Abstract
Aminonucleoside of puromycin (PAN) is known to cause altered glomerular permeability, resulting in a nephrotic syndrome in rats. The early sequence of this lesion was studied quantitatively, with the application of a new morphometric technique for determining epithelial foot process widths and a sensitive assay for quantifying urinary albumin excretion. Twenty-four hours following a single intraperitoneal injection of PAN, significant widening of foot processes was documented. Within 36 hours significant increases in urinary albumin excretion were observed. When control rats were examined, there was no clear correlation between epithelial foot process width and quantitative albumin excretion. However, in the PAN-treated animals, abnormal albuminuria only appeared in association with appreciable foot process expansion. These studies indicate that quantitative alterations occur in the rat glomerular capillary wall as early as 24 hours after PAN. Further studies of altered glomerular permeability may use these sensitive measures to more precisely define the temporal sequence and elucidate possible subgroups of experimental glomerular injury.
Full text
PDF


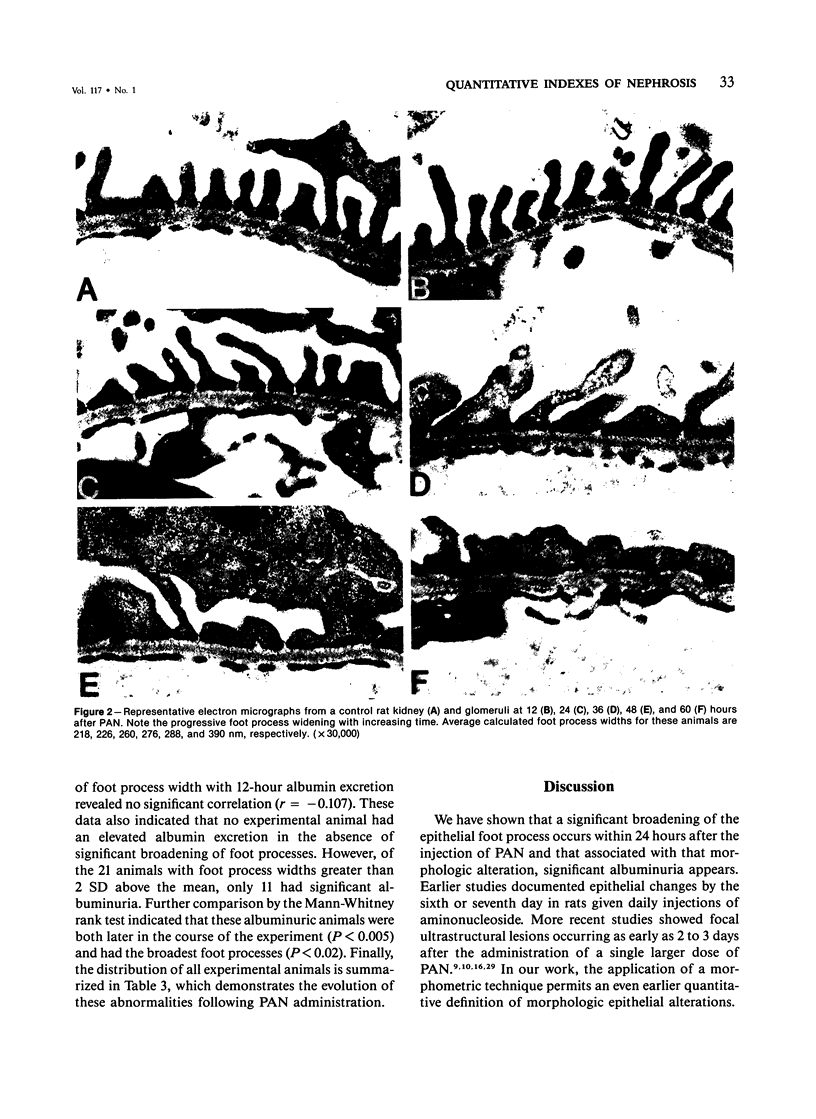



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M. A scanning and transmission electron microscopic comparison of puromycin aminonucleoside-induced nephrosis to hyperalbuminemia-induced proteinuria with emphasis on kidney podocyte pedicel loss. Lab Invest. 1977 Feb;36(2):183–197. [PubMed] [Google Scholar]
- Arakawa M. A scanning electron microscopy of the glomerulus of normal and nephrotic rats. Lab Invest. 1970 Nov;23(5):489–496. [PubMed] [Google Scholar]
- Blau E. B., Michael A. F. Rat glomerular glycoprotein composition and metabolism in aminonucleoside nephrosis. Proc Soc Exp Biol Med. 1972 Oct;141(1):164–172. doi: 10.3181/00379727-141-36737. [DOI] [PubMed] [Google Scholar]
- Bohrer M. P., Baylis C., Robertson C. R., Brenner B. M., Troy J. L., Willis W. T. Mechanisms of the puromycin-induced defects in the transglomerular passage of water and macromolecules. J Clin Invest. 1977 Jul;60(1):152–161. doi: 10.1172/JCI108751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. The permeability of glomerular capillaries of aminonuceoside nephrotic rats to graded dextrans. J Exp Med. 1975 Jul 1;142(1):61–83. doi: 10.1084/jem.142.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Reid J. J., Farquhar M. G. Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest. 1976 Jan;34(1):43–59. [PubMed] [Google Scholar]
- FARQUHAR M. G., PALADE G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J Exp Med. 1961 Nov 1;114:699–716. doi: 10.1084/jem.114.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARQUHAR M. G., VERNIER R. L., GOOD R. A. An electron microscope study of the glomerulus in nephrosis, glomerulonephritis, and lupus erythematosus. J Exp Med. 1957 Nov 1;106(5):649–660. doi: 10.1084/jem.106.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMAN J. D., FISHER E. R. Renal lesions of aminonucleoside nephrosis as revealed by electron microscopy. Lab Invest. 1959 Mar-Apr;8(2):371–385. [PubMed] [Google Scholar]
- FIEGELSON E. B., DRAKE J. W., RECANT L. Experimental aminonucleoside nephrosis in rats. J Lab Clin Med. 1957 Sep;50(3):437–446. [PubMed] [Google Scholar]
- FRENK S., ANTONOWICZ I., CRAIG J. M., METCOFF J. Experimental nephrotic syndrome induced in rats by aminonucleoside; renal lesions and body electrolyte composition. Proc Soc Exp Biol Med. 1955 Jul;89(3):424–427. doi: 10.3181/00379727-89-21833. [DOI] [PubMed] [Google Scholar]
- Gundersen H. J., Seefeldt T., Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res. 1980;205(1):147–155. doi: 10.1007/BF00234450. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. E., Uzqueda H. R. The influence of citrate and phosphate on the Mancini single radial immunodiffusion technique and suggested improvements for the determination of urinary albumin. Clin Chim Acta. 1978 Dec 15;90(3):249–257. doi: 10.1016/0009-8981(78)90264-4. [DOI] [PubMed] [Google Scholar]
- Hoyer J. R., Mauer S. M., Michael A. F. Unilateral renal disease in the rat. I. Clinical, morphologic, and glomerular mesangial functional features of the experimental model produced by renal perfusion with aminonucleoside. J Lab Clin Med. 1975 May;85(5):756–768. [PubMed] [Google Scholar]
- Jensen E. B., Gundersen H. J., Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979 Jan;115(1):19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Mauer S. M., Brown D. M., Matas A. J., Steffes M. W. Effects of pancreatic islet transplantation on the increased urinary albumin excretion rates in intact and uninephrectomized rats with diabetes mellitus. Diabetes. 1978 Sep;27(9):959–964. doi: 10.2337/diab.27.9.959. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Blau E., Vernier R. L. Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab Invest. 1970 Dec;23(6):649–657. [PubMed] [Google Scholar]
- Mynderse L. A., Hassell J. R., Kleinman H. K., Martin G. R., Martinez-Hernandez A. Loss of heparan sulfate proteoglycan from glomerular basement membrane of nephrotic rats. Lab Invest. 1983 Mar;48(3):292–302. [PubMed] [Google Scholar]
- Oken D. E., Cotes S. C., Mende C. W. Micropuncture study of tubular transport of albumin in rats with aminonucleoside nephrosis. Kidney Int. 1972;1(1):3–11. doi: 10.1038/ki.1972.2. [DOI] [PubMed] [Google Scholar]
- Olson J. L., Rennke H. G., Venkatachalam M. A. Alterations in the charge and size selectivity barrier of the glomerular filter in aminonucleoside nephrosis in rats. Lab Invest. 1981 Mar;44(3):271–279. [PubMed] [Google Scholar]
- Pinto J. A., Brewer D. B. Combined light and electron-microscope morphometric studies of acute puromycin aminonucleoside nephropathy in rats. J Pathol. 1975 Jul;116(3):149–164. doi: 10.1002/path.1711160304. [DOI] [PubMed] [Google Scholar]
- Powell H. R. Relationship between proteinuria and epithelial cell changes in minimal lesion glomerulopathy. Nephron. 1976;16(4):310–317. doi: 10.1159/000180616. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Karnovsky M. J. An ultrastructural study of the mechanisms of proteinuria in aminonucleoside nephrosis. Kidney Int. 1975 Oct;8(4):219–232. doi: 10.1038/ki.1975.105. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Leventhal M., Karnovsky M. J. A freeze-fracture study of the junctions between glomerular epithelial cells in aminonucleoside nephrosis. Lab Invest. 1975 Mar;32(3):397–403. [PubMed] [Google Scholar]
- Ryan G. B., Rodewald R., Karnovsky M. J. An ultrastructural study of the glomerular slit diaphragm in aminonucleoside nephrosis. Lab Invest. 1975 Nov;33(5):461–468. [PubMed] [Google Scholar]
- Steffes M. W., Leffert J. D., Basgen J. M., Brown D. M., Mauer S. M. Epithelia cell foot process width in intact and uninephrectomized diabetic and nondiabetic rats. Lab Invest. 1980 Sep;43(3):225–230. [PubMed] [Google Scholar]
- VERNIER R. L., PAPERMASTER B. W., GOOD R. A. Aminonucleoside nephrosis. I. Electron microscopic study of the renal lesion in rats. J Exp Med. 1959 Jan 1;109(1):115–126. doi: 10.1084/jem.109.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Cotran R. S., Karnovsky M. J. An ultrastructural study of glomerular permeability in aminonucleoside nephrosis using catalase as a tracer protein. J Exp Med. 1970 Dec 1;132(6):1168–1180. doi: 10.1084/jem.132.6.1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Karnovsky M. J., Cotran R. S. Glomerular permeability. Ultrastructural studies in experimental nephrosis using horseradish peroxidase as a tracer. J Exp Med. 1969 Aug 1;130(2):381–399. doi: 10.1084/jem.130.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON S. G., HACKEL D. B., HORWOOD S., NASH G., HEYMANN W. Aminonucleoside nephrosis in rats. Pediatrics. 1958 Jun;21(6):963–973. [PubMed] [Google Scholar]




