Abstract
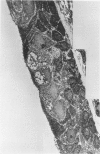
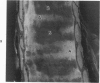
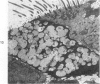
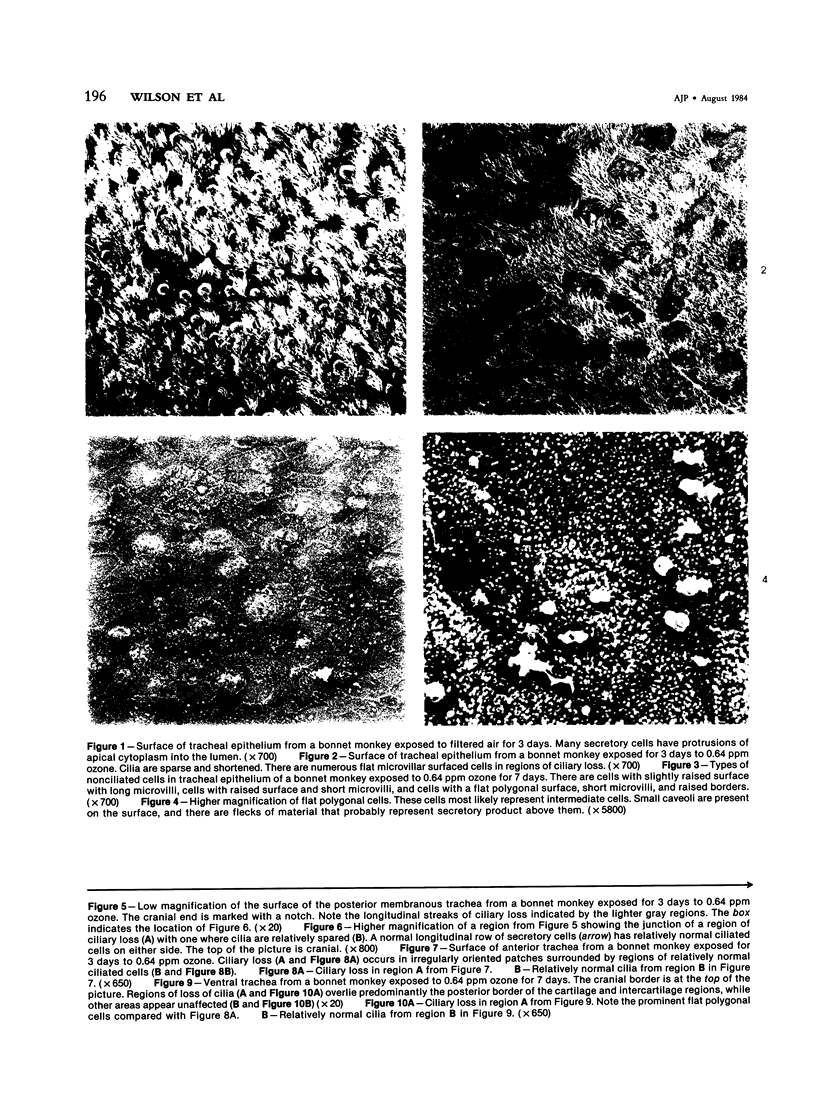
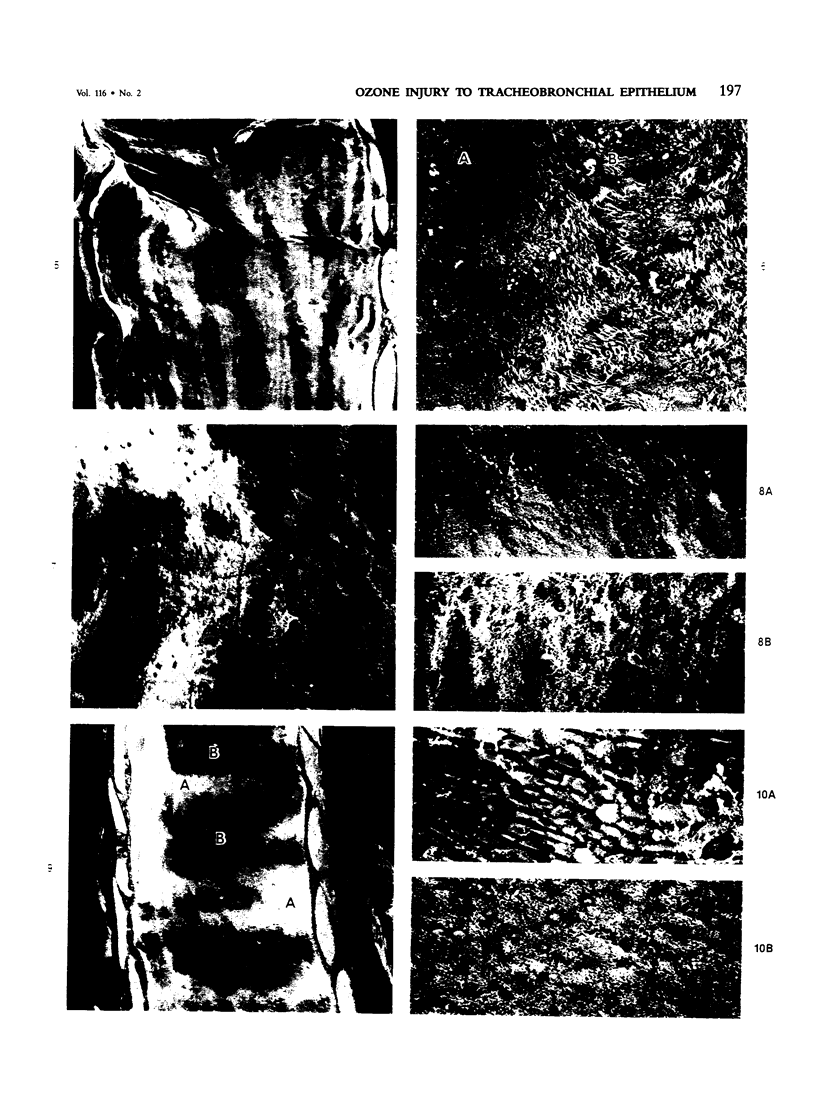
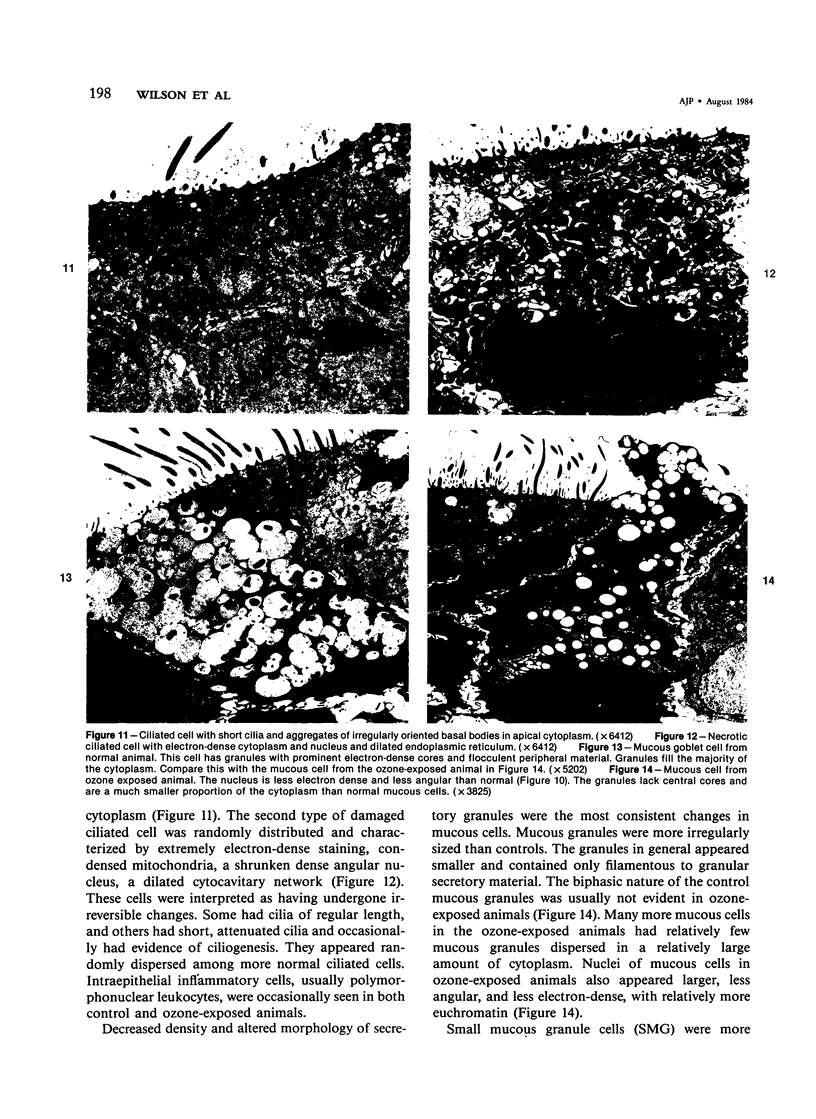
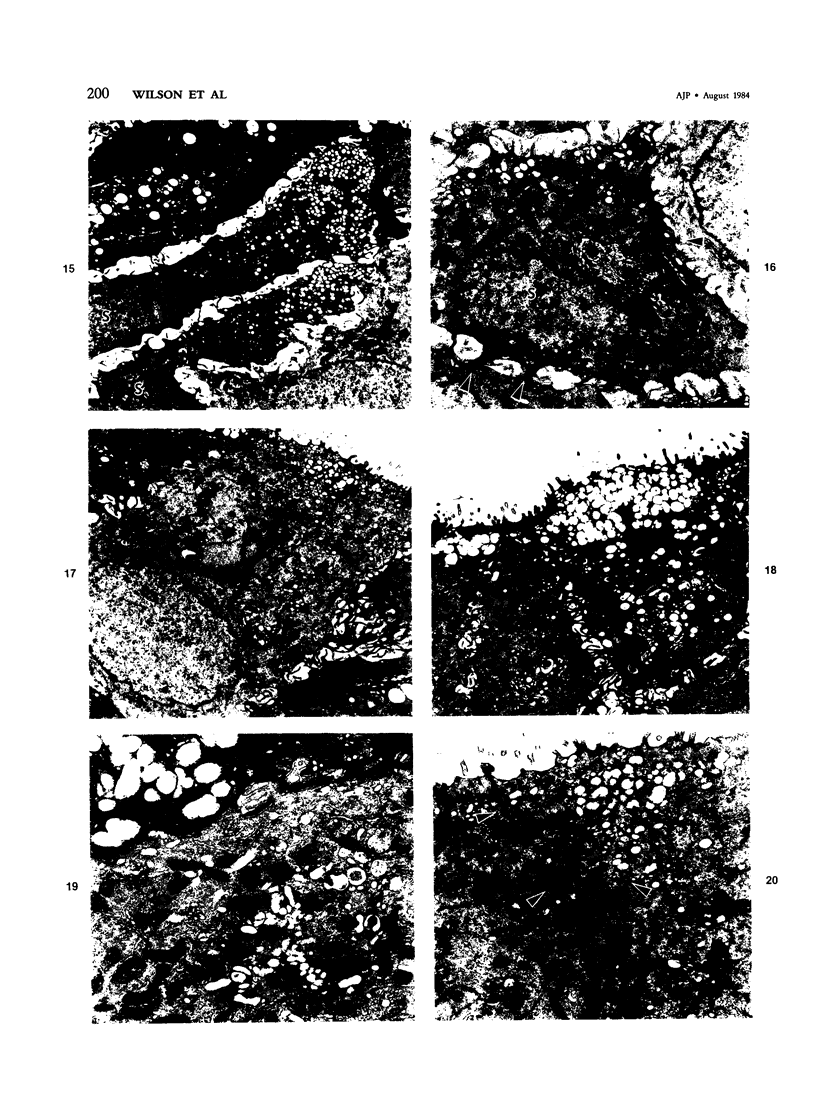
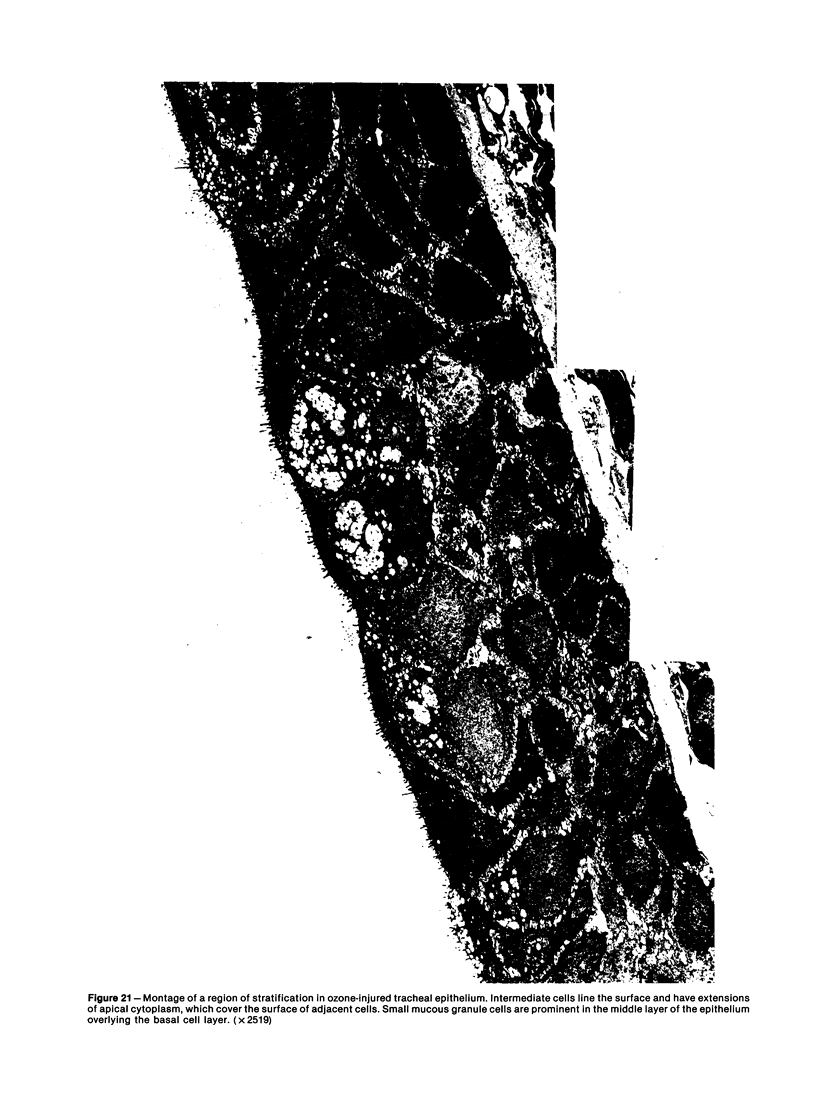
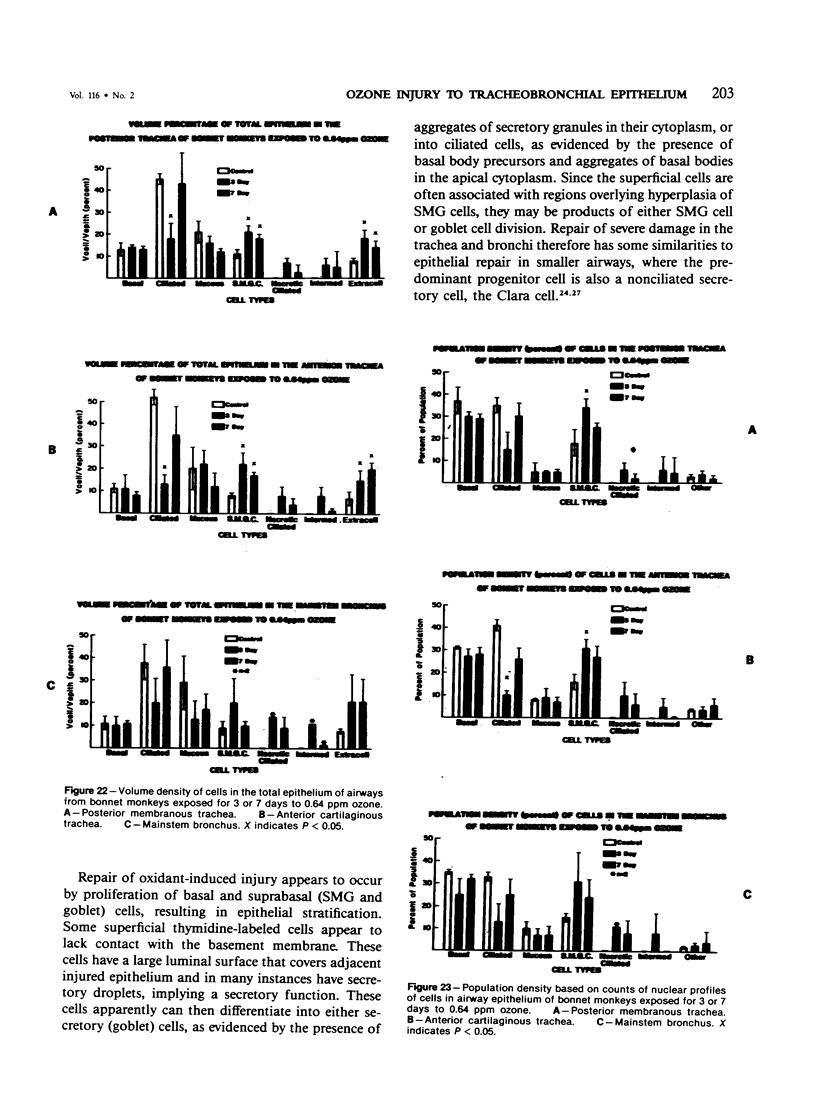
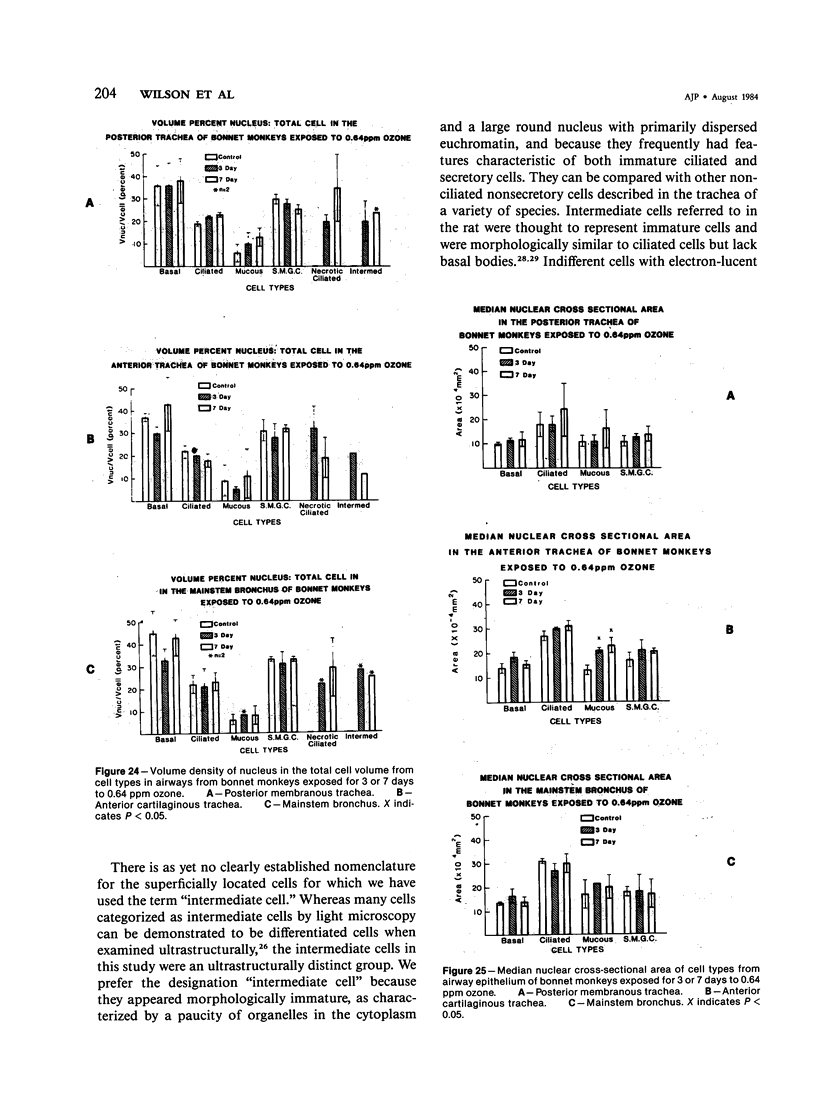
The tracheal epithelium of a variety of laboratory species is widely used as a model system in studies of epithelial biology and respiratory carcinogenesis. The purpose of this study was to evaluate the response of the tracheal epithelium to cytotoxic injury in a primate species that may have an epithelium more representative of that in man than smaller laboratory species. This study evaluated changes in the light-microscopic, surface, and ultrastructural appearance of the tracheobronchial epithelium of bonnet monkeys exposed for 3 or 7 days to 0.64 ppm ozone. Population densities, epithelial volumetric densities, and thymidine labeling indexes were determined for cells from posterior membranous and anterior cartilaginous trachea and mainstem bronchus. Ozone-induced epithelial changes were characterized by decreased numbers of ciliated cells, loss of cilia, and necrosis of ciliated cells. There were alterations in mucous (goblet) cell granules. There was an increase in extracellular space and focal epithelial stratification that was associated with increased numbers of small mucous granule cells and the presence of an epithelial cell type not seen in control animals (intermediate cells). There was an increase in cytoplasmic filaments and desmosomal attachments in basal cells, small mucous granule cells, and intermediate cells. Regional differences in lesion distribution were demonstrated by scanning electron microscopy. Longitudinal streaks of ciliary loss were evident in posterior membranous trachea, but ciliary loss in the ventral trachea was most prominent over the posterior border of the cartilaginous rings. The thymidine labeling index and numbers of necrotic ciliated cells were greater after 3 days than after 7 days of continuous exposure. Foci of stratification were often associated with increased numbers of labeled nuclei in the suprabasal region of the epithelium. The results of this study suggest that small mucous granule cells and intermediate cells are important participants in the repair of chemically injured airway epithelium; stratification and increased amounts of cytoplasmic filament bundles and desmosomal attachments, rather than being evidence of squamous metaplasia or dysplastic change, might be stereotypic responses of airway epithelium to injury; and the ciliated cell population becomes less susceptible to ozone-induced necrosis with continuing exposure.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asmundsson T., Kilburn K. H., McKenzie W. N. Injury and metaplasia of airway cells due to SO2. Lab Invest. 1973 Jul;29(1):41–53. [PubMed] [Google Scholar]
- Barrett L. A., McDowell E. M., Hill T. A., Pyeatte J. C., Harris C. C., Trump B. F. Induction of atypical squamous metaplasia with benzo[a]pyrene in cultured hamster tracheas. Pathol Res Pract. 1980;168(1-3):134–145. doi: 10.1016/S0344-0338(80)80213-5. [DOI] [PubMed] [Google Scholar]
- Becci P. J., McDowell E. M., Trump B. F. The respiratory epithelium. IV. Histogenesis of epidermoid metaplasia and carcinoma in situ in the hamster. J Natl Cancer Inst. 1978 Aug;61(2):577–586. [PubMed] [Google Scholar]
- Bindreiter M., Schuppler J., Stockinger L. Zellproliferation und Differenzierung im Trachealepithel der Ratte. Exp Cell Res. 1968 May;50(2):377–382. [PubMed] [Google Scholar]
- Boatman E. S., Sato S., Frank R. Acute effects of ozone on cat lungs. II. Structural. Am Rev Respir Dis. 1974 Aug;110(2):157–169. doi: 10.1164/arrd.1974.110.2.157. [DOI] [PubMed] [Google Scholar]
- Breeze R. G., Wheeldon E. B. The cells of the pulmonary airways. Am Rev Respir Dis. 1977 Oct;116(4):705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Schwartz L. W., Tyler W. S. Acute respiratory bronchiolitis: an ultrastructural and autoradiographic study of epithelial cell injury and renewal in rhesus monkeys exposed to ozone. Am J Pathol. 1980 Mar;98(3):811–840. [PMC free article] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral-Anderson L. J., Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978 Jun;38(6):648–653. [PubMed] [Google Scholar]
- Frasca J. M., Auerbach O., Parks V. R., Jamieson J. D. Electron microscopic observations of the bronchial epithelium of dogs. II. Smoking dogs. Exp Mol Pathol. 1968 Dec;9(3):380–399. doi: 10.1016/0014-4800(68)90027-0. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Sporn M. B., Kaufman D. G., Smith J. M., Baker M. S., Saffiotti U. Acute ultrastructural effects of benzo[a]pyrene and ferric oxide the hamster tracheobronchial epithelium. Cancer Res. 1971 Dec;31(12):1977–1989. [PubMed] [Google Scholar]
- Jeffery P. K., Reid L. New observations of rat airway epithelium: a quantitative and electron microscopic study. J Anat. 1975 Nov;120(Pt 2):295–320. [PMC free article] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Topping D. C., Heckman C. A., Nettesheim P. Ultrastructural characteristics of carcinogen-induced nondysplastic changes in tracheal epithelium. Am J Pathol. 1980 Jan;98(1):61–82. [PMC free article] [PubMed] [Google Scholar]
- Lane B. P., Gordon R. Regeneration of rat tracheal epithelium after mechanical injury. I. The relationship between mitotic activity and cellular differentiation. Proc Soc Exp Biol Med. 1974 Apr;145(4):1139–1144. doi: 10.3181/00379727-145-37968. [DOI] [PubMed] [Google Scholar]
- Last J. A., Jennings M. D., Schwartz L. W., Cross C. E. Glycoprotein secretion by tracheal explants cultured from rats exposed to ozone. Am Rev Respir Dis. 1977 Oct;116(4):695–703. doi: 10.1164/arrd.1977.116.4.695. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Barrett L. A., Glavin F., Harris C. C., Trump B. F. The respiratory epithelium. I. Human bronchus. J Natl Cancer Inst. 1978 Aug;61(2):539–549. [PubMed] [Google Scholar]
- McDowell E. M., Combs J. W., Newkirk C. A quantitative light and electron microscopic study of hamster tracheal epithelium with special attention to so-called intermediate cells. Exp Lung Res. 1983 Apr;4(3):205–226. doi: 10.3109/01902148309046061. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Combs J. W., Newkirk C. Changes in secretory cells of hamster tracheal epithelium in response to acute sublethal injury: a quantitative study. Exp Lung Res. 1983 Apr;4(3):227–243. doi: 10.3109/01902148309046062. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Penha P. D., Werthamer S. Pulmonary lesions induced by long-term exposure to ozone. II. Ultrastructure observations of proliferative and regressive lesions. Arch Environ Health. 1974 Nov;29(5):282–289. doi: 10.1080/00039896.1974.10666588. [DOI] [PubMed] [Google Scholar]
- Philpott D. E., Harrison G. A., Turnbill C., Black S. Ultrastructural changes in tracheal epithelial cells exposed to oxygen. Aviat Space Environ Med. 1977 Sep;48(9):812–818. [PubMed] [Google Scholar]
- Plopper C. G., Halsebo J. E., Berger W. J., Sonstegard K. S., Nettesheim P. Distribution of nonciliated bronchiolar epithelial (Clara) cells in intra- and extrapulmonary airways of the rabbit. Exp Lung Res. 1983 Sep;5(2):79–98. doi: 10.3109/01902148309061506. [DOI] [PubMed] [Google Scholar]
- Reznik-Schüller H., Reznik G., Mohr U. Effects of cigarette smoke on the bronchial epithelium of Syrian hamsters: ultrastructural studies. J Natl Cancer Inst. 1975 Aug;55(2):353–369. [PubMed] [Google Scholar]
- Schwartz L. W., Dungworth D. L., Mustafa M. G., Tarkington B. K., Tyler W. S. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976 Jun;34(6):565–578. [PubMed] [Google Scholar]
- Wells A. B. The kinetics of cell proliferation in the tracheobronchial epithelia of rats with and without chronic respiratory disease. Cell Tissue Kinet. 1970 Apr;3(2):185–206. doi: 10.1111/j.1365-2184.1970.tb00265.x. [DOI] [PubMed] [Google Scholar]