Abstract
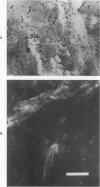
In rats, the healing process of a full-thickness dermal freeze injury differs from that of a burn wound. Whereas burn wounds heal by wound contraction, the movement of surrounding normal skin over the defect, freeze wounds heal without wound contraction. That absence of contraction may be due to the freeze wound's lack of myofibroblasts, the cells reportedly associated with wound contraction. Myofibroblasts can be demonstrated histologically by staining the F-actin filaments of the stress fibers with NBD-phallacidin, a fluorescent reagent specific to F-actin filaments. Fibroblasts in normal dermis have no staining stress fibers. However, staining myofibroblasts are uniformly distributed in the granulation tissue of the healing burn and in the islands of granulation tissue between residual connective tissue fibers in the healing freeze wound. These residual dermal fibers were identified by their patterns of birefringence. Residual connective tissue matrix persists following cold trauma and acts like an internal splint. Burn trauma destroys cells and the connective tissue matrix, which is completely replaced with granulation tissue which undergoes wound contraction. Freeze trauma kills the cellular components of dermis, while some residual connective tissue fibers endure. This study shows that the connective tissue matrix can play an important role in the control of wound contraction.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., Francis D. W., Nakajima H. Cyclic birefringence changes in pseudopods of Chaos carolinensis revealing the localization of the motive force in pseudopod extension. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1153–1161. doi: 10.1073/pnas.54.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BILLINGHAM R. E., MEDAWAR P. B. Contracture and intussusceptive growth in the healing of extensive wounds in mammalian skin. J Anat. 1955 Jan;89(1):114–123. [PMC free article] [PubMed] [Google Scholar]
- Bertolami C., Donoff R. B. The effect of full-thickness skin grafts on the actomyosin content of contracting wounds. J Oral Surg. 1979 Jul;37(7):471–476. [PubMed] [Google Scholar]
- Byers H. R., Fujiwara K. Stress fibers in cells in situ: immunofluorescence visualization with antiactin, antimyosin, and anti-alpha-actinin. J Cell Biol. 1982 Jun;93(3):804–811. doi: 10.1083/jcb.93.3.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich H. P., Trelstad R. L., Fallon J. T. Dermal vascular patterns in response to burn or freeze injury in rats. Exp Mol Pathol. 1981 Jun;34(3):281–289. doi: 10.1016/0014-4800(81)90045-9. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R., Majno G. Granulation tissue as a contractile organ. A study of structure and function. J Exp Med. 1972 Apr 1;135(4):719–734. doi: 10.1084/jem.135.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G., Ryan G. B., Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971 May 15;27(5):549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Heaysman J. E., Pegrum S. M. Early contacts between fibroblasts. An ultrastructural study. Exp Cell Res. 1973 Mar 30;78(1):71–78. doi: 10.1016/0014-4827(73)90039-6. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Li A. K., Ehrlich H. P., Trelstad R. L., Koroly M. J., Schattenkerk M. E., Malt R. A. Differences in healing of skin wounds caused by burn and freeze injuries. Ann Surg. 1980 Feb;191(2):244–248. doi: 10.1097/00000658-198002000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Gabbiani G., Hirschel B. J., Ryan G. B., Statkov P. R. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971 Aug 6;173(3996):548–550. doi: 10.1126/science.173.3996.548. [DOI] [PubMed] [Google Scholar]
- Nagai R., Yoshimoto R. N., Kamiya N. Cyclic production of tension force in the plasmodial strand of Physarum polycephalum and its relation to microfilament morphology. J Cell Sci. 1978 Oct;33:205–225. doi: 10.1242/jcs.33.1.205. [DOI] [PubMed] [Google Scholar]
- Rungger-Brändle E., Gabbiani G. The role of cytoskeletal and cytocontractile elements in pathologic processes. Am J Pathol. 1983 Mar;110(3):361–392. [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S., Moore P. L., Allen R. D. The contractile basis of amoeboid movement. I. The chemical control of motility in isolated cytoplasm. J Cell Biol. 1973 Nov;59(2 Pt 1):378–394. doi: 10.1083/jcb.59.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland T., Faulstich H. Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit Rev Biochem. 1978 Dec;5(3):185–260. doi: 10.3109/10409237809149870. [DOI] [PubMed] [Google Scholar]
- Wong A. J., Pollard T. D., Herman I. M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983 Feb 18;219(4586):867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]