Abstract
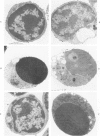
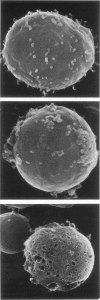
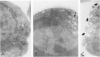
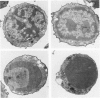
In vivo, apoptotic cells are swiftly recognized by phagocytes, presumably because of changes on their surface. This article describes surface changes in rat cortical thymocytes undergoing apoptosis induced by glucocorticoid treatment in vitro. Homogeneous populations of thymocytes early in apoptosis were prepared by isopyknic centrifugation. These cells were compared with purified nonapoptotic cells in terms of several surface characteristics, including binding to macrophages, surface ultrastructure, microelectrophoretic mobility (a measure of surface charge density), and ability to bind four lectins and four monoclonal antibodies to thymocyte antigens. Apoptotic cells bound to macrophages more avidly than did nonapoptotic cells by a process not dependent upon serum factors. Their surfaces lost microvilli and became " blistered ," apparently through fusion of vesicles of endoplasmic reticulum with the plasma membrane. The surface charge density of apoptotic cells was less than that of nonapoptotic cells. Surface antigens and lectin-binding sites were less abundant on apoptotic than on normal cells, in proportion to the general reduction in cell size observed in apoptosis. Differences between apoptotic and normal cells were not detected, however, in the relative quantities of exposed galactose, N-acetyl galactosamine, N-acetyl glucosamine, N-acetyl neuraminic acid, or of several surface antigens, including the major sialoglycoproteins of the thymocyte membrane. It appears that although several changes occur in the surface of apoptotic cells, many cell membrane structures remain intact. The changes responsible for the recognition of apoptotic cells by phagocytes are more subtle than those detectable by the binding of lectin and antibody probes, but preliminary data suggest that a lectin-sugar interaction is involved.
Full text
PDF

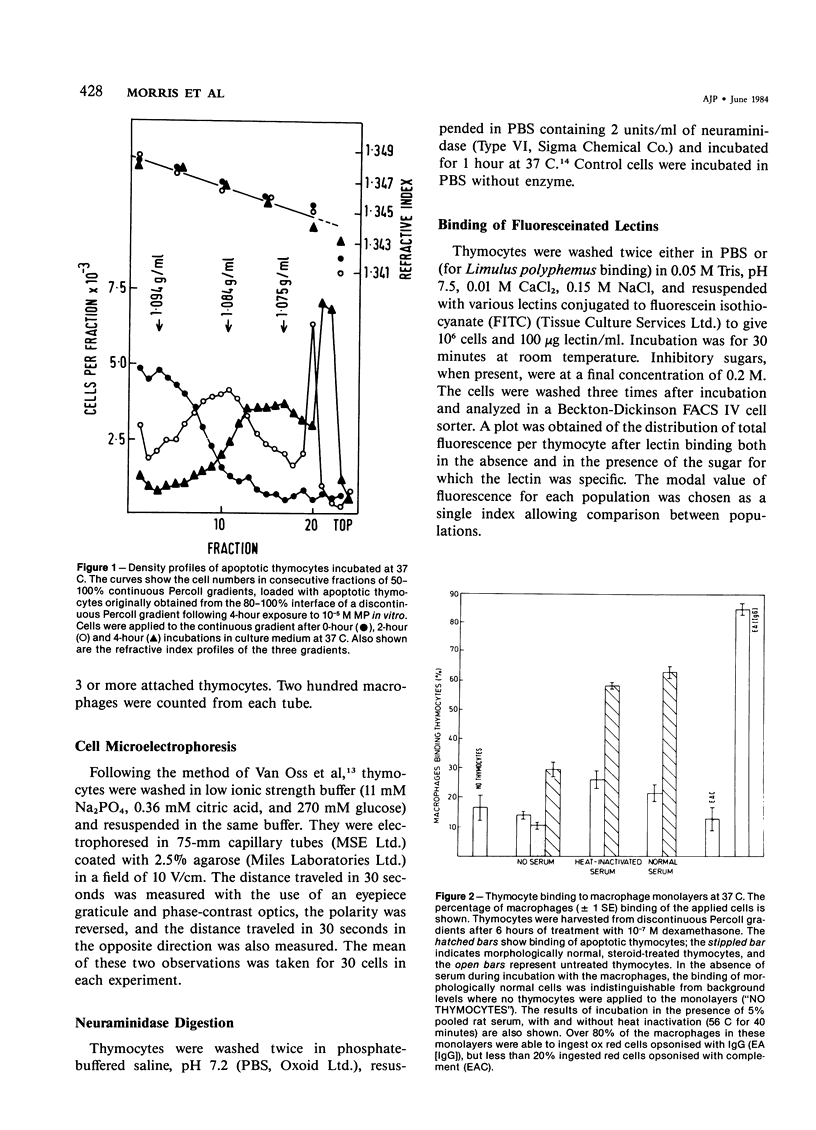

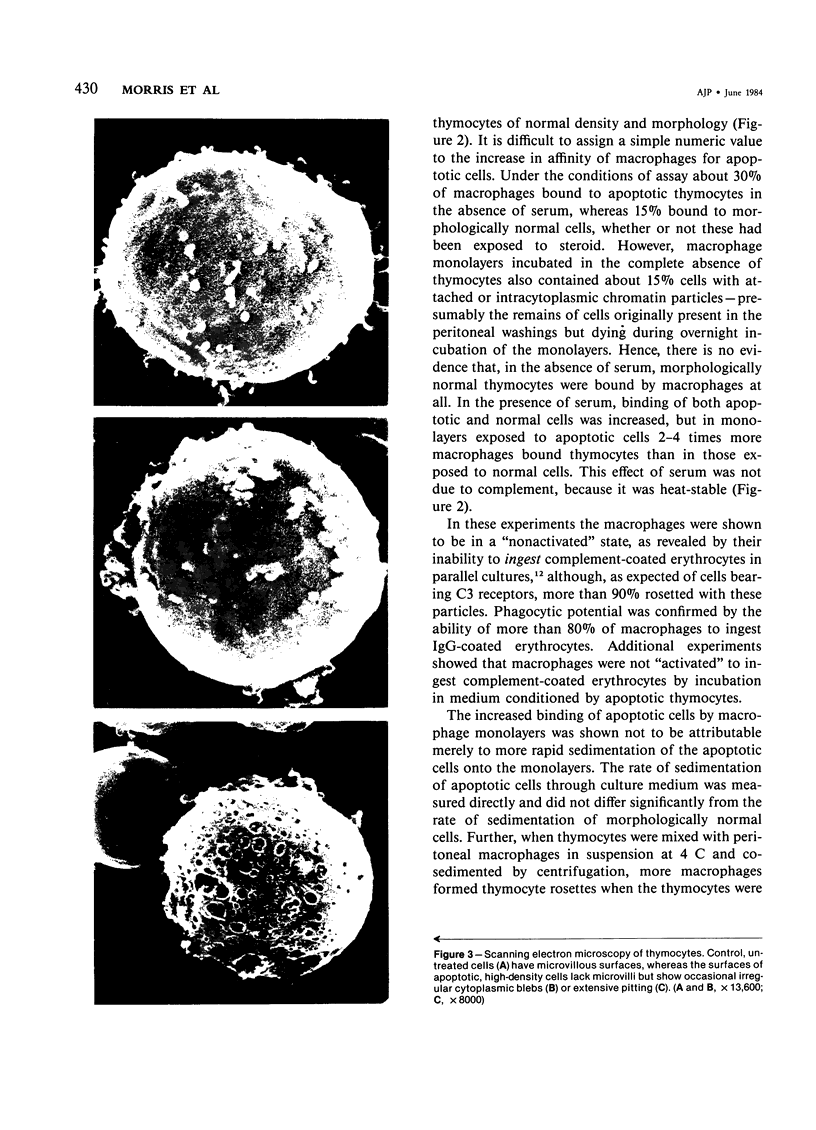


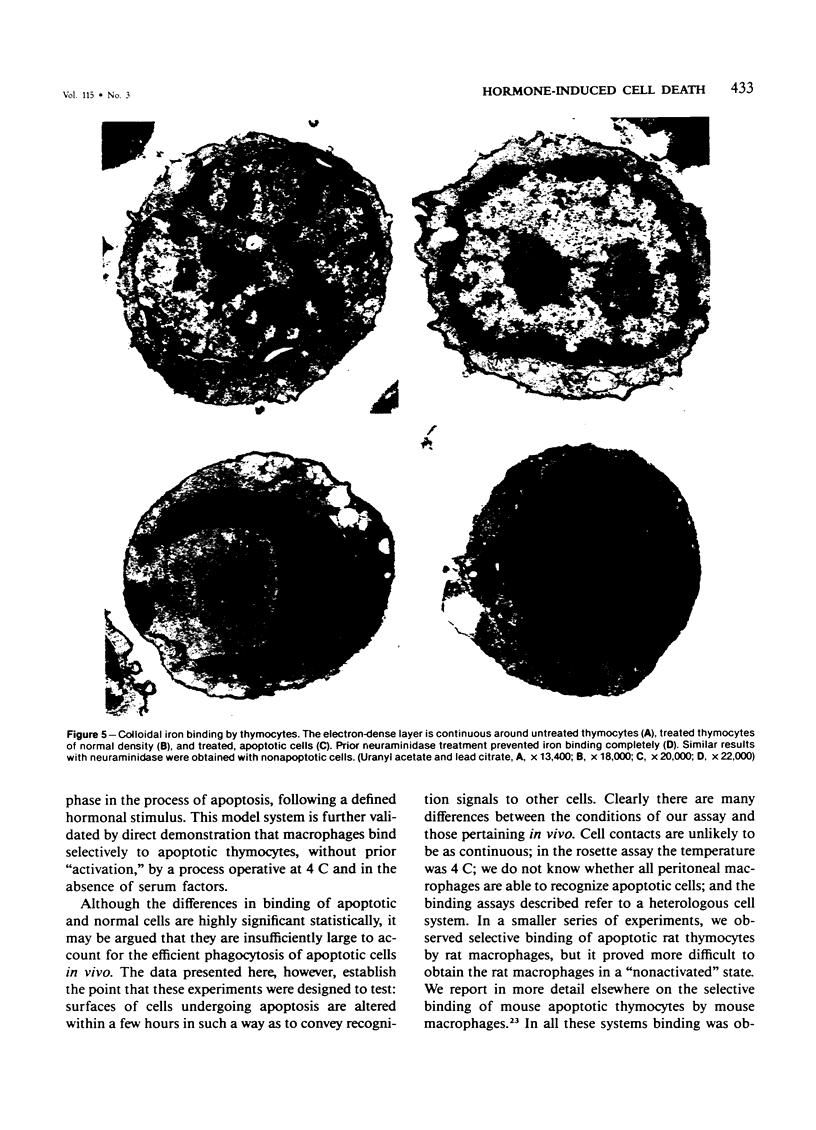
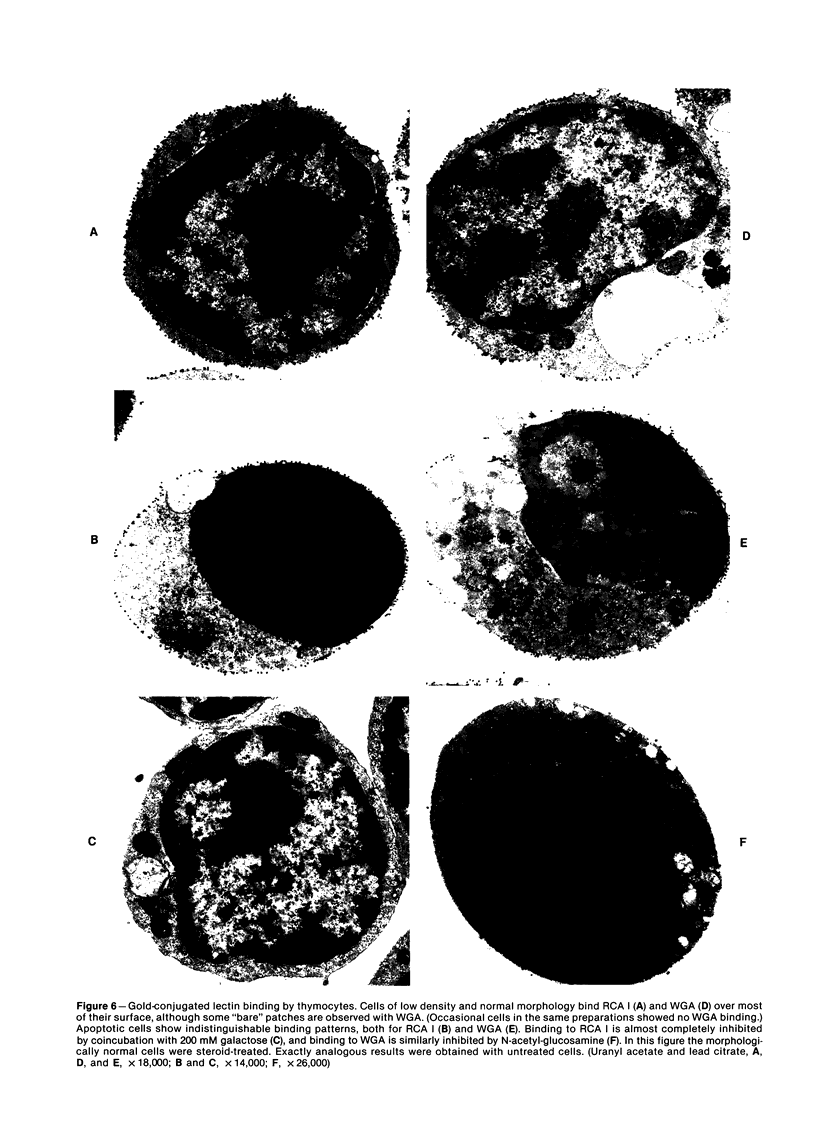

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balduini C. L., Sinigaglia F., Ascari E., Balduini C. Ageing of rabbit red cells in vitro: membrane modifications and their possible role in red cell survival in vivo. Acta Haematol. 1981;65(4):263–269. doi: 10.1159/000207190. [DOI] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med. 1975 Jun 1;141(6):1278–1290. doi: 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- Burger M. M., Goldberg A. R. Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci U S A. 1967 Feb;57(2):359–366. doi: 10.1073/pnas.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czop J. K., Fearon D. T., Austen K. F. Membrane sialic acid on target particles modulates their phagocytosis by a trypsin-sensitive mechanism on human monocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3831–3835. doi: 10.1073/pnas.75.8.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GASIC G., BERWICK L. HALE STAIN FOR SIALIC ACID-CONTAINING MUCINS. ADAPTATION TO ELECTRON MICROSCOPY. J Cell Biol. 1963 Oct;19:223–228. doi: 10.1083/jcb.19.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galili U., Leizerowitz R., Moreb J., Gamliel H., Gurfel D., Polliack A. Metabolic and ultrastructural aspects of the in vitro lysis of chronic lymphocytic leukemia cells by glucocorticoids. Cancer Res. 1982 Apr;42(4):1433–1440. [PubMed] [Google Scholar]
- Horisberger M., Rosset J. Colloidal gold, a useful marker for transmission and scanning electron microscopy. J Histochem Cytochem. 1977 Apr;25(4):295–305. doi: 10.1177/25.4.323352. [DOI] [PubMed] [Google Scholar]
- Hudgin R. L., Pricer W. E., Jr, Ashwell G., Stockert R. J., Morell A. G. The isolation and properties of a rabbit liver binding protein specific for asialoglycoproteins. J Biol Chem. 1974 Sep 10;249(17):5536–5543. [PubMed] [Google Scholar]
- Hurle J. M., Lafarga M., Hinchliffe J. R. The surface coat of embryonic limb mesenchymal cells during morphogenetic cell death. Exp Cell Res. 1981 Jun;133(2):465–470. doi: 10.1016/0014-4827(81)90343-8. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Knyszynski A. In vitro recognition of "old" red blood cells by macrophages from syngeneic mice: characteristics of the macrophage-red blood cell interaction. J Reticuloendothel Soc. 1980 Apr;27(4):411–419. [PubMed] [Google Scholar]
- Lutz H. U., Fehr J. Total sialic acid content of glycophorins during senescence of human red blood cells. J Biol Chem. 1979 Nov 25;254(22):11177–11180. [PubMed] [Google Scholar]
- Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv Carbohydr Chem Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- Nagamura Y., Kolb H. Presence of a lectin-like receptor for D-galactose on rat peritoneal macrophages. FEBS Lett. 1980 Jun 16;115(1):59–62. doi: 10.1016/0014-5793(80)80726-5. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J., Binder M. Coloidal gold, ferritin and peroxidase as markers for electron microscopic double labeling lectin techniques. J Histochem Cytochem. 1978 Mar;26(3):163–169. doi: 10.1177/26.3.632554. [DOI] [PubMed] [Google Scholar]
- Roth J., Brown D., Orci L. Regional distribution of N-acetyl-D-galactosamine residues in the glycocalyx of glomerular podocytes. J Cell Biol. 1983 May;96(5):1189–1196. doi: 10.1083/jcb.96.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow B. A., West N. B., Norman R. L., Brenner R. M. Hormonal control of apoptosis in hamster uterine luminal epithelium. Am J Anat. 1979 Sep;156(1):15–35. doi: 10.1002/aja.1001560103. [DOI] [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Fike R. M., Good R. J., Reinig J. M. Cell microelectrophoresis simplified by the reduction and uniformization of the electroosmotic backflow. Anal Biochem. 1974 Jul;60(1):242–251. doi: 10.1016/0003-2697(74)90150-x. [DOI] [PubMed] [Google Scholar]
- Vladutiu G. D., Fike R. M., Amigone V. T. Influence of sialic acid on cell surface properties in I-cell disease fibroblasts. In Vitro. 1981 Jul;17(7):588–592. doi: 10.1007/BF02618456. [DOI] [PubMed] [Google Scholar]
- Weir D. M., Ogmundsdóttir H. M. Non-specific recognition mechanisms by mononuclear phagocytes. Clin Exp Immunol. 1977 Nov;30(2):323–329. [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G. Hormone-induced cell death. Purification ad properties of thymocytes undergoing apoptosis after glucocorticoid treatment. Am J Pathol. 1982 Oct;109(1):78–87. [PMC free article] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]
- Yamada T., Ohyama H. Changes in surface morphology of rat thymocytes accompanying interphase death. J Radiat Res. 1980 Jun;21(2):190–196. doi: 10.1269/jrr.21.190. [DOI] [PubMed] [Google Scholar]