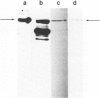
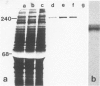
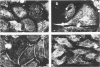
Abstract
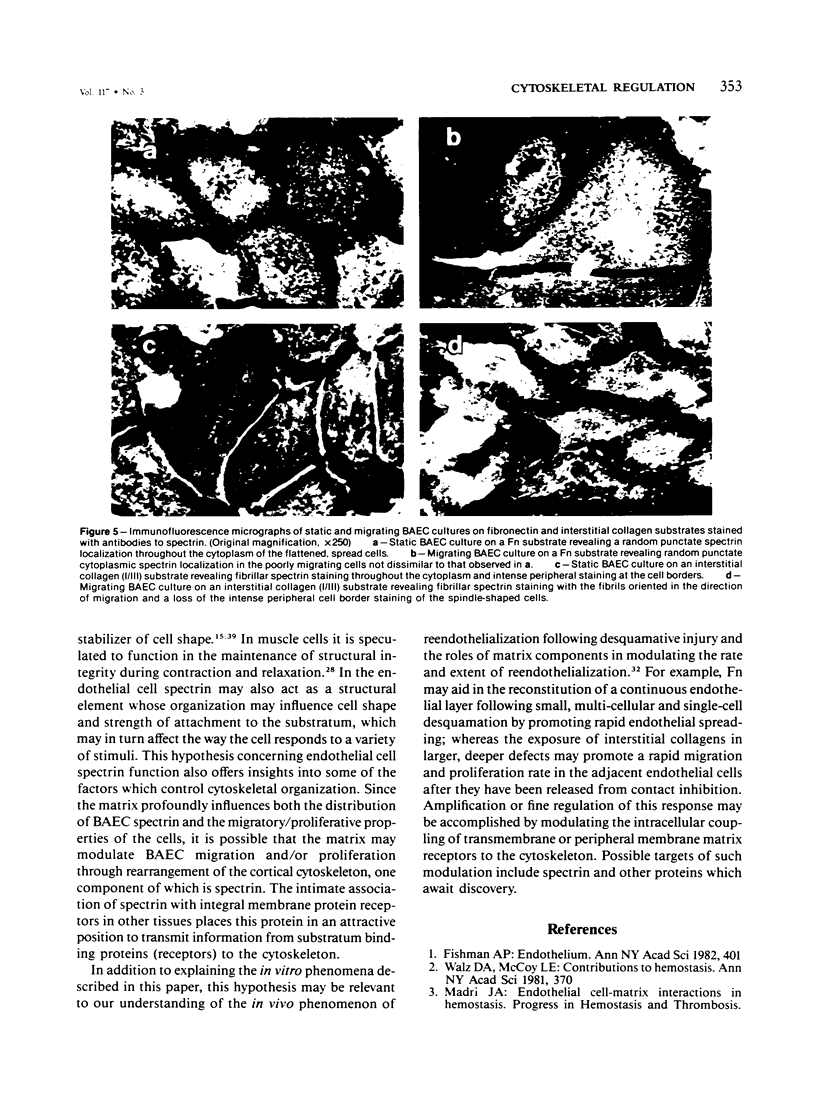
Endothelial cells have a complex cytoskeleton that is responsive to a variety of stimuli such as shear and desquamative injury. The extracellular matrix is known to influence several aspects of cellular behavior, including attachment, spreading, and migration and may, in part, initiate and control vascular responses in growth, differentiation, wound repair, and neoplasia. It is likely that linkage between surface receptors responsible for sensing the matrix and the cytoskeleton may be relevant to understanding the mechanisms of these responses. Spectrin is a high-molecular-weight heterodimer recently identified in many cells that appears to link surface receptors to cortical actin filaments. We have confirmed the existence of spectrin in cultured aortic endothelial cells by metabolic labeling and immunoprecipitation and demonstrated that its organization and intracellular distribution is sensitive to the extracellular matrix. When bovine calf aortic endothelial cells (BAEC) are cultured to confluency on a fibronectin (Fn) substrate, they assume a flattened, spread morphology and exhibit a punctate spectrin distribution with no discernible peripheral localization. In contrast, BAECs cultured on a Type I/III collagen (I/III) substrate exhibit a fibrillar spectrin pattern with significant peripheral localization. When migrating cells were examined, the distribution of spectrin was strikingly different. The cells on the Fn substrate showed no changes in spectrin localization, whereas the cells on I/III exhibited a significant rearrangement, with spectrin being in a coarse fibrillar form, with the fibrils aligned parallel to the direction of migration. The differences in arrangement of this cytoskeletal component on the two substrata reflect the ability of the substrate to perturb the cytoskeletal organization and modulate some aspects of cell behavior such as spreading, proliferation, and migration. These data are consistent with the concept that the nonerythroid spectrins may function as transducers of information from membrane receptors to the cytoskeleton.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Brown S. S., Malinoff H. L., Wicha M. S. Connectin: cell surface protein that binds both laminin and actin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5927–5930. doi: 10.1073/pnas.80.19.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Davis J., Bennett V. Brain spectrin. Isolation of subunits and formation of hybrids with erythrocyte spectrin subunits. J Biol Chem. 1983 Jun 25;258(12):7757–7766. [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., Haudenschild C. Angiogenesis in vitro. Nature. 1980 Dec 11;288(5791):551–556. doi: 10.1038/288551a0. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Intermediate-sized filaments of human endothelial cells. J Cell Biol. 1979 Jun;81(3):570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P. Fodrin is the general spectrin-like protein found in most cells whereas spectrin and the TW protein have a restricted distribution. Cell. 1983 Sep;34(2):503–512. doi: 10.1016/0092-8674(83)90383-5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Gonzalez R., Fujii D. K. Are factors originating from serum, plasma, or cultured cells involved in the growth-promoting effect of the extracellular matrix produced by cultured bovine corneal endothelial cells? J Cell Physiol. 1983 Feb;114(2):191–202. doi: 10.1002/jcp.1041140208. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., Spector W., Wong M. K., Lacey C. In vitro reendothelialization. Microfilament bundle reorganization in migrating porcine endothelial cells. Arteriosclerosis. 1984 Mar-Apr;4(2):91–96. doi: 10.1161/01.atv.4.2.91. [DOI] [PubMed] [Google Scholar]
- Gotlieb A. I., Subrahmanyan L., Kalnins V. I. Microtubule-organizing centers and cell migration: effect of inhibition of migration and microtubule disruption in endothelial cells. J Cell Biol. 1983 May;96(5):1266–1272. doi: 10.1083/jcb.96.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haudenschild C. C., Schwartz S. M. Endothelial regeneration. II. Restitution of endothelial continuity. Lab Invest. 1979 Nov;41(5):407–418. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Kadish J., Wilkins L., Stemerman M. B., Weinstein R. Organizational behavior of human umbilical vein endothelial cells. J Cell Biol. 1982 Sep;94(3):511–520. doi: 10.1083/jcb.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Barwick K. W. Use of avidin-biotin complex in an ELISA system: a quantitative comparison with two other immunoperoxidase detection systems using keratin antisera. Lab Invest. 1983 Jan;48(1):98–107. [PubMed] [Google Scholar]
- Madri J. A., Dreyer B., Pitlick F. A., Furthmayr H. The collagenous components of the subendothelium. Correlation of structure and function. Lab Invest. 1980 Oct;43(4):303–315. [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Williams S. K. Capillary endothelial cell cultures: phenotypic modulation by matrix components. J Cell Biol. 1983 Jul;97(1):153–165. doi: 10.1083/jcb.97.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Nelson W. J., Lazarides E. Switching of subunit composition of muscle spectrin during myogenesis in vitro. 1983 Jul 28-Aug 3Nature. 304(5924):364–368. doi: 10.1038/304364a0. [DOI] [PubMed] [Google Scholar]
- Palfrey H. C., Schiebler W., Greengard P. A major calmodulin-binding protein common to various vertebrate tissues. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3780–3784. doi: 10.1073/pnas.79.12.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Reidy M. A., Clowes A. W., Schwartz S. M. Endothelial regeneration. V. Inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983 Nov;49(5):569–575. [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Rogers K. A., Kalnins V. I. Comparison of the cytoskeleton in aortic endothelial cells in situ and in vitro. Lab Invest. 1983 Dec;49(6):650–654. [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schor A. M., Schor S. L., Kumar S. Importance of a collagen substratum for stimulation of capillary endothelial cell proliferation by tumour angiogenesis factor. Int J Cancer. 1979 Aug;24(2):225–234. doi: 10.1002/ijc.2910240215. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., Rao C. N., Kalebic T., Margulies I. M., Liotta L. A. Laminin receptor on human breast carcinoma cells. Proc Natl Acad Sci U S A. 1983 Jan;80(2):444–448. doi: 10.1073/pnas.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. E., Gimbrone M. A., Jr, Fujiwara K. Factors influencing the expression of stress fibers in vascular endothelial cells in situ. J Cell Biol. 1983 Aug;97(2):416–424. doi: 10.1083/jcb.97.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong A. J., Pollard T. D., Herman I. M. Actin filament stress fibers in vascular endothelial cells in vivo. Science. 1983 Feb 18;219(4586):867–869. doi: 10.1126/science.6681677. [DOI] [PubMed] [Google Scholar]