Abstract
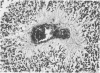
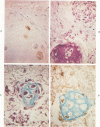
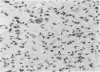
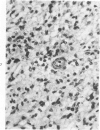
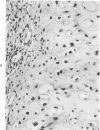
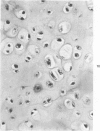
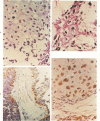
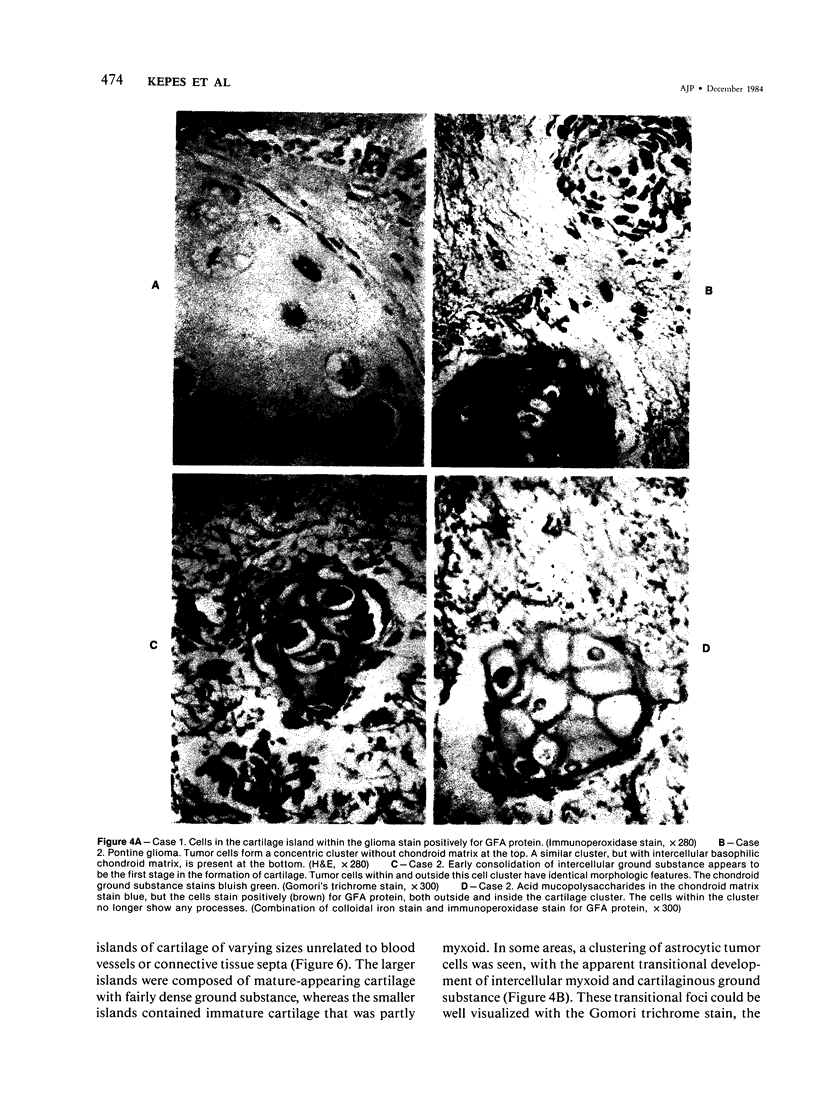
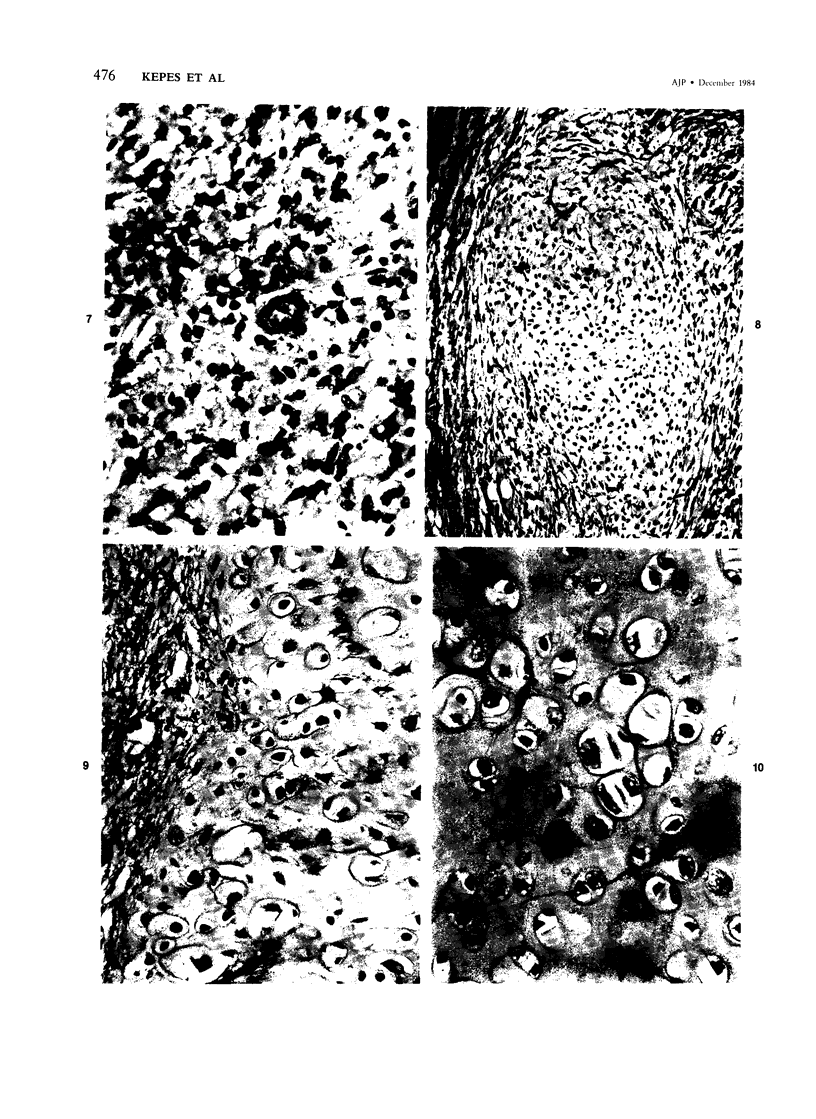
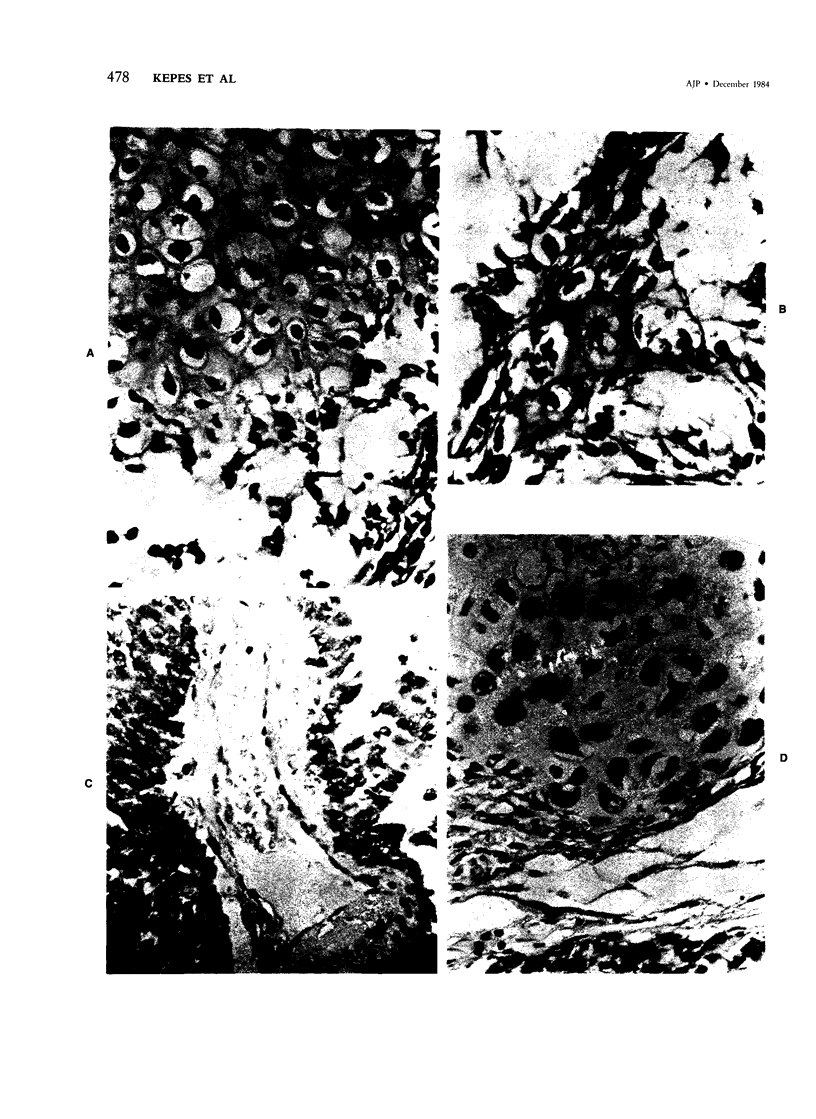
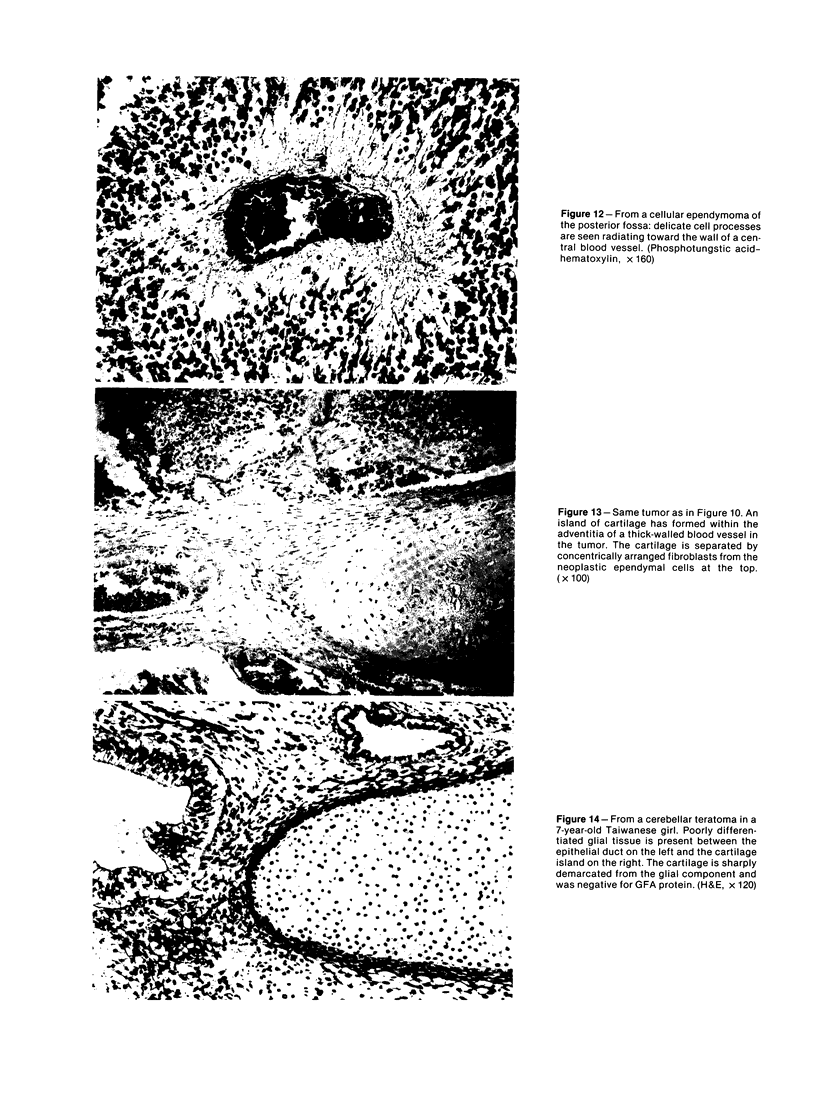
The occasional presence of focal cartilage in gliomas is generally attributed to metaplasia of the mesenchymal supportive elements. While this mechanism undoubtedly exists, the present report describes a different mode of development of cartilage in four gliomas occurring in young individuals. Two of the tumors were pontine astrocytomas, one was a mixed ependymoma and astrocytoma involving the fourth ventricle and the brainstem, and one was an extraspinal malignant astrocytoma in the lumbar region of a young boy who earlier had been diagnosed as having a pontine glioma for which he received radiation treatment. In all four tumors, transitions from astrocytic to cartilaginous elements were seen, characterized by an increasing deposition of chondroid ground substance between the astrocytes and a gradual morphologic changes of the glial cells to more rounded forms with a vacuolated cytoplasm, indistinguishable from chondrocytes of mesenchymal origin. Many of these cells retained positive staining for glial fibrillary acidic protein by the immunoperoxidase method, attesting to their astrocytic nature. The production of cartilage by neoplastic astrocytes may be related to their ability to secrete, in certain circumstances and occasionally in large amounts, basement membrane material and other forms of mucopolysaccharides, which may become condensed to form a chondroid ground substance. The process appears analogous to that of cartilage formation by epithelial cells in pleomorphic adenomas of the salivary glands.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer R. N., Becker L. E. Cerebral medulloepithelioma with bone, cartilage, and striated muscle. Light microscopic and immunohistochemical study. J Neuropathol Exp Neurol. 1983 May;42(3):256–267. doi: 10.1097/00005072-198305000-00004. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Hay E. D. Secretion of collagen by embryonic neuroepithelium at the time of spinal cord--somite interaction. Dev Biol. 1971 Dec;26(4):578–605. doi: 10.1016/0012-1606(71)90142-4. [DOI] [PubMed] [Google Scholar]
- Deck J. H., Rubinstein L. J. Glial fibrillary acidic protein in stromal cells of some capillary hemangioblastomas: significance and possible implications of an immunoperoxidase study. Acta Neuropathol. 1981;54(3):173–181. doi: 10.1007/BF00687739. [DOI] [PubMed] [Google Scholar]
- Feigin I. The mucopolysaccharides of the ground substance of the human brain. J Neuropathol Exp Neurol. 1980 Jan;39(1):1–12. doi: 10.1097/00005072-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Friede R. L. Gliofibroma. A peculiar neoplasia of collagen forming glia-like cells. J Neuropathol Exp Neurol. 1978 May;37(3):300–313. doi: 10.1097/00005072-197805000-00007. [DOI] [PubMed] [Google Scholar]
- Girard N., Tayot J., Delpech B., Delpech A., Clement J. C., Creissard P., Laumonier R. Brain glycoprotein in tumours of the nervous system. J Neuropathol Exp Neurol. 1980 Jan;39(1):88–98. doi: 10.1097/00005072-198001000-00008. [DOI] [PubMed] [Google Scholar]
- Heyerdahl Strøm E., Skullerud K. Pleomorphic xanthoastrocytoma: report of 5 cases. Clin Neuropathol. 1983;2(4):188–191. [PubMed] [Google Scholar]
- Hübner G., Klein H. J., Kleinsasser O., Schiefer H. G. Role of myoepithelial cells in the development of salivary gland tumors. Cancer. 1971 May;27(5):1255–1261. doi: 10.1002/1097-0142(197105)27:5<1255::aid-cncr2820270533>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Iglesias J. R., Richardson E. P., Jr, Collia F., Santos A., Garcia M. C., Redondo C. Prenatal intramedullary gliofibroma. A light and electron microscope study. Acta Neuropathol. 1984;62(3):230–234. doi: 10.1007/BF00691857. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980 Aug 14;286(5774):736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Ruoslahti E., Schold S. C., Bigner D. D. Fibronectin and glial fibrillary acidic protein expression in normal human brain and anaplastic human gliomas. Cancer Res. 1982 Jan;42(1):168–177. [PubMed] [Google Scholar]
- Kepes J. J., Fulling K. H., Garcia J. H. The clinical significance of "adenoid" formations of neoplastic astrocytes, imitating metastatic carcinoma, in gliosarcomas. A review of five cases. Clin Neuropathol. 1982;1(4):139–150. [PubMed] [Google Scholar]
- Kepes J. J., Rubinstein L. J., Eng L. F. Pleomorphic xanthoastrocytoma: a distinctive meningocerebral glioma of young subjects with relatively favorable prognosis. A study of 12 cases. Cancer. 1979 Nov;44(5):1839–1852. doi: 10.1002/1097-0142(197911)44:5<1839::aid-cncr2820440543>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Kepes J. J., Striebinger C. M., Brackett C. E., Kishore P. Gliomas (astrocytomas) of the brain-stem with spinal intra- and extradural metastases: report of three cases. J Neurol Neurosurg Psychiatry. 1976 Jan;39(1):66–76. doi: 10.1136/jnnp.39.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhajda F. P., Mendelsohn G., Taxy J. B., Long D. M. Pleomorphic xanthoastrocytoma: report of a case with light and electron microscopy. Ultrastruct Pathol. 1981 Jan-Mar;2(1):25–32. doi: 10.3109/01913128109031500. [DOI] [PubMed] [Google Scholar]
- Liesi P., Dahl D., Vaheri A. Laminin is produced by early rat astrocytes in primary culture. J Cell Biol. 1983 Mar;96(3):920–924. doi: 10.1083/jcb.96.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews T., Moossy J. Gliomas containing bone and cartilage. J Neuropathol Exp Neurol. 1974 Jul;33(3):456–471. doi: 10.1097/00005072-197407000-00011. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Dahl D., Virtanen I. Differential diagnosis of chordoma, chondroid, and ependymal tumors as aided by anti-intermediate filament antibodies. Am J Pathol. 1983 Aug;112(2):160–169. [PMC free article] [PubMed] [Google Scholar]
- Nakazato Y., Ishizeki J., Takahashi K., Yamaguchi H., Kamei T., Mori T. Localization of S-100 protein and glial fibrillary acidic protein-related antigen in pleomorphic adenoma of the salivary glands. Lab Invest. 1982 Jun;46(6):621–626. [PubMed] [Google Scholar]
- Newsome D. A., Kenyon K. R. Collagen production in vitro by the retinal pigmented epithelium of the chick embryo. Dev Biol. 1973 Jun;32(2):387–400. doi: 10.1016/0012-1606(73)90249-2. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Ramsey H. J. Fine structure of the surface of the cerebral cortex of human brain. J Cell Biol. 1965 Aug;26(2):323–334. doi: 10.1083/jcb.26.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlinson D. G., Rubinstein L. J., Herman M. M. In vitro characteristics of a myxopapillary ependymoma of the filum terminale maintained in tissue and organ culture systems. Light and electron microscopic observations. Acta Neuropathol. 1974 Mar 26;27(3):185–200. doi: 10.1007/BF00687629. [DOI] [PubMed] [Google Scholar]
- Richman A. V., Balis G. A., Maniscalco J. E. Primary intracerebral tumor with mixed chondrosarcoma and glioblastoma--gliosarcoma or sarcoglioma? J Neuropathol Exp Neurol. 1980 May;39(3):329–335. doi: 10.1097/00005072-198005000-00007. [DOI] [PubMed] [Google Scholar]
- Rubinstein L. J., Brucher J. M. Focal ependymal differentiation in choroid plexus papillomas. An immunoperoxidase study. Acta Neuropathol. 1981;53(1):29–33. doi: 10.1007/BF00697181. [DOI] [PubMed] [Google Scholar]
- Sakai H., Kawano N., Okada K., Tanabe T., Yada K., Yagishita S. [A case of pleomorphic xanthoastrocytoma (Kepes) (author's transl)]. No Shinkei Geka. 1981 Dec;9(13):1519–1524. [PubMed] [Google Scholar]
- Sarmiento J., Ferrer I., Pons L., Ferrer E. Cerebral mixed tumour: osteo-condrosarcoma--glioblastoma multiforme. Acta Neurochir (Wien) 1979;50(3-4):335–341. doi: 10.1007/BF01808532. [DOI] [PubMed] [Google Scholar]
- Shuangshoti S., Dharmmaponpilas J. Neoplasm of mixed mesenchymal and neuroepithelial origin in the posterior cranial fossa: combined chondroma, ependymoma and astrocytoma. J Med Assoc Thai. 1979 Apr;62(4):208–215. [PubMed] [Google Scholar]
- Sipe J. C., Rubinstein L. J., Herman M. M., Bignami A. Ethylnitrosourea-induced astrocytomas. Morphologic observations on rat tumors maintained in tissue and organ culture systems. Lab Invest. 1974 Dec;31(6):571–579. [PubMed] [Google Scholar]
- Siqueira E. B., Bucy P. C. Case report. Chondroma arising within a mixed clioma. J Neuropathol Exp Neurol. 1966 Oct;25(4):667–673. doi: 10.1097/00005072-196610000-00012. [DOI] [PubMed] [Google Scholar]
- Smith B., Butler M. Acid mucopolysaccharides in tumours of the myelin sheath cells, the oligodendroglioma and the neurilemmoma. Acta Neuropathol. 1973 Jan 30;23(2):181–185. doi: 10.1007/BF00685771. [DOI] [PubMed] [Google Scholar]
- Soeur M., Monseu G., Ketelbant P., Flament-Durand J. Intramedullary ependymoma producing collagen. A clinical and pathological study. Acta Neuropathol. 1979 Jul 13;47(2):159–160. doi: 10.1007/BF00717041. [DOI] [PubMed] [Google Scholar]
- Takeuchi J., Sobue M., Yoshida M., Esaki T., Kato Y. Pleomorphic adenoma of the salivary gland. With special reference to histochemical and electron microscopic studies and biochemical analysis of glycosaminoglycans in vivo and in vitro. Cancer. 1975 Nov;36(5):1771–1789. doi: 10.1002/1097-0142(197511)36:5<1771::aid-cncr2820360532>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Taratuto A. L., Molina H., Monges J. Choroid plexus tumors in infancy and childhood. Focal ependymal differentiation. An immunoperoxidase study. Acta Neuropathol. 1983;59(4):304–308. doi: 10.1007/BF00691497. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Kang A. H., Cohen A. M., Hay E. D. Collagen synthesis in vitro by embryonic spinal cord epithelium. Science. 1973 Jan 19;179(4070):295–297. doi: 10.1126/science.179.4070.295. [DOI] [PubMed] [Google Scholar]
- Velasco M. E., Roessmann U., Gambetti P. The presence of glial fibrillary acidic protein in the human pituitary gland. J Neuropathol Exp Neurol. 1982 Mar;41(2):150–163. doi: 10.1097/00005072-198203000-00005. [DOI] [PubMed] [Google Scholar]
- Zimmerman L. E., Font R. L., Andersen S. R. Rhabdomyosarcomatous differentiation in malignant intraocular medulloepitheliomas. Cancer. 1972 Sep;30(3):817–835. doi: 10.1002/1097-0142(197209)30:3<817::aid-cncr2820300334>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]