Abstract
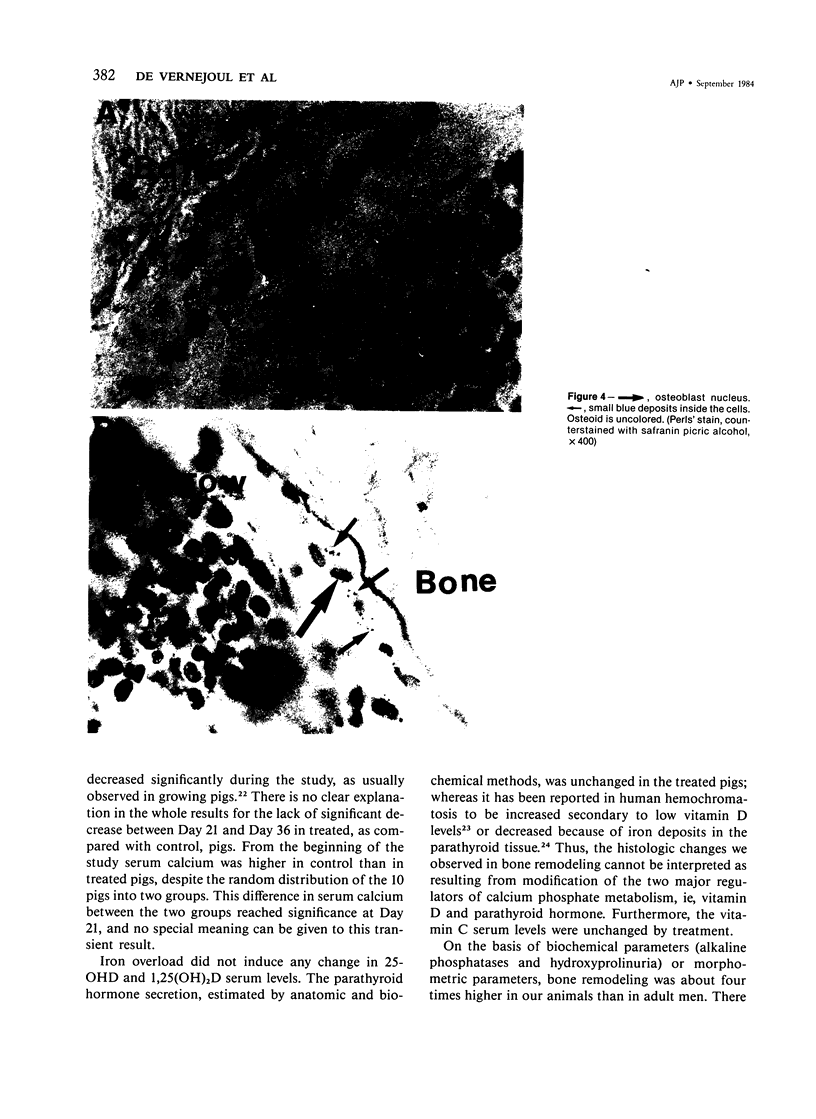
For study of the effects of an iron overload on bone remodeling, 5 control pigs were compared with 5 pigs given a total dose of 10.8 g of parenteral iron in 36 days. Treated pigs developed an iron tissue overload demonstrated by a marked increase in bone and liver iron. Except for a modest increase in SGOT, there was no biochemical or histologic sign of liver damage. Serum levels of 25-hydroxyvitamin D and 1,25-dihydroxyvitamin D were unchanged in the treated pigs. There was no accumulation of iron in the parathyroid glands and the serum immunoreactive parathyroid hormone level was unchanged in the treated animals. Bone histomorphometry after double tetracycline labeling showed that in the treated pigs osteoblast cell surfaces, double and total labeled surfaces, appositional rate, and formation at tissue level were significantly decreased, and reversal surfaces were increased. Mineralization was not impaired because the osteoid thickness was unchanged. From the morphometric measurements it was concluded that osteoblast recruitment and the collagen synthesis rate were decreased. Mean wall thickness, which indicates the amount of bone synthesized, was also lowered. In contrast, the osteoclastic resorption surfaces and the depth of lacunae resulting from osteoclast resorption were unchanged by treatment. Despite this imbalance between formation and resorption, trabecular bone mass estimated on trabecular bone volume and bone ash was unchanged after 36 days' treatment. Perls' stain revealed that iron deposits were present in osteoblast and osteoclast cells and also inside the bone matrix, because there was a linear deposit along the trabecular surfaces, cement line, and osteoid-mineralized bone interface. Therefore, because treatment induced no modification of the major humoral regulators of bone metabolism, it is suggested that iron, which was present in bone cells and matrix, could play a role in bone remodeling.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry M., Sherlock S. Measurement of liver-iron concentration in needle-biopsy specimens. Lancet. 1971 Jan 16;1(7690):100–103. doi: 10.1016/s0140-6736(71)90838-5. [DOI] [PubMed] [Google Scholar]
- Brink B., Disler P., Lynch S., Jacobs P., Charlton R., Bothwell T. Patterns of iron storage in dietary iron overload and idiopathic hemochromatosis. J Lab Clin Med. 1976 Nov;88(5):725–731. [PubMed] [Google Scholar]
- Brissot P., Bourel M., Herry D., Verger J. P., Messner M., Beaumont C., Regnouard F., Ferrand B., Simon M. Assessment of liver iron content in 271 patients: a reevaluation of direct and indirect methods. Gastroenterology. 1981 Mar;80(3):557–565. [PubMed] [Google Scholar]
- Cooperberg A. A., Rosenberg A., Schwartz J. P. Diagnostic value of bone marrow iron deposits in idiopathic hemochromatosis. Arch Intern Med. 1977 Jun;137(6):748–751. [PubMed] [Google Scholar]
- DELBARRE F. [Osteoporosis in hemochromatosis]. Sem Hop. 1960 Dec 20;36:3279–3294. [PubMed] [Google Scholar]
- Darby A. J., Meunier P. J. Mean wall thickness and formation periods of trabecular bone packets in idiopathic osteoporosis. Calcif Tissue Int. 1981;33(3):199–204. doi: 10.1007/BF02409438. [DOI] [PubMed] [Google Scholar]
- Frost H. M. Tetracycline-based histological analysis of bone remodeling. Calcif Tissue Res. 1969;3(3):211–237. doi: 10.1007/BF02058664. [DOI] [PubMed] [Google Scholar]
- Gokal R., Millard P. R., Weatherall D. J., Callender S. T., Ledingham J. G., Oliver D. O. Iron metabolism in haemodialysis patients. A study of the management of iron therapy and overload. Q J Med. 1979 Jul;48(191):369–391. [PubMed] [Google Scholar]
- JEE W. S., NOLAN P. D. ORIGIN OF OSTEOCLASTS FROM THE FUSION OF PHAGOCYTES. Nature. 1963 Oct 19;200:225–226. doi: 10.1038/200225a0. [DOI] [PubMed] [Google Scholar]
- Lisboa P. E. Experimental hepatic cirrhosis in dogs caused by chronic massive iron overload. Gut. 1971 May;12(5):363–368. doi: 10.1136/gut.12.5.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long R. G., Meinhard E., Skinner R. K., Varghese Z., Wills M. R., Sherlock S. Clinical, biochemical, and histological studies of osteomalacia, osteoporosis, and parathyroid function in chronic liver disease. Gut. 1978 Feb;19(2):85–90. doi: 10.1136/gut.19.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt A. M. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res. 1982;4(1):1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- Pawlotsky Y., Lancien Y., Roudier G., Hany Y., Louboutin J. Y., Ferrand B., Bourel M. Histomorphométrie osseuse et manifestations ostéo-articulaires de l'hémochromatose idiopathique. Rev Rhum Mal Osteoartic. 1979 Feb;46(2):91–99. [PubMed] [Google Scholar]
- Powell L. W. Changing concepts in haemochromatosis. Postgrad Med J. 1970 Apr;46(534):200–209. doi: 10.1136/pgmj.46.534.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preece M. A., TomlinsonS, Ribot C. A., Pietrek J., Korn H. T., Davies D. M., Ford J. A., Dunnigan M. G., O'Riordan J. L. Studies of vitamin D deficiency in man. Q J Med. 1975 Oct;44(176):575–589. [PubMed] [Google Scholar]
- RICHTER G. W., BESSIS M. C. COMMENTARY ON HEMOSIDERIN. Blood. 1965 Mar;25:370–374. [PubMed] [Google Scholar]
- Ullrey D. E., Miller E. R., Brent B. E., Bradley B. L., Hoefer J. A. Swine hematology from birth to maturity. IV. Serum calcium, magnesium, sodium, potassium, copper, zinc and inorganic phosphorus. J Anim Sci. 1967 Sep;26(5):1024–1029. doi: 10.2527/jas1967.2651024x. [DOI] [PubMed] [Google Scholar]
- Valberg L. S., Simon J. B., Manley P. N., Corbett W. E., Ludwig J. Distribution of storage iron as body stores expand in patients with hemochromatosis. J Lab Clin Med. 1975 Sep;86(3):479–489. [PubMed] [Google Scholar]
- Wapnick A. A., Lynch S. R., Seftel H. C., Charlton R. W., Bothwell T. H., Jowsey J. The effect of siderosis and ascorbic acid depletion on bone metabolism, with special reference to osteoporosis in the Bantu. Br J Nutr. 1971 May;25(3):367–376. doi: 10.1079/bjn19710101. [DOI] [PubMed] [Google Scholar]
- de Vernejoul M. C., Girot R., Gueris J., Cancela L., Bang S., Bielakoff J., Mautalen C., Goldberg D., Miravet L. Calcium phosphate metabolism and bone disease in patients with homozygous thalassemia. J Clin Endocrinol Metab. 1982 Feb;54(2):276–281. doi: 10.1210/jcem-54-2-276. [DOI] [PubMed] [Google Scholar]