Abstract
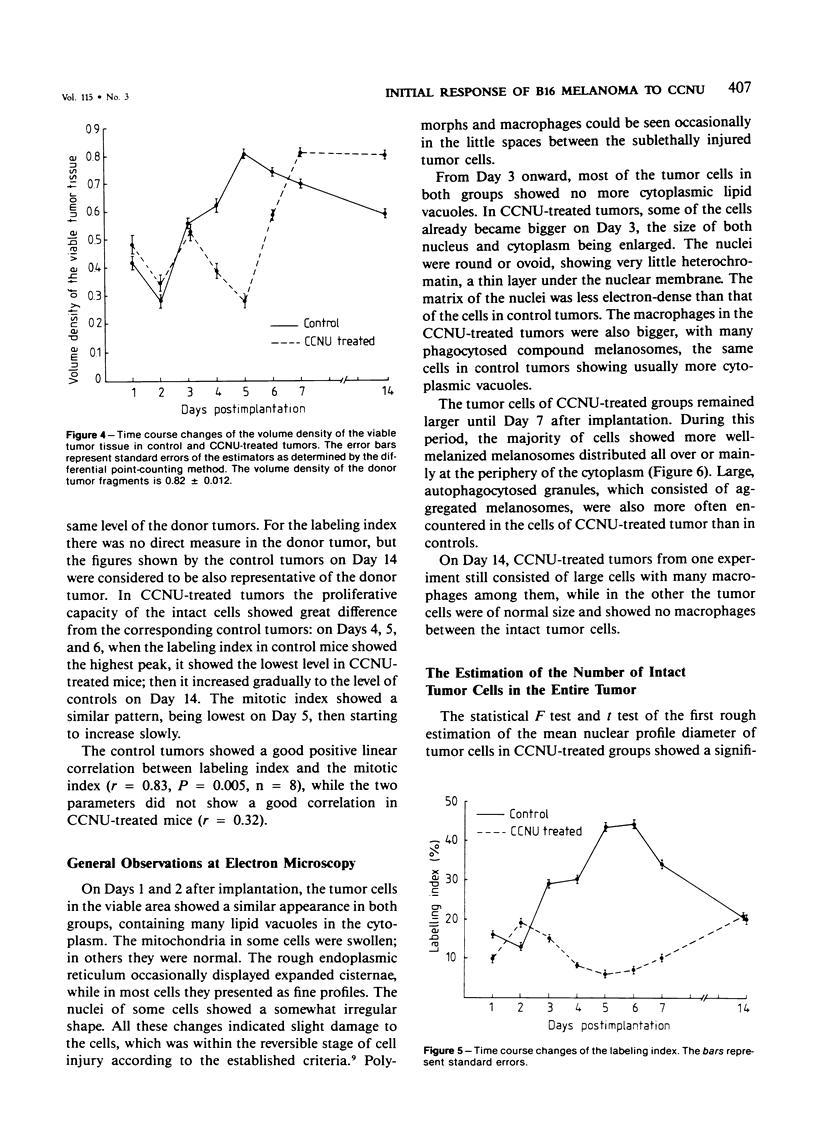
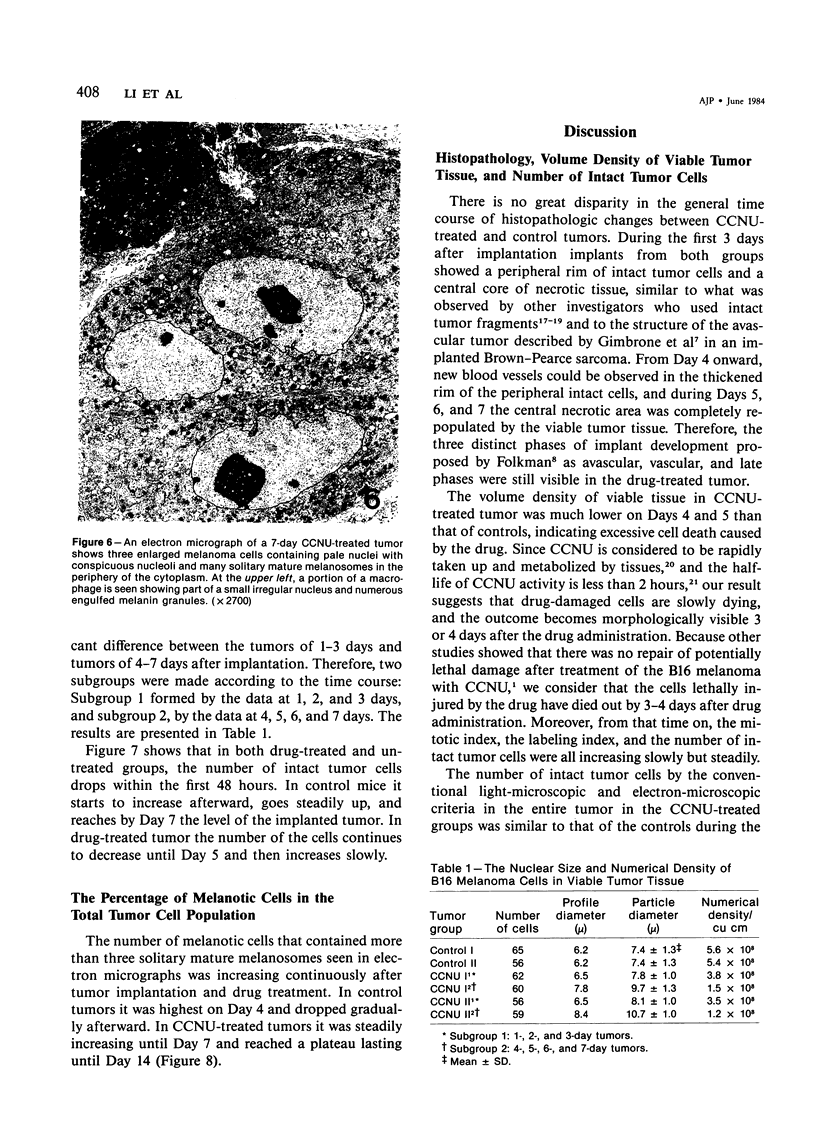
The changes of implanted B16 melanoma fragments in situ following early treatment with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU) were studied by quantitative histopathologic methods from Day 1 to Day 7 and at Day 14 after transplantation. During the first 3-day period there were no apparent differences between the two groups in all the parameters studied. The most striking differences were observed on Day 5 after implantation, when the drug-treated tumor showed the lowest number of morphologically intact tumor cells and the lowest level of proliferative capacity, with a high proportion of melanotic cells. The late infiltration of host macrophages was more abundant in drug-treated tumors than in controls due to an enhanced production and liberation of melanin granules. The results suggest that a 7-day growth delay of drug-treated tumors is characterized not only by a reduced number (one order of magnitude) of intact tumor cells but also by a severely suppressed proliferative capacity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barkla D. H., Tutton P. J. Temporal morphologic changes in human colorectal carcinomas following xenografting. Am J Pathol. 1983 Mar;110(3):315–321. [PMC free article] [PubMed] [Google Scholar]
- Castaldi G., Zavagli G., Fiocchi O., Trotta F. Giant histiocytes after cyclophosphamide. Experientia. 1970 Mar 15;26(3):300–301. doi: 10.1007/BF01900109. [DOI] [PubMed] [Google Scholar]
- Chabner B. A., Myers C. E., Coleman C. N., Johns D. G. The clinical pharmacology of antineoplastic agents (second of two parts). N Engl J Med. 1975 May 29;292(22):1159–1168. doi: 10.1056/NEJM197505292922206. [DOI] [PubMed] [Google Scholar]
- Elias H., Hyde D. M. An elementary introduction to stereology (quantitative microscopy). Am J Anat. 1980 Dec;159(4):412–446. doi: 10.1002/aja.1001590407. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972 Aug 1;136(2):261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. P., Stanley J. A. The response of hypoxic B16 melanoma cells to in vivo treatment with chemotherapeutic agents. Cancer Res. 1975 May;35(5):1147–1153. [PubMed] [Google Scholar]
- Kline I., Gang M., Tyrer D. D., Mantel N., Venditti J. M., Goldin A. Duration of drug levels in mice as indicated by residual antileukemic efficacy. Chemotherapy. 1968;13(1):28–41. doi: 10.1159/000220528. [DOI] [PubMed] [Google Scholar]
- Kreider J. W., Schmoyer M. E. Spontaneous maturation and differentiation of B16 melanoma cells in culture. J Natl Cancer Inst. 1975 Sep;55(3):641–647. doi: 10.1093/jnci/55.3.641. [DOI] [PubMed] [Google Scholar]
- Paulus G., Hong L. X., Atassi G., Buyssens N. Degree of differentiation and blood vessel proximity in B16 melanoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(2):229–238. doi: 10.1007/BF02892850. [DOI] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H. Cell yield and cell survival following chemotherapy of the B16 melanoma. Br J Cancer. 1978 Nov;38(5):591–598. doi: 10.1038/bjc.1978.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens T. C., Peacock J. H. Tumour volume response, initial cell kill and cellular repopulation in B16 melanoma treated with cyclophosphamide and 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Br J Cancer. 1977 Sep;36(3):313–321. doi: 10.1038/bjc.1977.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrathall J. R., Newcomb E. W., Balint R., Zeitz L., Silagi S. Suppression of melanoma cell tyrosinase activity and tumorigenicity after incorporation of bromouracil for one or two cell divisions. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):581–592. doi: 10.1002/jcp.1040860503. [DOI] [PubMed] [Google Scholar]