Abstract
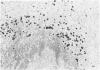
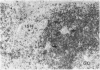
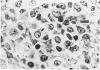
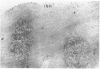
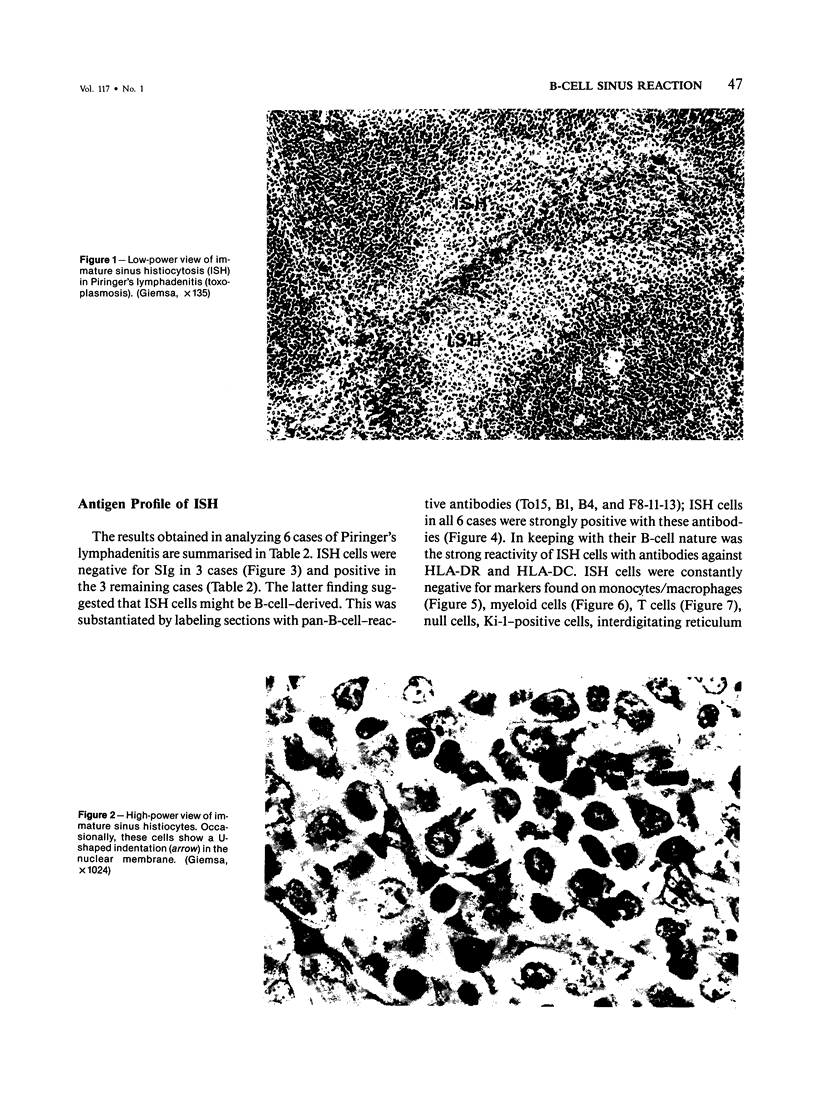
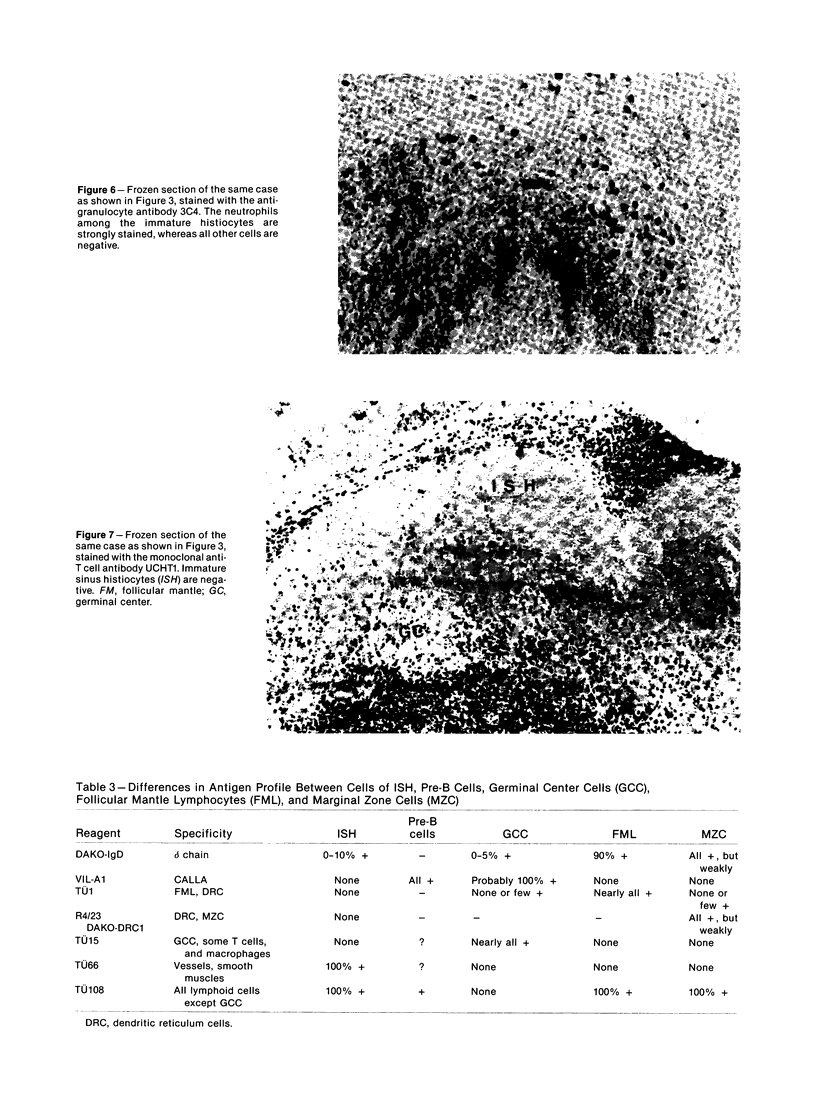
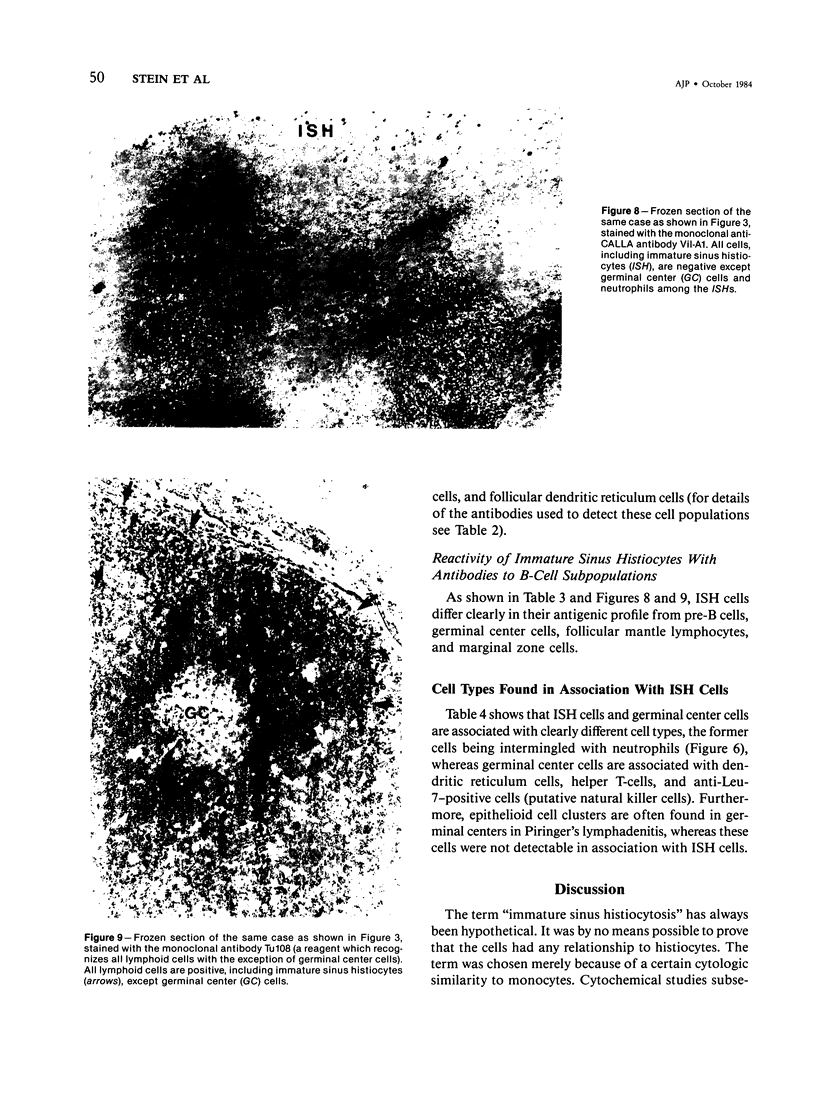
The true nature of cells of "immature sinus histiocytosis" (ISH) is uncertain because they lack the typical features of normal histiocytes when analyzed by enzyme cytochemistry or electron microscopy. In the present study the antigenic profile of ISH cells has been analyzed by immunohistologic techniques in six cases of Piringer's lymphadenitis with the use of a large panel of monoclonal and polyclonal antibodies reactive with the major cell types of the hematolymphoid system. The results obtained indicate that ISH cells consistently lack markers found on cells of the monocyte/macrophage series, myeloid cells, interdigitating reticulum cells, follicular dendritic reticulum cells, T cells, or Ki-1-positive cells. They constantly express B-cell antigens and HLA-DR and (on a variable proportion of cells) surface immunoglobulin. The application of antibodies reactive with different B-cell subsets showed that the cells of ISH do not correspond to any previously described B-cell population, eg, pre-B cells, germinal center cells, follicular mantle lymphocytes, or marginal zone cells. Furthermore, ISH cells and germinal center cells are found in association with clearly different cell types. These findings indicate that ISH cells represent a B-cell population at a previously undescribed differentiation stage, occurring only under certain circumstances (eg, in toxoplasmosis or AIDS). It is proposed that the term "immature sinus histiocytosis" be replaced by "B-cell sinus reaction."
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Fabre J. W. Identification with a monoclonal antibody of a predominantly B lymphocyte-specific determinant of the human leukocyte common antigen. Evidence for structural and possible functional diversity of the human leukocyte common molecule. J Exp Med. 1981 Apr 1;153(4):753–765. doi: 10.1084/jem.153.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman R. F., Remington J. S. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med. 1973 Oct 25;289(17):878–881. doi: 10.1056/NEJM197310252891702. [DOI] [PubMed] [Google Scholar]
- Knapp W., Majdic O., Bettelheim P., Liszka K. VIL-A1, a monoclonal antibody reactive with common acute lymphatic leukemia cells. Leuk Res. 1982;6(2):137–147. doi: 10.1016/0145-2126(82)90019-4. [DOI] [PubMed] [Google Scholar]
- LENNERT K. Die Frühveränderungen der Lymphogranulomatose. Frankf Z Pathol. 1958 Mar;69(1):103–122. [PubMed] [Google Scholar]
- LENNERT K., REMMELE W. Karyometrische Untersuchungen an Lymphknotenzellen des Menschen. II. Reticulum- und Endothelzellen. Acta Haematol. 1958 Nov;20(5):301–317. doi: 10.1159/000205496. [DOI] [PubMed] [Google Scholar]
- Lennert K., Schwarze E. W., Krüger G. Lymphknotenveränderungen durch Virusinfektionen. Verh Dtsch Ges Pathol. 1981;65:151–171. [PubMed] [Google Scholar]
- Miettinen M., Franssila K. Malignant lymphoma simulating lymph node toxoplasmosis. Histopathology. 1982 Mar;6(2):129–140. doi: 10.1111/j.1365-2559.1982.tb02710.x. [DOI] [PubMed] [Google Scholar]
- Miettinen M. Histological differential diagnosis between lymph node toxoplasmosis and other benign lymph node hyperplasias. Histopathology. 1981 Mar;5(2):205–216. doi: 10.1111/j.1365-2559.1981.tb01778.x. [DOI] [PubMed] [Google Scholar]
- Motoi M., Stein H., Lennert K. Demonstration of lysozyme, alpha 1-antichymotrypsin, alpha 1-antitrypsin, albumin, and transferrin with the immunoperoxidase method in lymph node cells. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;35(1):73–82. doi: 10.1007/BF02889150. [DOI] [PubMed] [Google Scholar]
- Nadler L. M., Anderson K. C., Marti G., Bates M., Park E., Daley J. F., Schlossman S. F. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983 Jul;131(1):244–250. [PubMed] [Google Scholar]
- Naiem M., Gerdes J., Abdulaziz Z., Stein H., Mason D. Y. Production of a monoclonal antibody reactive with human dendritic reticulum cells and its use in the immunohistological analysis of lymphoid tissue. J Clin Pathol. 1983 Feb;36(2):167–175. doi: 10.1136/jcp.36.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab U., Stein H., Gerdes J., Lemke H., Kirchner H., Schaadt M., Diehl V. Production of a monoclonal antibody specific for Hodgkin and Sternberg-Reed cells of Hodgkin's disease and a subset of normal lymphoid cells. Nature. 1982 Sep 2;299(5878):65–67. doi: 10.1038/299065a0. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Schwab U., Lemke H., Mason D. Y., Ziegler A., Schienle W., Diehl V. Identification of Hodgkin and Sternberg-reed cells as a unique cell type derived from a newly-detected small-cell population. Int J Cancer. 1982 Oct 15;30(4):445–459. doi: 10.1002/ijc.2910300411. [DOI] [PubMed] [Google Scholar]
- Ziegler A., Uchańska-Ziegler B., Zeuthen J., Wernet P. HLA antigen expression at the single cell level on a K562 X B cell hybrid: an analysis with monoclonal antibodies using bacterial binding assays. Somatic Cell Genet. 1982 Nov;8(6):775–789. doi: 10.1007/BF01543018. [DOI] [PubMed] [Google Scholar]