Abstract
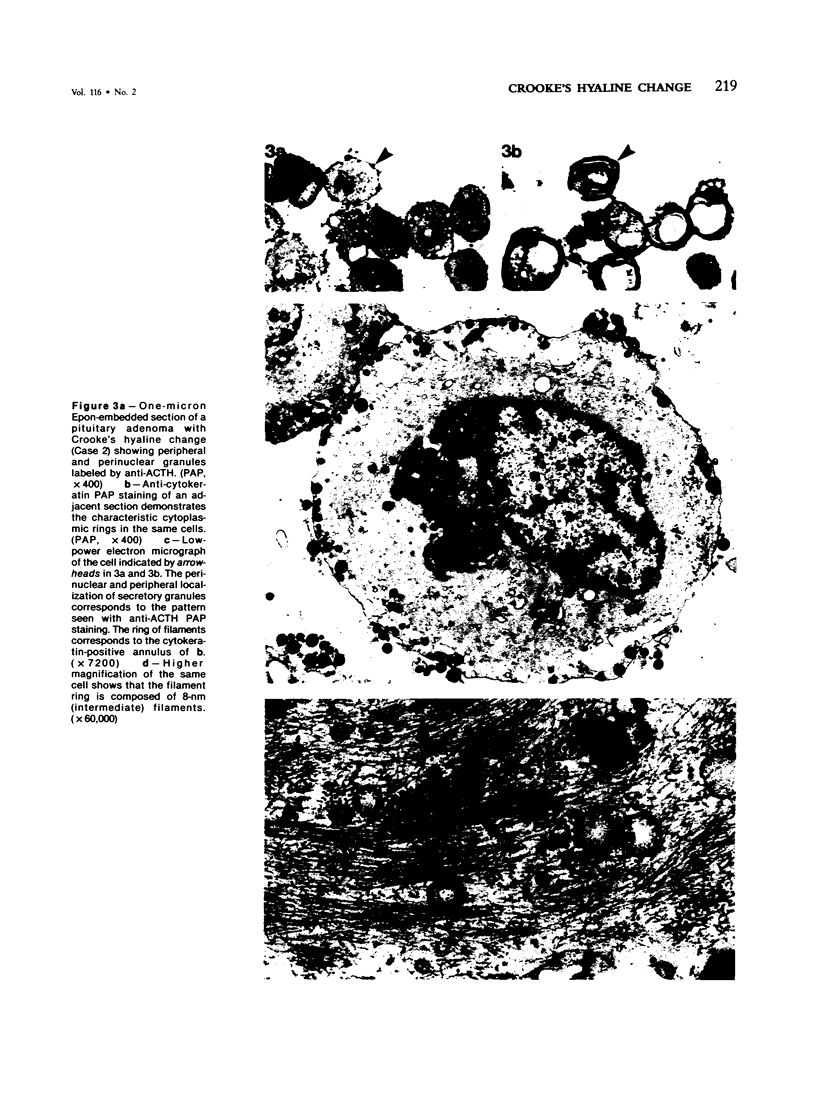
Crooke's hyaline change was studied by immunocytochemistry using an anti-adrenocorticotropic hormone (ACTH) antiserum and five different antisera against cytokeratins. Crooke's hyaline appears in basophil cells of the adenohypophysis in patients with hypercortisolism, presumably as a part of the negative feedback on corticotropin secretion. Previous studies have identified the hyaline material as a simple protein, apparently unrelated to ACTH, and electron microscopy has revealed a loss of secretory granules and an accumulation of 6-9-nm filaments in the cytoplasm of affected cells. In this study, the secretory granules in adenohypophysial cells exhibiting Crooke's hyaline change were labeled by anti-ACTH antibodies, while the hyaline material was positive for cytokeratin with each of the five antisera used. The results suggest that high levels of glucocorticoids may stimulate elaboration of cytokeratins in basophils while they suppress the production and release of ACTH.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Weber K., Hölscher A., Schauer A., Osborn M. Antibodies to intermediate filaments as diagnostic tools: human gastrointestinal carcinomas express prekeratin. Lab Invest. 1982 May;46(5):520–526. [PubMed] [Google Scholar]
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Asa S. L., Kovacs K., Bilbao J. M., Penz G. Immunohistochemical localization of keratin in craniopharyngiomas and squamous cell nests of the human pituitary. Acta Neuropathol. 1981;54(3):257–260. doi: 10.1007/BF00687750. [DOI] [PubMed] [Google Scholar]
- Asa S. L., Kovacs K., Bilbao J. M. The pars tuberalis of the human pituitary. A histologic, immunohistochemical, ultrastructural and immunoelectron microscopic analysis. Virchows Arch A Pathol Anat Histopathol. 1983;399(1):49–59. doi: 10.1007/BF00666218. [DOI] [PubMed] [Google Scholar]
- BENNETT W. A., KILBY R. A., SPRAGUE R. G. Anterior pituitary glands in patients treated with cortisone and corticotropin. Am J Pathol. 1957 Jan-Feb;33(1):155–173. [PMC free article] [PubMed] [Google Scholar]
- Bergland R. M., Torack R. M. An ultrastructural study of follicular cells in the human anterior pituitary. Am J Pathol. 1969 Nov;57(2):273–297. [PMC free article] [PubMed] [Google Scholar]
- DeCicco F. A., Dekker A., Yunis E. J. Fine structure of Crooke's hyaline change in the human pituitary gland. Arch Pathol. 1972 Jul;94(1):65–70. [PubMed] [Google Scholar]
- Felix I. A., Horvath E., Kovacs K. Massive Crooke's hyalinization in corticotroph cell adenomas of the human pituitary. A histological, immunocytological, and electron microscopic study of three cases. Acta Neurochir (Wien) 1981;58(3-4):235–243. doi: 10.1007/BF01407130. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- French S. W. Present understanding of the development of Mallory's body. Arch Pathol Lab Med. 1983 Sep;107(9):445–450. [PubMed] [Google Scholar]
- GOLDBERG G. M., ESHBAUGH D. E. Squamous cell nests of the pituitary gland as related to the origin of craniopharyngiomas. A study of their presence in the newborn and infants up to age four. Arch Pathol. 1960 Sep;70:293–299. [PubMed] [Google Scholar]
- GOLDEN A., BONDY P. K., SHELDON W. H. Pituitary basophile hyperplasia and Crooke's hyaline changes in man after ACTH therapy. Proc Soc Exp Biol Med. 1950 Jun;74(2):455–458. doi: 10.3181/00379727-74-17938. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Chiu F. C. Growth kinetics, cell shape, and the cytoskeleton of primary astrocyte cultures. J Neurochem. 1984 Jan;42(1):175–184. doi: 10.1111/j.1471-4159.1984.tb09714.x. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Yen S. H., Chiu F. C., Peress N. S. Lewy bodies of Parkinson's disease contain neurofilament antigens. Science. 1983 Sep 9;221(4615):1082–1084. doi: 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER I. J. Squamous metaplasia of cells of the anterior pituitary gland. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):141–145. doi: 10.1002/path.1700690120. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepes J. J., Sayler J., Hiszczynskyj R. Squamous metaplasia following necrosis of the adenohypophysis and of a chromophobe adenoma of the pituitary. Virchows Arch A Pathol Anat Histol. 1982;395(1):69–76. doi: 10.1007/BF00443485. [DOI] [PubMed] [Google Scholar]
- Kovacs K., Horvath E., Stratmann I. E., Ezrin C. Cytoplasmic microfilaments in the anterior lobe of the human pituitary gland. Acta Anat (Basel) 1974;87(3):414–426. doi: 10.1159/000144190. [DOI] [PubMed] [Google Scholar]
- LAQUEUR G. L. Cytological changes in human hypophyses after cortisone and ACTH treatment. Science. 1950 Oct 13;112(2911):429–430. doi: 10.1126/science.112.2911.429-a. [DOI] [PubMed] [Google Scholar]
- LUSE S. A., KERNOHAN J. W. Squamous-cell nests of the pituitary gland. Cancer. 1955 May-Jun;8(3):623–628. doi: 10.1002/1097-0142(1955)8:3<623::aid-cncr2820080328>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Martin R., Cetin Y., Fehm H. L., Fahlbusch R., Voigt K. H. Multiple cellular forms of corticotrophs in surgically removed pituitary adenomas and periadenomatous tissue in Cushing's disease. Am J Pathol. 1982 Mar;106(3):332–341. [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- PEARSE A. G. E. Cytological and cytochemical investigations on the foetal and adult hypophysis in various physiological and pathological states. J Pathol Bacteriol. 1953 Apr;65(2):355–370. doi: 10.1002/path.1700650208. [DOI] [PubMed] [Google Scholar]
- Phillips M. J. Mallory bodies and the liver. Lab Invest. 1982 Oct;47(4):311–313. [PubMed] [Google Scholar]
- Porcile E., Racadot J. Ultrastructure des cellules de Crooke observées dans l'hypophyse humaine au cours de la maladie de Cushing. C R Acad Sci Hebd Seances Acad Sci D. 1966 Oct 3;263(14):948–951. [PubMed] [Google Scholar]
- Robert F., Pelletier G., Hardy J. Pituitary adenomas in Cushing's disease. A histologic, ultrastructural, and immunocytochemical study. Arch Pathol Lab Med. 1978 Sep;102(9):448–455. [PubMed] [Google Scholar]
- Schlegel R., Banks-Schlegel S., Pinkus G. S. Immunohistochemical localization of keratin in normal human tissues. Lab Invest. 1980 Jan;42(1):91–96. [PubMed] [Google Scholar]
- Schmid E., Schiller D. L., Grund C., Stadler J., Franke W. W. Tissue type-specific expression of intermediate filament proteins in a cultured epithelial cell line from bovine mammary gland. J Cell Biol. 1983 Jan;96(1):37–50. doi: 10.1083/jcb.96.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubart U. K., Fields K. L. Identification of a calcium-regulated insulinoma cell phosphoprotein as an islet cell keratin. J Cell Biol. 1984 Mar;98(3):1001–1009. doi: 10.1083/jcb.98.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]