissues, we have previously reported a threefold increase in proteasome content in aged rat muscle (Ferrington et al., 2005; Husom et al., 2004). Additionally, aging can differentially affect tissues in close proximity, as has been shown comparing regions of the brain (Mishto et al., 2006; Zeng et al., 2005) and in the present study for the retina. Second, the three catalytic sites can be differentially affected in aged tissues, suggesting different mechanisms of inhibition. Exclusive inhibition of the chymotrypsin-like (Bulteau et al., 2000; Keller et al., 2000; Louie et al., 2002; Ponnapan et al., 1999), caspase-like (Anselmi et al., 1998; Conconi and Friguet, 1997) and trypsin-like (Hayashi and Goto, 1998; Radak et al., 2002) activities has been reported. Taken together, the tissue-specific differences in how aging affects proteasome function suggests exposure to environmental stressors (i.e., light, free radicals), protective mechanisms (i.e., heat shock proteins, antioxidants), or differences in proteasome subtypes (i.e., constitutive versus immunoproteasome) that are unique to each cell type can influence the extent and specificity of the effect.
Consequences of Proteasome Inhibition
Studies using catalytic site-specific mutants of yeast proteasome and site-specific inhibitors have demonstrated that the chymotrypsin-like activity primarily determines the rate of protein degradation (Craiu et al., 1997; Lee and Goldberg, 1996). Therefore, the inhibition of this activity probably has the most significant consequences for key processes involved in cell survival. For example, cells that overexpressed a mutant β5 subunit where the active site threonine was mutated to an alanine were significantly hypersensitive to oxidative stress (Li et al., 2004). These results show that inhibition of the chymotrypsin-like activity can critically affect cell survival under stressful conditions. Considering that the retina in vivo is particularly prone to oxidative stress due to the high oxygen tension and metabolic activity as well as the presence of photosensitive pigments that generate reactive oxygen species (Boulton et al., 2001), it is possible that this tissue periodically experiences sufficient stress to surpass a threshold for maintaining cellular redox homeostasis. Therefore, the loss in chymotrypsin-like activity in aged retina could result in reduced tolerance of oxidative stress via several mechanisms, including the decreased clearance of oxidatively damaged proteins. Our previous reports of increased content of oxidatively damaged proteins in aged retina (Kapphahn et al., 2006; Louie et al., 2002) could potentially result from the age-dependent loss in proteasome function.
Another consequence of inhibited proteasome function is the inability to regulate key signaling pathways. In the retina, a critical role for proteasome in regulating pathways that respond to light damage (Grimm et al., 2000; Wenzel et al., 2001) and in melatonin production (Iovone et al., 2002) have been established. Thus, a loss in proteasome function could result in dysregulation of the circadian cycles of melatonin production and impact on the retina’s ability to survive following exposure to damaging light.
Mechanisms of Chymotrypsin-like Inhibition
Evidence from previous studies showing partial reversal of peroxide-induced inhibition of chymotrypsin-like activity by DTT and the inhibition of chymotrypsin-like activity by sulfhydryl blocking compounds (Demasi et al., 2003; Andersson et al., 1999), suggest that at least one of the sites critical for maintenance of chymotrypsin-like activity is a cysteine residue. In fact, recent data suggests that the reversible S-glutathionylation of cysteine may be an important physiological regulator of the chymotrypsin-like activity (Demasi et al., 2001; Demasi et al., 2003). The current results further implicate the importance of cysteine residues for chymotrypsin-like activity by showing that the sulfhydryl-reactive compound NEM reduced degradation of both the LLVY peptide and the model substrate.
The ability of NEM to mimic the effect of aging on proteasome function suggests an age-dependent increase in cysteine modification as a potential mechanism. Our lab recently showed that DTT partially rescued peptide hydrolysis and degradation of an oxidized model protein for proteasome from aged muscle (Ferrington et al., 2005), which is consistent with cysteine oxidation contributing to the loss in function. In the current study of retinal proteasome, we observed no age-related difference in activation with DTT. Rather, the mechanism of inhibition in the retina may include modification of cysteine residues that are not reversed by DTT. Examples of irreversible modifications include oxidation of the sulhydryl to sulfonic acid and the covalent attachment of 4-hydroxynonenal (HNE) (Hamann et al., 2002; Schaur, 2003). HNE is a product of lipid peroxidation that can covalently modify cysteine, lysine, and histidine residues (Davies and Dean, 1997). The extensive modification of retinal proteins by HNE provides evidence that HNE is present and highly reactive in the retina (Kapphahn et al., 2006; Tanito et al., 2005). Our lab (Ferrington and Kapphahn, 2004) and others (Farout et al., 2006) have shown that HNE can inhibit proteasome function and that the loss in function is most dramatic for the chymotrypsin-like site. Thus, the age-related inhibition of the chymotrypsin-like activity could arise from modification of critical residues through oxidation by HNE or other cysteine-reactive reagents. However, the in vivo relevance to aging is at this time speculative, but warrants further investigation.
In summary, the cumulative results of the current study and our previous report (Louie et al., 2002) have confirmed the age-related reduction in the chymotrypsin-like activity and model protein substrate degradation in the neural retina of two different rat models of aging. In a parallel study of proteasome in the RPE, no age-dependent changes in proteasome content, activity, or proteasome regulatory proteins were detected. These results demonstrate both tissue-specific and catalytic-site specific differences in how aging affects retinal proteasome function. We could partially recapitulate aging by in vitro modification of cysteine residues with NEM. Further studies are required to confirm that irreversible modification of functionally critical cysteine residues is mechanistically involved in the age-induced loss in function.
Acknowledgments
We acknowledge the following organizations for their financial support: National Institutes of Health grant (EY013623), the Minnesota Lions and Lionesses, and an unrestricted grant to the Department of Ophthalmology from the Research to Prevent Blindness Foundation. We would like to thank Shannon Kavanaugh and Babitomiwa Giwa for their technical assistance and Curt Nordgaard and Stacy Hussong for careful reading of the manuscript.
Abbreviations
- ANOVA
Analysis of Variance
- BCA
Bicinchoninic acid
- CHAPS
3-[(3-cholamidopropyl)dimethylamino]-1-propanesulfonate
- DTT
dithiotreitol
- HNE
4-hydroxynonenal
- NEM
N-ethylmaleimide
- HSP
heat shock protein
- RPE
retinal pigment epithelium
- SDS
sodium dodecylsulfate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson M, Sjostrand J, Karlsson JO. Differential inhibition of three peptidase activities of the proteasome in human lens epithelium by heat and oxidation. Exp Eye Res. 1999;69:129–138. doi: 10.1006/exer.1999.0688. [DOI] [PubMed] [Google Scholar]
- Anselmi B, Conconi M, Veyrat-Durebex C, Turlin E, Biville F, Alliot J, Friguet B. Dietary self-selection can compensate an age-related decrease of rat liver 20S proteasome activity observed with standard diet. J Gerontol A Biol Sci Med Sci. 1998;53:B173–B179. doi: 10.1093/gerona/53a.3.b173. [DOI] [PubMed] [Google Scholar]
- Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2000;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- Carrard G, Bulteau AL, Petropoulos I, Friguet B. Impairment of proteasome structure and function in aging. Int J Biochem Cell Bio. 2002;34:1461–1474. doi: 10.1016/s1357-2725(02)00085-7. [DOI] [PubMed] [Google Scholar]
- Conconi M, Friguet B. Proteasome inactivation upon aging and on oxidation-effect of HSP90. Mol Bio Rep. 1997;24:45–50. doi: 10.1023/a:1006852506884. [DOI] [PubMed] [Google Scholar]
- Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. Lactacystin and clasto-lactacystin beta lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- Dahlmann B, Ruppert T, Kloetzel PM, Kuehn L. Subtypes of the 20S proteasomes from skeletal muscle. Biochimie. 2001;83:295–299. doi: 10.1016/s0300-9084(01)01240-8. [DOI] [PubMed] [Google Scholar]
- Davies KJA. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Dean RT. Radical-mediated protein oxidation. Oxford University; Oxford, UK: 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartino GN, Proske RJ, Moomaw CR, Strong AA, Song X, Hisamatsu H, Tanaka K, Slaughter CA. Identification, purification, and characterization of a PA700-dependent activator of the proteasome. J Biol Chem. 1996;271:3112–3118. doi: 10.1074/jbc.271.6.3112. [DOI] [PubMed] [Google Scholar]
- Demasi M, Silva GM, Netto LES. 20S proteasome from Saccharomyces cerevisiae is responsive to redox modification and is S-glutathionylated. J Biol Chem. 2003;278:679–685. doi: 10.1074/jbc.M209282200. [DOI] [PubMed] [Google Scholar]
- Demasi M, Shringarpure R, Davies KJA. Glutathiolation of the proteasome is enhanced by proteolytic inhibitors. Arch Biochem Biophys. 2001;389:254–263. doi: 10.1006/abbi.2001.2332. [DOI] [PubMed] [Google Scholar]
- Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- Ehring B, Meyer TH, Eckerskorn C, Lottspeich F, Tampe R. Effects of major histocompatibility-complex-encoded subunits of the peptidase and proteolytic activities of human 20S proteasomes: Cleavage of proteins and antigenic peptides. Eur J Biochem. 1996;235:404–415. doi: 10.1111/j.1432-1033.1996.00404.x. [DOI] [PubMed] [Google Scholar]
- Farout L, Mary J, Vinh J, Szweda LI, Friguet B. Inactivation of the proteasome by 4-hydroxy-2-nonenal is site specific and dependent on 20S proteasome subtypes. Arch Biochem Biophys. 2006;453:133–140. doi: 10.1016/j.abb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J. 2005;19:644–646. doi: 10.1096/fj.04-2578fje. [DOI] [PubMed] [Google Scholar]
- Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Gaczynska M, Osmulski PA, Ward WF. Caretaker or undertaker? The role of the proteasome in aging. Mech Ageing Dev. 2001;122:235–254. doi: 10.1016/s0047-6374(00)00246-3. [DOI] [PubMed] [Google Scholar]
- Gordon WC, Bazan NG. Retina. In: Harding JJ, editor. Biochemistry of the Eye. Chapman and Hall; London: 1997. pp. 144–244. [Google Scholar]
- Grimm C, Wenzel A, Hafezi F, Reme CE. Gene expression in the mouse retina: the effect of damaging light. Mol Vis. 2000;6:252–260. [PubMed] [Google Scholar]
- Hallermalm K, Seki K, Wei C, Catelli C, Rivoltini L, Kiessling R, Levitskaya J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood. 2001;98:1108–1115. doi: 10.1182/blood.v98.4.1108. [DOI] [PubMed] [Google Scholar]
- Hamann M, Zhang T, Hendrich S, Thomas JA. Quantitation of protein sulfinic and sulfonic acid, irreversibly oxidized protein cysteine sites in cellular proteins. Methods Enzymol. 2002;348:146–156. doi: 10.1016/s0076-6879(02)48634-x. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Goto S. Age-related changes in the 20S and 26S proteasome activities in the liver of male F344 rats. Mech Ageing Dev. 1998;102:55–66. doi: 10.1016/s0047-6374(98)00011-6. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. Ubiquitin-dependent protein degradation. Annu Rev Genet. 1996;30:405–439. doi: 10.1146/annurev.genet.30.1.405. [DOI] [PubMed] [Google Scholar]
- Husom AD, Peters EA, Kolling EA, Fugere NA, Thompson LV, Ferrington DA. Altered proteasome function and subunit composition in aged muscle. Arch Biochem Biophys. 2004;421:67–76. doi: 10.1016/j.abb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Iuvone PM, Brown AD, Haquae R, Weller J, Zawilska JB, Chaurasia SS, Ma M, Klein DC. Retinal melatonin production: Role of proteasomal proteolysis in circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci. 2002;43:564–572. [PubMed] [Google Scholar]
- Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: Identification of molecular targets. Exp Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000;113:61–70. doi: 10.1016/s0047-6374(99)00101-3. [DOI] [PubMed] [Google Scholar]
- Kisselev AF, Akopian TN, Woo KM, Goldberg AL. The size of peptides generated from protein by mammalian 26S and 20S proteasome. J Biol Chem. 1999;274:3363–3371. doi: 10.1074/jbc.274.6.3363. [DOI] [PubMed] [Google Scholar]
- Kloetzel PM, Soza A, Stohwasser R. The role of the proteasome system and the proteasome activator PA28 complex in the cellular immune response. Biol Chem. 1999;380:293–297. doi: 10.1515/BC.1999.040. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee DH, Goldberg AL. Selective inhibitors of the protesome-dependent and vacuolar pathways of protein degradation in Saccharomyces cervisiae. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- Li Z, Arnaud L, Rockwell P, Figueiredo-Pereira ME. A single amino acid substitution in a proteasome subunit triggers aggregation of ubiquitinated proteins in stressed neuronal cells. J Neurochem. 2004;90:19–28. doi: 10.1111/j.1471-4159.2004.02456.x. [DOI] [PubMed] [Google Scholar]
- Louie JL, Kapphahn RJ, Ferrington DA. Proteasome function and protein oxidation in the aged retina. Exp Eye Res. 2002;75:271–284. [PubMed] [Google Scholar]
- McNerlan SE, Rea IM, Alexander HD. A whole blood method for measurement of intracellular TNF-alpha, IFN-gamma and IL-2 expression in stimulated CD3+ lymphocytes: differences between young and elderly subjects. Exp Gerontol. 2002;37:227–234. doi: 10.1016/s0531-5565(01)00188-7. [DOI] [PubMed] [Google Scholar]
- Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M, Licastro F, Sorbi S, Pession A, Ohm T, Grune T, Franceschi C. Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging. 2006;27:54–66. doi: 10.1016/j.neurobiolaging.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Nelson JE, Altschuller-Felberg C, Loukissa A, Cardozo C. Proteasome from cytokine-treated human cells shows stimulated BrAAP activity and depressed PGPH activity. Biochem Cell Biol. 2000;78:115–118. [PubMed] [Google Scholar]
- Ponnappan U, Zhong M, Trebilcock GU. Decreased proteasome-mediated degradation in T-cells for the elderly: a role in immune senescence. Cell Immunol. 1999;192:167–74. doi: 10.1006/cimm.1998.1418. [DOI] [PubMed] [Google Scholar]
- Radak Z, Takahashi R, Kumiyama A, Nakamoto H, Ohno H, Ookawara T, Goto S. Effect of aging and late onset dietary restriction on antioxidant enzymes and proteasome activities and protein carbonylation of rat skeletal muscle and tendon. Exp Gerontol. 2002;37:1423–1430. doi: 10.1016/s0531-5565(02)00116-x. [DOI] [PubMed] [Google Scholar]
- Reidlinger J, Pike AM, Savory PJ, Murray RZ, Rivett AJ. Catalytic properties of 26S and 20S proteasomes and radiolabeling of MB1, LMP7, and C7 subunits associated with trypsin-like and chymotrypsin-like activities. J Biol Chem. 1997;272:24899–24905. doi: 10.1074/jbc.272.40.24899. [DOI] [PubMed] [Google Scholar]
- Reinheckel T, Sitte N, Ullrich O, Kuckelkorn U, Davies KJ, Grune T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem J. 1998;335:637–642. doi: 10.1042/bj3350637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- Tanito M, Elliott MH, Kotake Y, Anderson RE. Protein modifications by 4-hydroxynonenal and 4-hydroxyhexenal in light-exposed rat retina. Invest Ophthalmol Vis Sci. 2005;46:3859–3868. doi: 10.1167/iovs.05-0672. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol Biol Sci. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Voges D, Zwickl P, Baumeister W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. doi: 10.1146/annurev.biochem.68.1.1015. [DOI] [PubMed] [Google Scholar]
- Weisse I. Changes in the Aging Rat Retina. Ophthalmic Res. 1995;27:154–163. doi: 10.1159/000267862. [DOI] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Seelinger MW, Jaissle G, Hafezi F, Kretschmer R, Zrenner E, Reme CE. Prevention of photoreceptor apoptosis by activation of glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001;42:1653–1659. [PubMed] [Google Scholar]
- Whittier JE, Xiong Y, Rechsteiner MC, Squier TC. HSP90 enhances degradation of oxidized calmodulin by the 20S proteasome. J Biol Chem. 2004;279:46135–46142. doi: 10.1074/jbc.M406048200. [DOI] [PubMed] [Google Scholar]
- Zeng BY, Medhurst AD, Jackson M, Rose S, Jenner P. Proteasomal activity in brain differs between species and brain regions and changes with age. Mech Ageing Dev. 2005;126:760–766. doi: 10.1016/j.mad.2005.01.008. [DOI] [PubMed] [Google Scholar]


