Abstract
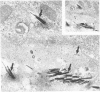
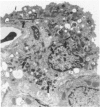
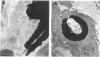
Inhalation of chrysotile asbestos fibers causes interstitial lung disease in animals and man. For examination of the anatomic localization of inhaled asbestos and its relationship to alveolar tissue responses of the lung during and after chronic exposure, male and female Fischer 344 rats were exposed to aerosolized chrysotile for 7 hours/day, 5 days/week for 3 or 12 months. A number of exposed animals were kept in filtered air for an additional 12 months. Lung tissue from randomly selected animals in each group was studied by morphometric analysis of electron micrographs. Our findings show that during exposure to asbestos fibers, macrophages and alveolar epithelial cells contain statistically significant amounts of asbestos and are associated with histologic changes indicating marked epithelial injury. Increased amounts of fibers are also localized in the lung interstitium with continued exposure to asbestos and are associated with a progressive interstitial fibrotic reaction. Following cessation of exposure, macrophages and epithelial cells are cleared of fibers and resolve toward normal proportions. However, significant clearance of fibers from the lung interstitium does not occur after cessation of exposure, and there is a continuing process of fibrogenesis. These data provide new insights related to the pathogenesis of diffuse lung disease and the role each alveolar tissue compartment plays in the early and late phases of the disease.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Dose response of the pulmonary macrophagic system to various particulates and its relationship to transepithelial passage of free particles. Exp Lung Res. 1981 Aug;2(3):165–175. doi: 10.3109/01902148109052312. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Barry B. E., Wong K. C., Brody A. R., Crapo J. D. Reaction of rat lungs to inhaled chrysotile asbestos following acute and subchronic exposures. Exp Lung Res. 1983 Jul;5(1):1–21. doi: 10.3109/01902148309061501. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Adkins B., Jr, O'Connor R. W. Chrysotile asbestos inhalation in rats: deposition pattern and reaction of alveolar epithelium and pulmonary macrophages. Am Rev Respir Dis. 1981 Jun;123(6):670–679. doi: 10.1164/arrd.1981.123.6.670. [DOI] [PubMed] [Google Scholar]
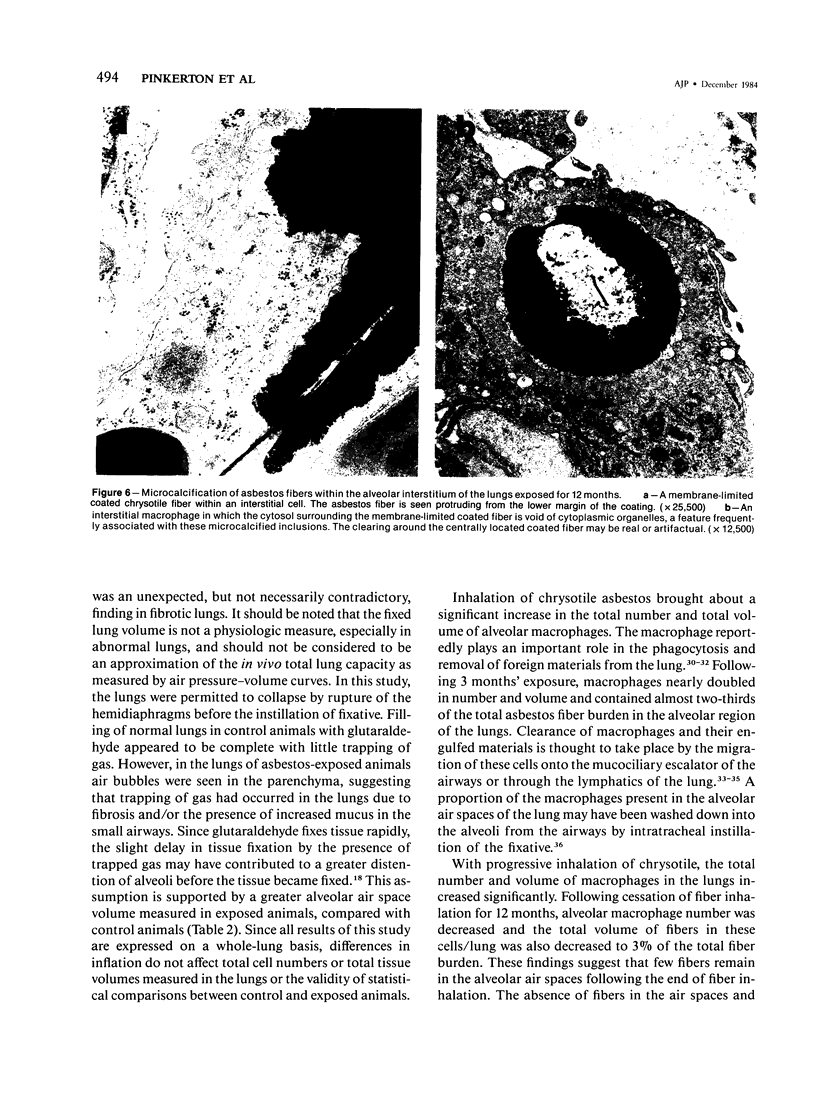
- Brody A. R., Hill L. H. Interstitial accumulation of inhaled chrysotile asbestos fibers and consequent formation of microcalcifications. Am J Pathol. 1982 Oct;109(1):107–114. [PMC free article] [PubMed] [Google Scholar]
- Brody A. R., Hill L. H., Stirewalt W. S., Adler K. B. Actin-containing microfilaments of pulmonary epithelial cells provide a mechanism for translocating asbestos to the interstitium. Chest. 1983 May;83(5 Suppl):11S–12S. doi: 10.1378/chest.83.5_supplement.11s. [DOI] [PubMed] [Google Scholar]
- Brody A. R., Roe M. W. Deposition pattern of inorganic particles at the alveolar level in the lungs of rats and mice. Am Rev Respir Dis. 1983 Oct;128(4):724–729. doi: 10.1164/arrd.1983.128.4.724. [DOI] [PubMed] [Google Scholar]
- Chesky J. A., Rockstein M. Life span characteristics in the male Fischer rat. Exp Aging Res. 1976 Sep;2(5):399–407. doi: 10.1080/03610737608257998. [DOI] [PubMed] [Google Scholar]
- Coleman G. L., Barthold W., Osbaldiston G. W., Foster S. J., Jonas A. M. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977 May;32(3):258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Brody A. R., O'Neil J. J. Morphological, morphometric and x-ray microanalytical studies on lung tissue of rats exposed to chrysotile asbestos in inhalation chambres. IARC Sci Publ. 1980;(30):273–283. [PubMed] [Google Scholar]
- Crapo J. D., Greeley D. A. Estimation of the mean caliper diameter of cell nuclei. II. Various cell types in rat lung. J Microsc. 1978 Sep;114(1):41–48. doi: 10.1111/j.1365-2818.1978.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Peters-Golden M., Marsh-Salin J., Shelburne J. S. Pathologic changes in the lungs of oxygen-adapted rats: a morphometric analysis. Lab Invest. 1978 Dec;39(6):640–653. [PubMed] [Google Scholar]
- Davis J. M. Asbestos dust as a nucleation center in the calcification of old fibrous tissue lesions, and the possible association of this process to the formation of asbestos bodies. Exp Mol Pathol. 1970 Apr;12(2):133–147. doi: 10.1016/0014-4800(70)90045-6. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Beckett S. T., Bolton R. E., Collings P., Middleton A. P. Mass and number of fibres in the pathogenesis of asbestos-related lung disease in rats. Br J Cancer. 1978 May;37(5):673–688. doi: 10.1038/bjc.1978.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. M., Gross P., De Treville R. T. "Ferruginous bodies" in guinea pigs. Fine structure produced experimentally from minerals other than asbestos. Arch Pathol. 1970 Apr;89(4):364–373. [PubMed] [Google Scholar]
- Esterly J. R., Faulkner C. S., 2nd The granular pneumonocyte. Absence of phagocytic activity. Am Rev Respir Dis. 1970 Jun;101(6):869–876. doi: 10.1164/arrd.1970.101.6.869. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Hayatdavoudi G., Crapo J. D., Miller F. J., O'Neil J. J. Factors determining degree of inflation in intratracheally fixed rat lungs. J Appl Physiol Respir Environ Exerc Physiol. 1980 Feb;48(2):389–393. doi: 10.1152/jappl.1980.48.2.389. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Mortality and growth characteristics of rat strains commonly used in aging research. Exp Aging Res. 1980 Jun;6(3):219–233. doi: 10.1080/03610738008258359. [DOI] [PubMed] [Google Scholar]
- Miller K. The effects of asbestos on macrophages. CRC Crit Rev Toxicol. 1978 Sep;5(4):319–354. doi: 10.3109/10408447809081010. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Barry B. E., O'Neil J. J., Raub J. A., Pratt P. C., Crapo J. D. Morphologic changes in the lung during the lifespan of Fischer 344 rats. Am J Anat. 1982 Jun;164(2):155–174. doi: 10.1002/aja.1001640206. [DOI] [PubMed] [Google Scholar]
- Pinkerton K. E., Brody A. R., McLaurin D. A., Adkins B., Jr, O'Connor R. W., Pratt P. C., Crapo J. D. Characterization of three types of chrysotile asbestos after aerosolization. Environ Res. 1983 Jun;31(1):32–53. doi: 10.1016/0013-9351(83)90060-9. [DOI] [PubMed] [Google Scholar]
- Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie. 1970 Jun;26(1):57–60. [PubMed] [Google Scholar]
- Siegrist H. G., Jr, Wylie A. G. Characterizing and discriminating the shape of asbestos particles. Environ Res. 1980 Dec;23(2):348–361. doi: 10.1016/0013-9351(80)90070-5. [DOI] [PubMed] [Google Scholar]
- Sorokin S. P., Brain J. D. Pathways of clearance in mouse lungs exposed to iron oxide aerosols. Anat Rec. 1975 Mar;181(3):581–625. doi: 10.1002/ar.1091810304. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Interaction of asbestos with alveolar cells. Environ Health Perspect. 1974 Dec;9:241–252. doi: 10.1289/ehp.749241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley T. D., Hext P. M., Richards R. J., McDermott M. Chrysotile-induced asbestosis: changes in the free cell population, pulmonary surfactant and whole lung tissue of rats. Br J Exp Pathol. 1976 Oct;57(5):505–514. [PMC free article] [PubMed] [Google Scholar]
- Timbrell V., Hyett A. W., Skidmore J. W. A simple dispenser for generating dust clouds from standard reference samples of asbestos. Ann Occup Hyg. 1968 Oct;11(4):273–281. doi: 10.1093/annhyg/11.4.273. [DOI] [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Pooley F. D. The comparative effects of three chrysotiles by injection and inhalation in rats. IARC Sci Publ. 1980;(30):363–372. [PubMed] [Google Scholar]
- Wagner J. C., Berry G., Skidmore J. W., Timbrell V. The effects of the inhalation of asbestos in rats. Br J Cancer. 1974 Mar;29(3):252–269. doi: 10.1038/bjc.1974.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody D., Woody E., Crapo J. D. Determination of the mean caliper diameter of lung nuclei by a method which is independent of shape assumptions. J Microsc. 1980 Apr;118(4):421–427. doi: 10.1111/j.1365-2818.1980.tb00291.x. [DOI] [PubMed] [Google Scholar]