Abstract
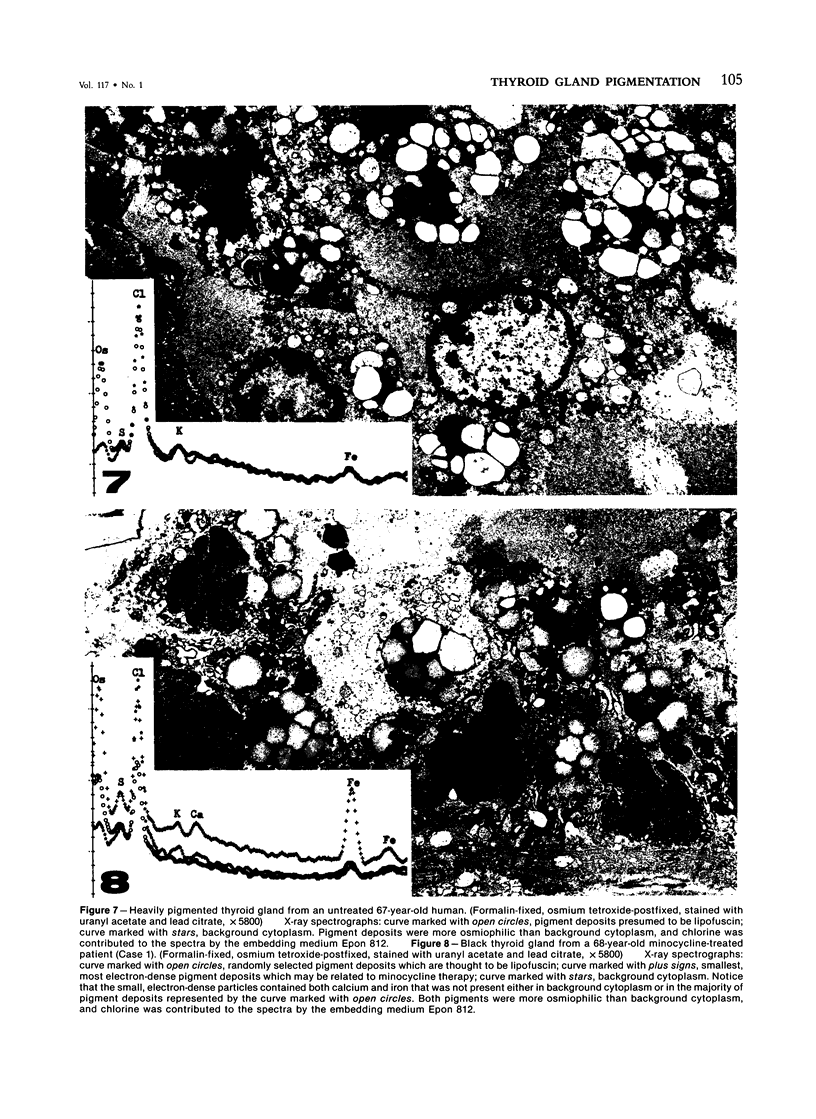
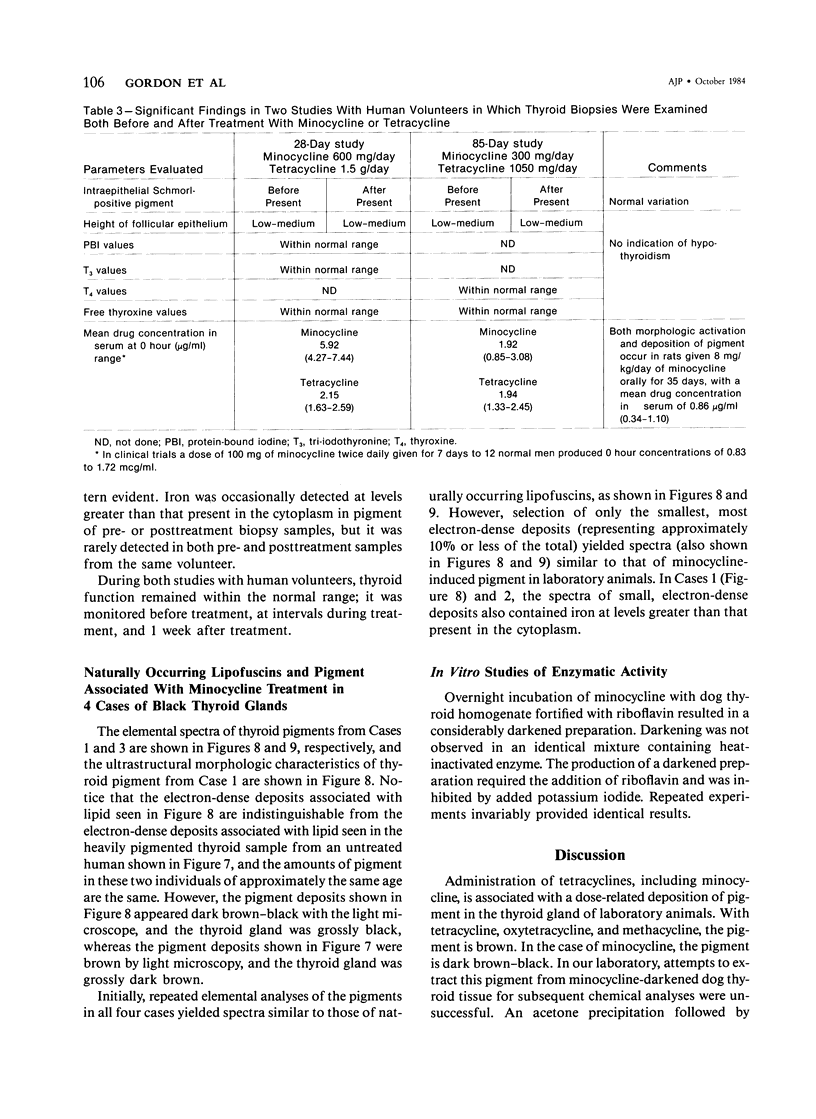
Thyroid pigments in black thyroid glands from minocycline-treated patients were compared by light and electron microscopy, histochemistry, and energy-dispersive x-ray analysis with minocycline-induced pigment in thyroid glands of laboratory animals, and with naturally occurring lipofuscins in untreated laboratory animals and humans. All thyroid samples examined contained nonbirefringent, Schmorl-positive pigment. However, the pigments in black thyroids from minocycline-treated patients resembled lipofuscins of untreated humans since both fluoresced and were Ziehl-Neelsen- and Sudan IV-positive. Minocycline induced pigment in rats was nonfluorescent and Ziehl-Neelsen- and Sudan IV-negative. Ultrastructurally, pigments in black thyroid glands of minocycline-treated humans resembled lipofuscins in untreated humans, and initial elemental analyses yielded similar spectra. Repeated analyses of the most electron-dense pigment deposits yielded spectra that resembled those of minocycline-induced pigment in laboratory animals-ie, both contained calcium. Black thyroid glands associated with minocycline administration contained predominantly lipofuscins with a small amount of another, possibly minocycline-related pigment. The absence of functional changes in patients and animals given minocycline suggests that discoloration of the thyroid gland associated with minocycline administration is innocuous. This is further supported by the lack of documented changes in thyroid physiology in patients that have received tetracyclines for a variety of indications in the last 30-odd years since their introduction to therapy.
Full text
PDF

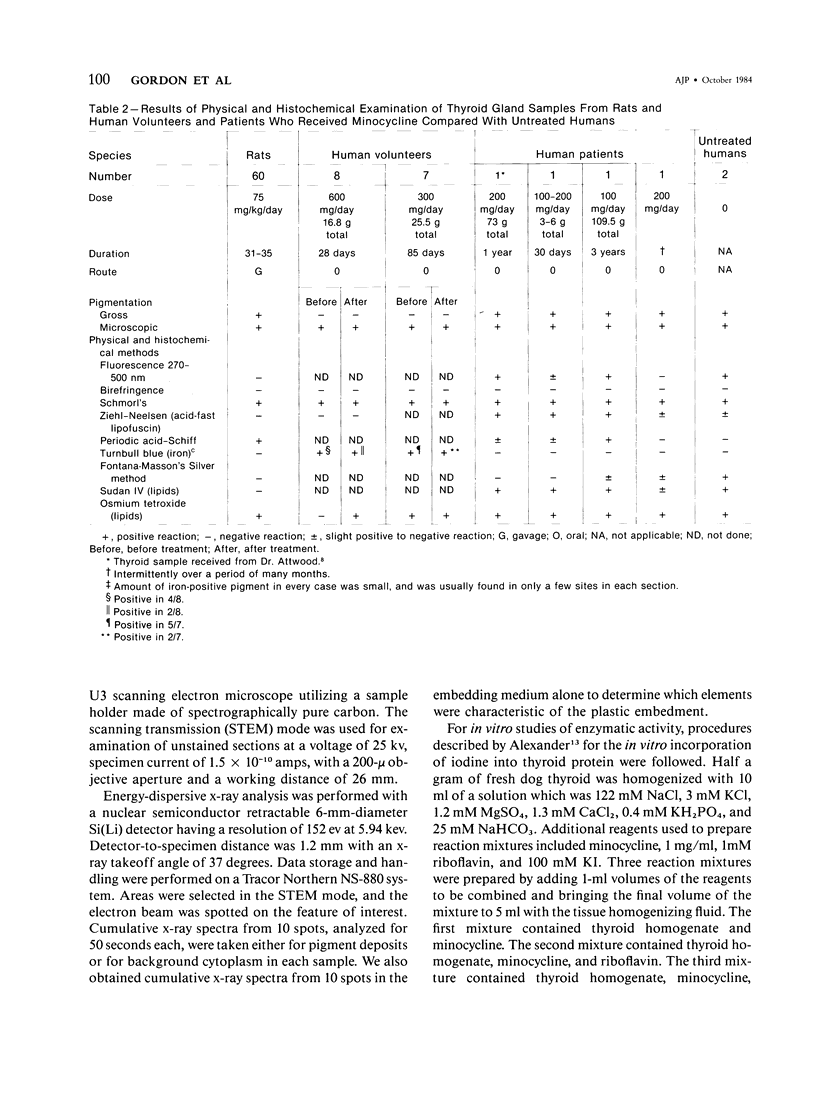
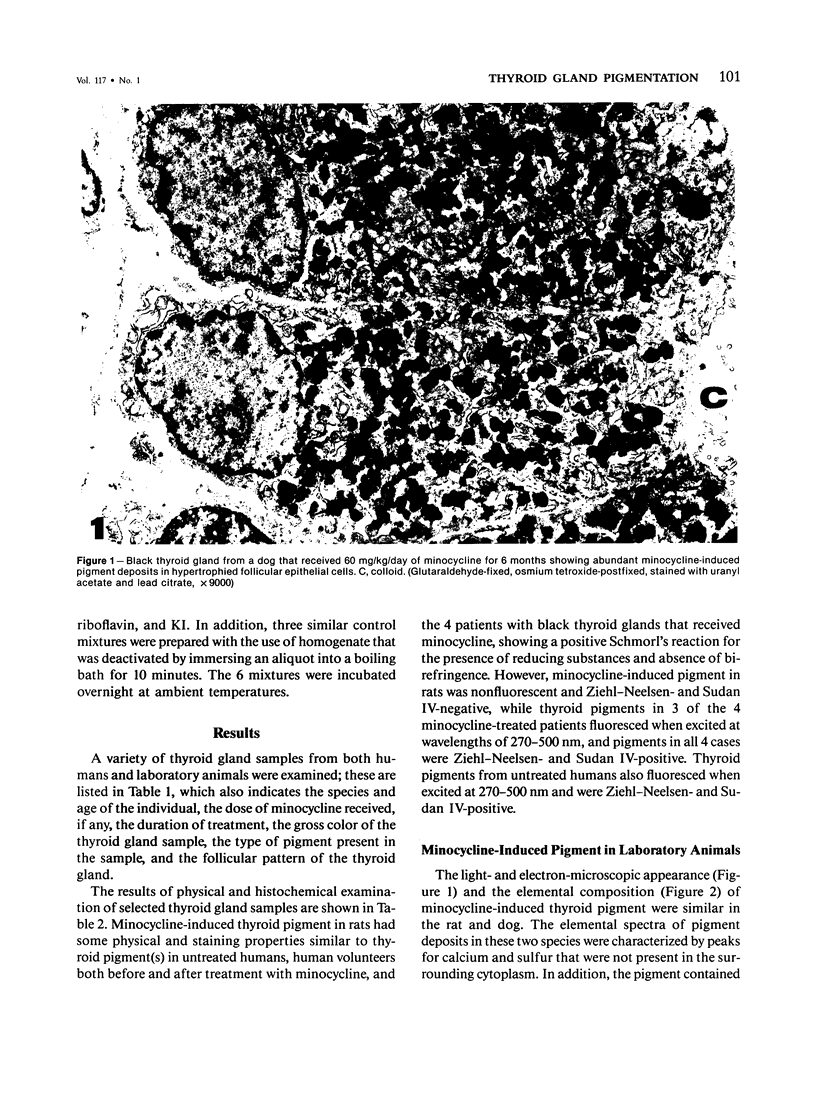






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER N. M. The mechanism of iodination reactions in thyroid glands. Endocrinology. 1961 Apr;68:671–679. doi: 10.1210/endo-68-4-671. [DOI] [PubMed] [Google Scholar]
- Attwood H. D., Dennett X. A black thyroid and minocycline treatment. Br Med J. 1976 Nov 6;2(6044):1109–1110. doi: 10.1136/bmj.2.6044.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler R. S., Kohnen P. W. Localized hemosiderosis as a sequela of acne. Arch Dermatol. 1978 Nov;114(11):1695–1697. [PubMed] [Google Scholar]
- Basler R. S. Minocycline therapy for acne. Arch Dermatol. 1979 Dec;115(12):1391–1391. [PubMed] [Google Scholar]
- Becker K. L., Katz S., Miale A., Jr Tetracycline and thyroid function. JAMA. 1967 Feb 6;199(6):416–417. [PubMed] [Google Scholar]
- Benitz K. F., Roberts G. K., Yusa A. Morphologic effects of minocycline in laboratory animals. Toxicol Appl Pharmacol. 1967 Jul;11(1):150–170. doi: 10.1016/0041-008x(67)90035-x. [DOI] [PubMed] [Google Scholar]
- Billano R. A., Ward W. Q., Little W. P. Minocycline and black thyroid. JAMA. 1983 Apr 8;249(14):1887–1887. [PubMed] [Google Scholar]
- Borel D. M., Reddy J. K. Excessive lipofuscin accumulation in the thyroid gland in mucoviscidosis. Arch Pathol. 1973 Oct;96(4):269–271. [PubMed] [Google Scholar]
- DEICHMANN W. B., BERNAL E., ANDERSON W. A., KEPLINGER M., LANDEEN K., MACDONALD W., MCMAHON R., STEBBINS R. THE CHRONIC ORAL TOXICITY OF OXYTETRACYCLINE HC1 AND TETRACYCLINE HC1 IN THE RAT, DOG AND PIG. Ind Med Surg. 1964 Nov;33:787–806. [PubMed] [Google Scholar]
- Fenske N. A., Millns J. L., Greer K. E. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA. 1980 Sep 5;244(10):1103–1106. [PubMed] [Google Scholar]
- Heimann P. Ultrastructure of human thyroid. A study of normal thyroid, untreated and treated diffuse toxic goiter. Acta Endocrinol (Copenh) 1966;53(Suppl):1+–1+. [PubMed] [Google Scholar]
- McGrae J. D., Jr, Zelickson A. S. Skin pigmentation secondary to minocycline therapy. Arch Dermatol. 1980 Nov;116(11):1262–1265. [PubMed] [Google Scholar]
- Möller H., Rausing A. Methacycline hyperpigmentation: a five-year follow-up. Acta Derm Venereol. 1980;60(6):495–501. [PubMed] [Google Scholar]
- Reid J. D. The black thyroid associated with minocycline therapy. A local manifestation of a drug-induced lysosome/substrate disorder. Am J Clin Pathol. 1983 Jun;79(6):738–746. doi: 10.1093/ajcp/79.6.738. [DOI] [PubMed] [Google Scholar]
- Ridgway H. A., Sonnex T. S., Kennedy C. T., Millard P. R., Henderson W. J., Gold S. C. Hyperpigmentation associated with oral minocycline. Br J Dermatol. 1982 Jul;107(1):95–102. doi: 10.1111/j.1365-2133.1982.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Sato S., Murphy G. F., Bernhard J. D., Mihm M. C., Jr, Fitzpatrick T. B. Ultrastructural and x-ray microanalytical observations of minocycline-related hyperpigmentation of the skin. J Invest Dermatol. 1981 Sep;77(3):264–271. doi: 10.1111/1523-1747.ep12482449. [DOI] [PubMed] [Google Scholar]
- Saul S. H., Dekker A., Lee R. E., Breitfeld V. The black thyroid. Its relation to minocycline use in man. Arch Pathol Lab Med. 1983 Apr;107(4):173–177. [PubMed] [Google Scholar]
- White S. W., Besanceney C. Systemic pigmentation from tetracycline and minocycline therapy. Arch Dermatol. 1983 Jan;119(1):1–2. doi: 10.1001/archderm.119.1.1. [DOI] [PubMed] [Google Scholar]