Abstract
A mononuclear cell chemoattractant of high specific activity produced by baboon (Papio cynocephalus) aortic medial smooth-muscle cells (SMCs) in culture has been partially characterized. Smooth-muscle cells, between the third and eighth passage, were grown to confluence in Medium 199 containing 10% fetal calf serum and then incubated for 24 hours in either serumless medium (Neuman and Tytell) or Medium 199 containing 0.2% bovine serum albumin. The 24-hour SMC-conditioned medium was fractionated on Sephadex G100-Superfine and potent chemoattractant activity (SMC-CF) eluted in the 10,000-12,000 dalton region. SMC-CF displayed chemotactic and chemokinetic activity for peripheral blood mononuclear cells but not for polymorphonuclear leukocytes. Production of SMC-CF by the cells was significantly inhibited in the presence of cycloheximide, and its activity was abolished after incubation with the bacterial protease subtilisin. Chromatofocusing experiments indicate that SMC-CF is a cationic protein with a pI of greater than 10.5. The role of SMC-CF may play as an inflammatory mediator in monocyte recruitment to the arterial intima in atherogenesis is discussed.
Full text
PDF

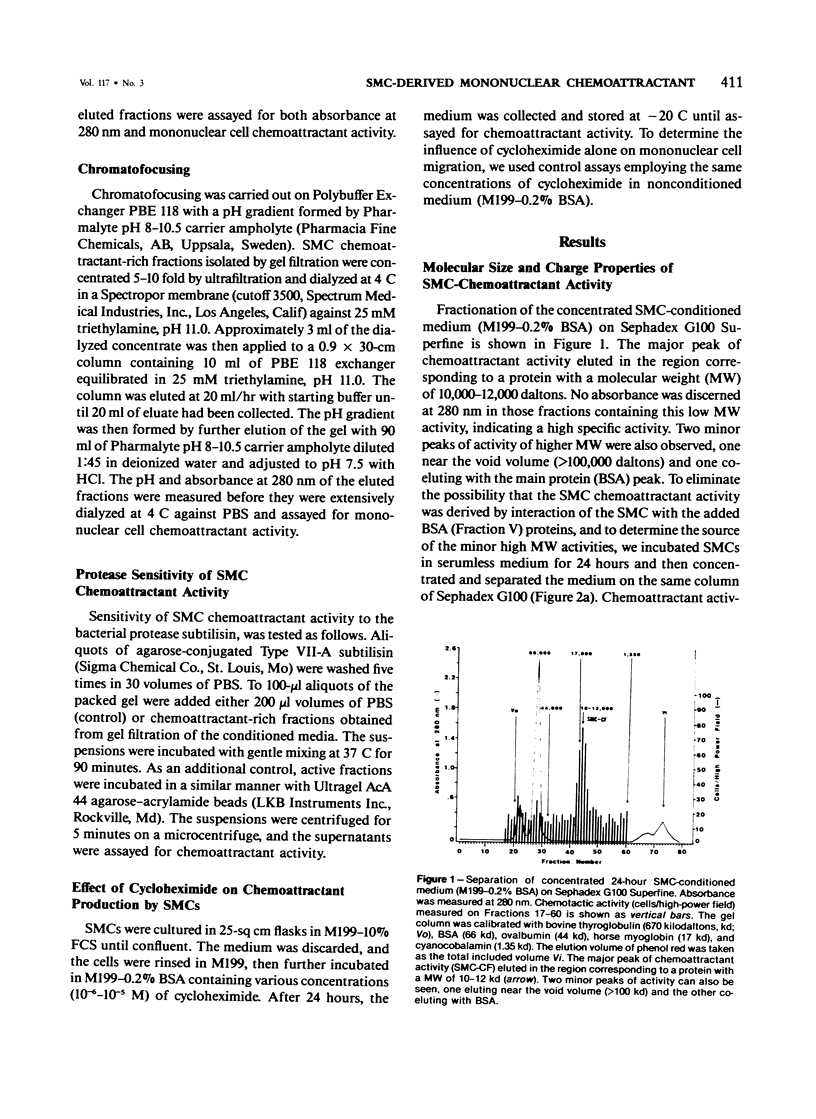

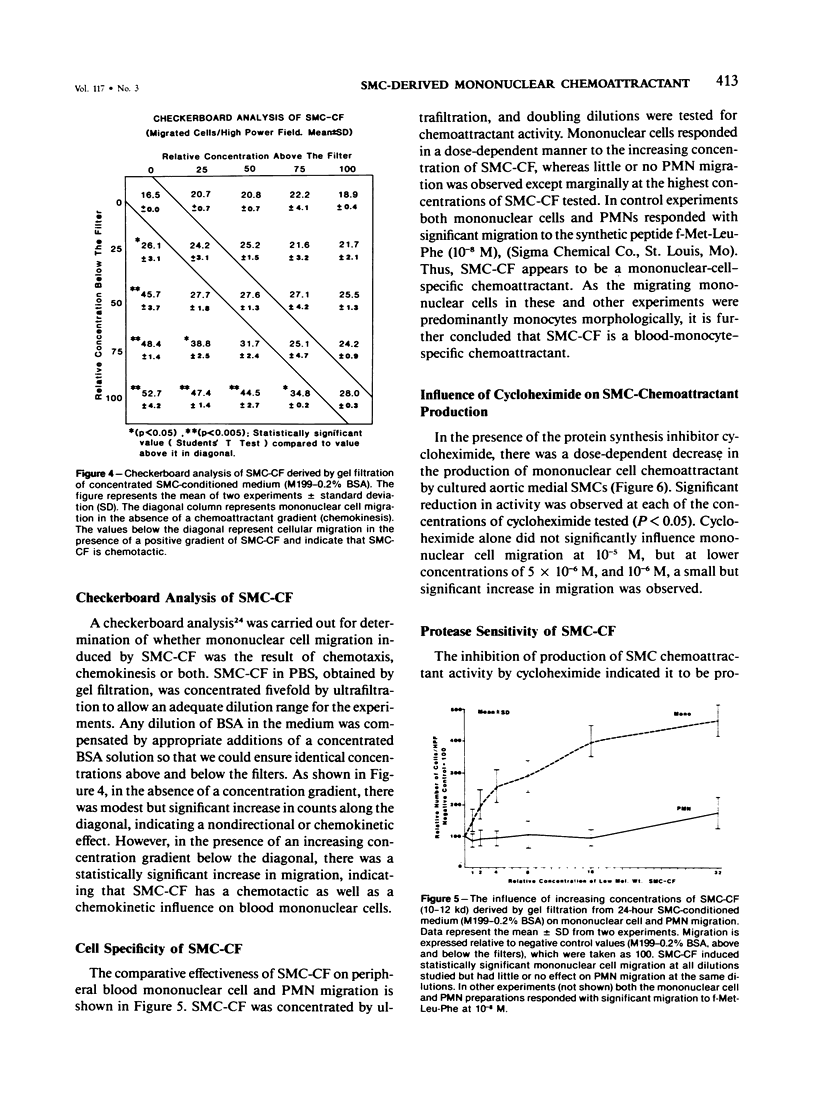




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman L. C., Snyderman R., Oppenheim J. J., Mergenhagen S. E. A human mononuclear leukocyte chemotactic factor: characterization, specificity and kinetics of production by homologous leukocytes. J Immunol. 1973 Mar;110(3):801–810. [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983 Jan;96(1):282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbolini G., Scilabra G. A., Botticelli A., Botticelli S. On the origin of foam cells in cholesterol-induced atherosclerosis of the rabbit. Virchows Arch B Cell Pathol. 1969;3(1):24–32. doi: 10.1007/BF02901924. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Chemotactic activity for mononuclear phagocytes of culture supernatants from murine and human tumor cells: evidence for a role in the regulation of the macrophage content of neoplastic tissues. Int J Cancer. 1983 Jan 15;31(1):55–63. doi: 10.1002/ijc.2910310110. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Collagen synthesis by monkey arterial smooth muscle cells during proliferation and quiescence in culture. Exp Cell Res. 1977 Jul;107(2):387–395. doi: 10.1016/0014-4827(77)90360-3. [DOI] [PubMed] [Google Scholar]
- Burke J. M., Ross R. Synthesis of connective tissue macromolecules by smooth muscle. Int Rev Connect Tissue Res. 1979;8:119–157. doi: 10.1016/b978-0-12-363708-6.50010-2. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chait A., Iverius P. H., Brunzell J. D. Lipoprotein lipase secretion by human monocyte-derived macrophages. J Clin Invest. 1982 Feb;69(2):490–493. doi: 10.1172/JCI110473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J., Campbell G. R., Ross R. The smooth muscle cell in culture. Physiol Rev. 1979 Jan;59(1):1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- DUFF G. L., McMILLAN G. C., RITCHIE A. C. The morphology of early atherosclerotic lesions of the aorta demonstrated by the surface technique in rabbits fed cholesterol; together with a description of the anatomy of the intima of the rabbit's aorta and the spontaneous lesions which occur in it. Am J Pathol. 1957 Sep-Oct;33(5):845–873. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Schwartz C. J. Endothelial cell injury in early mild hypercholesterolemia. Prog Biochem Pharmacol. 1977;13:213–219. [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Greenburg G. B., Hunt T. K. The proliferative response in vitro of vascular endothelial and smooth muscle cells exposed to wound fluids and macrophages. J Cell Physiol. 1978 Dec;97(3 Pt 1):353–360. doi: 10.1002/jcp.1040970310. [DOI] [PubMed] [Google Scholar]
- Hoover R. L., Folger R., Haering W. A., Ware B. R., Karnovsky M. J. Adhesion of leukocytes to endothelium: roles of divalent cations, surface charge, chemotactic agents and substrate. J Cell Sci. 1980 Oct;45:73–86. doi: 10.1242/jcs.45.1.73. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Davidson J. M., Rennard S., Szapiel S., Gadek J. E., Crystal R. G. Elastin fragments attract macrophage precursors to diseased sites in pulmonary emphysema. Science. 1981 May 22;212(4497):925–927. doi: 10.1126/science.7233186. [DOI] [PubMed] [Google Scholar]
- Jauchem J. R., Lopez M., Sprague E. A., Schwartz C. J. Mononuclear cell chemoattractant activity from cultured arterial smooth muscle cells. Exp Mol Pathol. 1982 Oct;37(2):166–174. doi: 10.1016/0014-4800(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Joris I., Zand T., Nunnari J. J., Krolikowski F. J., Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983 Dec;113(3):341–358. [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C., Taylor R. G., Jones N. D., St Clair R. W., Cornhill J. F. Endothelial surface characteristics in pigeon coronary artery atherosclerosis. I. Cellular alterations during the initial stages of dietary cholesterol challenge. Lab Invest. 1982 Feb;46(2):123–138. [PubMed] [Google Scholar]
- Muir L. W., Bornstein P., Ross R. A presumptive subunit of elastic fiber microfibrils secreted by arterial smooth-muscle cells in culture. Eur J Biochem. 1976 Apr 15;64(1):105–114. doi: 10.1111/j.1432-1033.1976.tb10278.x. [DOI] [PubMed] [Google Scholar]
- NEUMAN R. E., TYTELL A. A. Serumless medium for cultivation of cells of normal and malignant origin. Proc Soc Exp Biol Med. 1960 Jun;104:252–256. doi: 10.3181/00379727-104-25797. [DOI] [PubMed] [Google Scholar]
- Norris D. A., Clark R. A., Swigart L. M., Huff J. C., Weston W. L., Howell S. E. Fibronectin fragment(s) are chemotactic for human peripheral blood monocytes. J Immunol. 1982 Oct;129(4):1612–1618. [PubMed] [Google Scholar]
- Pierce C. W. Macrophages: modulators of immunity. Parke-Davis Award Lecture. Am J Pathol. 1980 Jan;98(1):10–28. [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Kang A. H. Collagen-and collagen peptide-induced chemotaxis of human blood monocytes. J Exp Med. 1976 Jun 1;143(6):1299–1307. doi: 10.1084/jem.143.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Keski-Oja J., Balian G., Kang A. H. Induction of fibroblast chemotaxis by fibronectin. Localization of the chemotactic region to a 140,000-molecular weight non-gelatin-binding fragment. J Exp Med. 1981 Feb 1;153(2):494–499. doi: 10.1084/jem.153.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STILL W. J., O'NEAL R. M. Electron microscopic study of experimental atherosclerosis in the rat. Am J Pathol. 1962 Jan;40:21–35. [PMC free article] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Schwartz E., Adamany A. M., Blumenfeld O. O. Extracellular proteins of the calf aortic media smooth muscle cells in culture. Biochim Biophys Acta. 1980 Aug 21;624(2):531–544. doi: 10.1016/0005-2795(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Schwartz E., Bienkowski R. S., Coltoff-Schiller B., Goldfischer S., Blumenfeld O. O. Changes in the components of extracellular matrix and in growth properties of cultured aortic smooth muscle cells upon ascorbate feeding. J Cell Biol. 1982 Feb;92(2):462–470. doi: 10.1083/jcb.92.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Mecham R. P. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Altman L. C., Hausman M. S., Mergenhagen S. E. Human mononuclear leukocyte chemotaxis: a quantitative assay for humoral and cellular chemotactic factors. J Immunol. 1972 Mar;108(3):857–860. [PubMed] [Google Scholar]
- Sobel J. D., Gallin J. I. Polymorphonuclear leukocyte and monocyte chemoattractants produced by human fibroblasts. J Clin Invest. 1979 Apr;63(4):609–618. doi: 10.1172/JCI109343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague E. A., Kelley J. L., Schwartz C. J. Growth, structure and function of baboon aortic smooth muscle cells in culture. Exp Mol Pathol. 1982 Aug;37(1):48–66. doi: 10.1016/0014-4800(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Stary H. C. The intimal macrophage in atherosclerosis. Artery. 1980;8(3):205–207. [PubMed] [Google Scholar]
- Stossel T. P. Phagocytosis (third of three parts). N Engl J Med. 1974 Apr 11;290(15):833–839. doi: 10.1056/NEJM197404112901506. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Secretory function of mononuclear phagocytes: a review. Am J Pathol. 1976 May;83(2):396–418. [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. The macrophage as a regulator of lymphocyte function. Hosp Pract. 1979 Nov;14(11):61-4, 69-74. doi: 10.1080/21548331.1979.11707644. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Newman L. J. A neutrophil chemotactic factor from human C'5. J Immunol. 1969 Jan;102(1):93–99. [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. II. Synthesis and secretion of glycosaminoglycans by arterial smooth muscle cells in culture. J Cell Biol. 1975 Dec;67(3):675–686. doi: 10.1083/jcb.67.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



