Abstract
An important class of cellular proteins, which includes members of the p21ras family, undergoes posttranslational farnesylation, a modification required for their partition to membranes. Specific farnesyl transferase inhibitors (FTIs) have been developed that selectively inhibit the processing of these proteins. FTIs have been shown to be potent inhibitors of tumor cell growth in cell culture and in murine models and at doses that cause little toxicity to the animal. These data suggest that these drugs might be useful therapeutic agents. We now report that, when FTI is combined with some cytotoxic antineoplastic drugs, the effects on tumor cells are additive. No interference is noted. Furthermore, FTI and agents that prevent microtubule depolymerization, such as taxol or epothilones, act synergistically to inhibit cell growth. FTI causes increased sensitivity to induction of metaphase block by these agents, suggesting that a farnesylated protein may regulate the mitotic check point. The findings imply that FTI may be a useful agent for the treatment of tumors with wild-type ras that are sensitive to taxanes.
Potent and specific peptidomimetic inhibitors of farnesyl transferase (FTIs) have been synthesized and characterized by several laboratories (1–4). These compounds originally were conceived as potential anti-neoplastic drugs because the Ras family of proteins is farnesylated. Members of the ras family of protooncogenes are mutated in 30% of human cancers, and the Ras protein plays an important role in the development and progression of many human cancers. Ras is isoprenylated through the addition of a C15 farnesyl moiety. This modification confers association with the plasma membrane. Mutants of Ras that do not become membrane-associated are not transforming, and FTIs cause the reversion of transformation of fibroblasts that express the Ha-ras gene (reviewed in ref. 5).
FTIs also inhibit the growth of a majority of human tumor cells in culture. In a variety of animal systems, including v-H-ras transgenic mice and xenograft models, FTIs inhibit tumor growth, causing complete tumor regression in some murine models (6, 7). However, it is not clear that the key defarnesylated target protein is Ras. Human tumor cells without ras mutation often are quite sensitive to FTIs (8). The membrane association of Ki-ras and N-ras proteins is much less sensitive than is that of Ha-ras, yet tumor cells containing mutated Ki-ras can be quite sensitive to the drug (7, 9). Remarkably, even though FTI affects the processing of wild-type Ras protein, the drug has little discernible toxicity in animals at doses that have major anti-tumor effects (6).
These data do not rule out the possibility that Ras inhibition plays an important role in FTI action, but they suggest that other targets may be involved (10). A number of other proteins are known to be farnesylated, including RhoB and Rap2, lamins A and B, phosphorylase kinase, rhodopsin kinase, cyclic GMP phosphodiesterase, and the γ subunit of transducin (5). Whatever the mechanism of inhibition of tumor cell growth, FTIs are novel drugs with wide therapeutic index in animals. Their role in the treatment of cancer patients has not been defined, but their low toxicity in animals, especially the absence of myelosuppression, suggests that they could be used effectively in combination with conventional chemotherapeutic agents. However, FTIs are cytostatic in some experimental models and could conceivably interfere with the effects of cytotoxic agents. We now have tested the effects of combinations of FTI and a variety of commonly used anti-cancer agents on human tumor cells in culture. FTI in combination with many of these agents causes potent and additive cell killing. Moreover, the effect of FTI in combination with taxol or an epothilone, agents that stabilize microtubule polymerization, is synergistic. Analysis of the mechanism of this interaction suggests that FTI enhances the mitotic block caused by exposure to these agents.
MATERIALS AND METHODS
Cell Culture and Growth Assays.
MCF-7 and MDA-MB-468 breast cancer cells were obtained from the American Type Culture Collection and maintained in a 1:1 mixture of DME-to-F12 media supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, 4 mM glutamine, and 10% heat-inactivated fetal bovine serum and incubated at 37°C at 5% CO2. Growth assays were performed by seeding 5,000 or 10,000 cells per well in 6-well clusters and incubating for 24 h before drug treatments. Various drug treatments then were administered as outlined for individual experiments, and cells were incubated for 8–10 days, at which time they were harvested by trypsinization and counted with a Coulter counter. Doxorubicin (Pharmacia), cisplatin (Bristol–Meyers), and taxol (Bristol–Meyers) were diluted appropriately in media to achieve the desired experimental conditions. The FTI L-744832 [Merck (6)] was dissolved in PBS, desoxyepothilone A was dissolved in dimethyl sulfoxide, and appropriate dilutions were made in media to achieve desired experimental conditions. Cells were exposed to chemotherapy for 4 h to approximate in vivo exposure of tumors to these drugs. FTI is used in continuous culture because preclinical studies indicate tumor regrowth upon cessation of therapy (6).
Cell Cycle Analysis.
Cell cycle distribution was studied in cells harvested by trypsinization, taking care to preserve the suspended and adherent cell populations. After washing in cold PBS, cell nuclei were prepared by the method of Nusse (11), and cell cycle distribution was determined by flow cytometric analysis by using red fluorescence of 488 nm of excited ethidium bromide-stained nuclei as a measure of DNA content. Linear displays of fluorescence emissions were used to study and compare cell cycle phases whereas logarithmically displayed emissions were used to best quantitate the cells with degraded sub-G1 DNA content characteristic of apoptotic cells.
To quantitate mitotic indices, cells were harvested as described above, fixed in 3% paraformaldehyde for 10 min at room temperature, and stained in 24 μg/ml bis-benzimide (Hoechst Pharmaceuticals 33258). An aliquot of cells was placed on a glass slide and viewed under fluorescence microscopy. Mitotic cells were identified by characteristic chromatin condensation, and mitotic indices were quantitated by counting 1,000 cells manually.
Taxol Uptake Studies.
To study taxol uptake, cells were treated with HPLC purified 3H-labeled taxol (Moravek Biochemicals, Brea, CA). After 24 h, cells were washed rapidly with PBS and lysed in 1% Nonidet P-40 lysis buffer. Lysates were counted in scintillation fluid. Experimental arms and appropriate controls all were performed in triplicate.
Immunohistochemistry.
MCF-7 cells were seeded on fibronectin-treated glass slides and 24 h later exposed to experimental arms as described. At the time of characterization, cells were fixed in 20% methanol at −20°C for 20 min and double-stained with anti-tubulin mouse mAbs (Sigma) and anti-centromere human serum (gift of K. Elkon, Cornell University, Ithaca, NY). Subsequent staining by using fluorescein isothiocyanate- and rhodamine-conjugated secondary antibodies was performed such that red fluorescence identified tubulin and green fluorescence identified centromeres.
RESULTS
Growth Inhibition by Combinations of Chemotherapy and FTI.
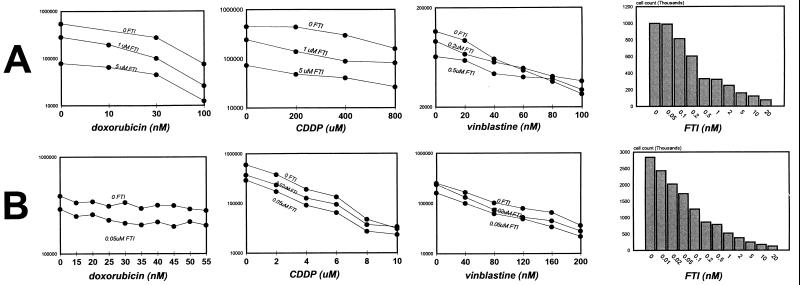
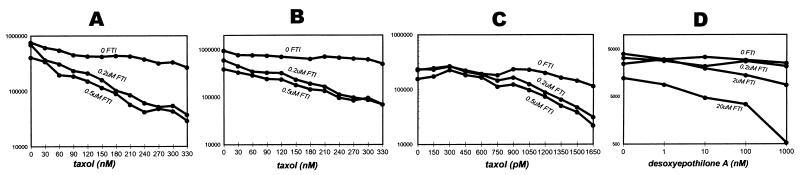
Treatment of cultured breast cancer cells containing wild-type ras with FTI causes a dose-dependent growth inhibition that is additive with the dose-dependent cytotoxicity of several chemotherapeutic agents. These include the DNA-intercalating antibiotic doxorubicin, the DNA-crosslinking agent cisplatin, the microtubule blocking agent vinblastine, and the DNA and RNA synthesis inhibitor fluorouracil (Fig. 1 and data not shown). The experiments shown here were performed at the lowest doses that show growth inhibitory activity for each agent so as to best illustrate additive and potentially synergistic activities. However, higher concentrations of FTI and of chemotherapeutic agents produce complete growth inhibition. In contrast to the additive effects seen with most chemotherapeutic drugs, when FTI is given in combination with the microtubule-stabilizing agent taxol, the combination is synergistic. Addition of FTI steepens the taxol dose–response curve (Fig. 2a). Although treatment with up to 300 nM taxol alone caused moderate growth inhibition in a 4-h treatment, the addition of <1 μM FTI produced an almost one and one-half log growth inhibition. Mathematical analyses performed on cell counts from the above growth assays at several degrees of growth inhibition by using the combination index of Chou and Talalay reveal indices that confirm synergistic effects (12). These effects are maintained when FT inhibition is initiated before taxol, indicating that the synergistic effect is independent of the sequence of exposure (Fig. 2b). In addition, increasing the duration that cells are exposed to both agents does not potentiate the synergy, indicating that the effect is saturated at these conditions (Fig. 2 a–c). Analysis of other cell lines shows that taxol and FTI have similar synergistic effects in T47D breast cancer and DU-145 prostate cancer cells and, to relatively lesser degrees, in MDA-MB-231 and MCF-7 breast cancer cells (data not shown). Thus, there is a mechanistic relationship between these two classes of agents that confers favorable anti-neoplastic effects. Taxol alters tubulin dynamics to promote and stabilize microtubules and arrests mitotic cells in metaphase (13, 14).
Figure 1.
Growth inhibition assays of FTI and chemotherapy combinations. MCF-7 and MDA- MB-468 breast cancer cells were seeded at 10,000 and 20,000 cells per well and the following day were exposed to the stated chemotherapeutic agent for 4 h. Cells then were washed and placed in media containing the FTI L-744832 [Merck (6)] and incubated for 7–10 days. Cells then were harvested by trypsinization and counted in a Coulter counter. The assays were performed at doses of chemotherapy and FTI that show only modest growth inhibition by themselves to best evaluate additive effects. Combinations of FTI with doxorubicin, cisplatin, and vinblastine are shown for MDA-MB-468 (A) and MCF-7 (B) cells. The growth-suppressive effects of FTI alone also are depicted on the right for comparison.
Figure 2.
Growth inhibition assays of FTI and taxol combination. MDA-MB-468 cells were seeded at 10,000 cells per well and incubated for 24 h, then treated in three scheduling variations. In A, cells were treated in a sequential schedule. Cells were exposed to taxol for 4 h, washed, and subsequently incubated in media containing FTI for 7–10 days. In B, cells were treated with FTI before taxol exposure; 24 h after initiating FTI treatment, cells also were exposed to taxol for 4 h and washed off, and FTI treatment continued until days 7–10. In C, the time of two drug exposures was maximized to determine whether the degree of synergy could be increased. Cells were placed in media containing both FTI and taxol and incubated for 7–10 days, exposing cells to both agents continuously. In D, cells were exposed to desoxyepothilone A for 4 h, then placed in media containing FTI for 7 days.
To examine whether the FTI synergy is unique to taxol or is shared by other agents that stabilize microtubules, we tested the effects of epothilones. The epothilones are a newly discovered class of compounds that bear little structural similarity to taxol. However, they have been found to stabilize microtubules and compete with taxol for binding to tubulin (15). They are potent inhibitors of mitosis and are active in cells that are resistant to taxol because of the multi-drug-resistant phenotype (15). The combination of FTI with desoxyepothilone A shows synergistic characteristics similar to the taxol–FTI combination (Fig. 2d), confirming that there is a mechanistic relationship between FTI and microtubule-stabilizing agents. FTIs previously have not been described to affect mitotic progression or microtubule dynamics. FTI treatment of tumor cells in monolayer does not produce an immediate cell cycle block but rather a gradual decline in proliferative rate over 5–7 days and eventual arrest with a multiphasic cell cycle distribution, including both G1 and G2/M phases (data not shown). The mechanism of growth retardation induced by FTIs is unclear but may involve disruption of signals mediated by farnesylated proteins such as Ras.
Potential Mechanism of Synergy.
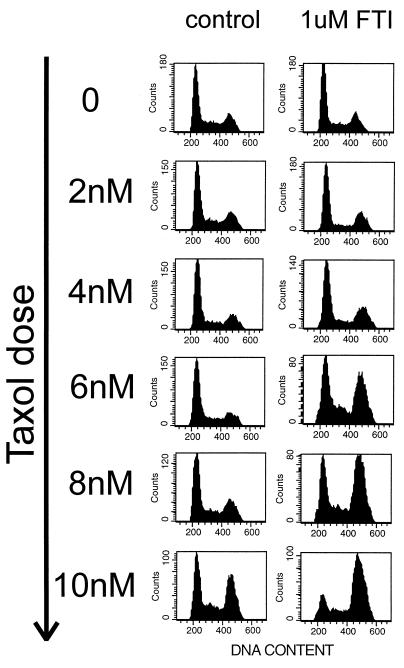
We examined whether the mechanism of synergy involves an effect of taxol on Ras-mediated signal transduction. Taxol treatment of MCF-7 and MDA-468 cells has no effects on the expression of the EGFR, HER2, Ras, ERK1, or ERK2 proteins. Although others have reported that taxol interferes with lipid incorporation into Ras (16), our studies of Ras processing by gel migration analysis do not corroborate this (data not shown). In addition, the EGF-induced activation of MAP kinase is not affected by taxol (data not shown). We proceeded to study whether FTI potentiates the anti-mitotic activity of taxol. In a 24-h assay, taxol causes mitotic arrest in these cells, an effect that becomes apparent at concentrations of 8–10 nM. The concentration dependence of this anti-mitotic effect is altered by the addition of FTI. This difference is most prominent in MCF-7 cells, the cells in which the growth assays show marginal synergy. In this cell line, FTI has no effects on mitosis, nor does 6 nM taxol. However, the combination causes a majority of the cells to block in G2/M (Fig. 3).
Figure 3.
The sensitivity of M phase progression to taxol in the presence and absence of FTI. MCF-7 cells were seeded at 1 million/10-cm dish and placed the following day in varying concentrations of taxol in the presence of 1 μM FTI or vehicle control (PBS). After 24 h of taxol exposure, cells were harvested and cell cycle distribution was determined as described in Materials and Methods. Increasing the concentration of FTI to 10-μM does not increase the twofold sensitization of M phase progression to taxol seen here (data not shown), indicating that the effect is maximal at 1 μM FTI. In MDA-MB-468 cells, taxol causes an M phase block with a much higher degree of apoptotic cell death and shows a similar FTI-induced sensitization of mitosis to taxol.
Further Characterization of the Synergistic Block.
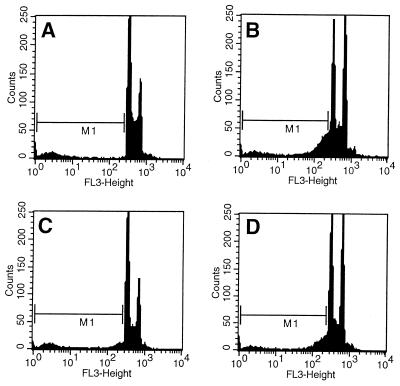
The block induced by FTI and taxol was found to be in M phase as determined by mitotic index analysis. Table 1 shows that, in the presence of FTI, mitotic accumulation begins at lower doses of taxol. Cells inhibited by the FTI and taxol combination at the lowest doses undergo DNA degradation characteristic of apoptosis similar to that seen with taxol alone at higher doses (Fig. 4). Immunohistochemical analysis with anti-tubulin and anti-centromere antibodies revealed that the FTI–taxol mitotic block is characterized by an abnormal chromosome alignment and disordered spindle apparatus that is consistent with metaphase arrest and indistinguishable from that induced by higher doses of taxol alone (Fig. 5). Occasional cells have disordered separation of chromatids that suggests abnormal progression into anaphase.
Table 1.
Mitotic index analysis of Fig. 3
| Taxol dose, nM | Control, % | 1 μM FTI, % |
|---|---|---|
| 4 | 3.3 | 12.3 |
| 6 | 8.8 | 16.9 |
| 8 | 20.5 | 27.6 |
| 10 | 30.0 | 36.0 |
Figure 4.
Apoptotic DNA degradation induced by taxol in the presence or absence of FTI. The logarithmic fluorescence intensity is shown for several arms of the experiment described in Fig. 3 to highlight cells with sub-G1 DNA content due to apoptotic DNA degradation (indicated by the M1 gate). The histograms correspond to A (0 taxol, 0 FTI) and B (8 nM taxol, 0 FTI), showing the characteristic DNA degradation seen with taxol-induced apoptosis in these cells. Histograms C (4 nM taxol, 0 FTI) and D (4 nM taxol, 1 μM FTI) demonstrate cells that become blocked in mitosis largely because of the addition of FTI, and this block also is characterized by apoptotic DNA degradation.
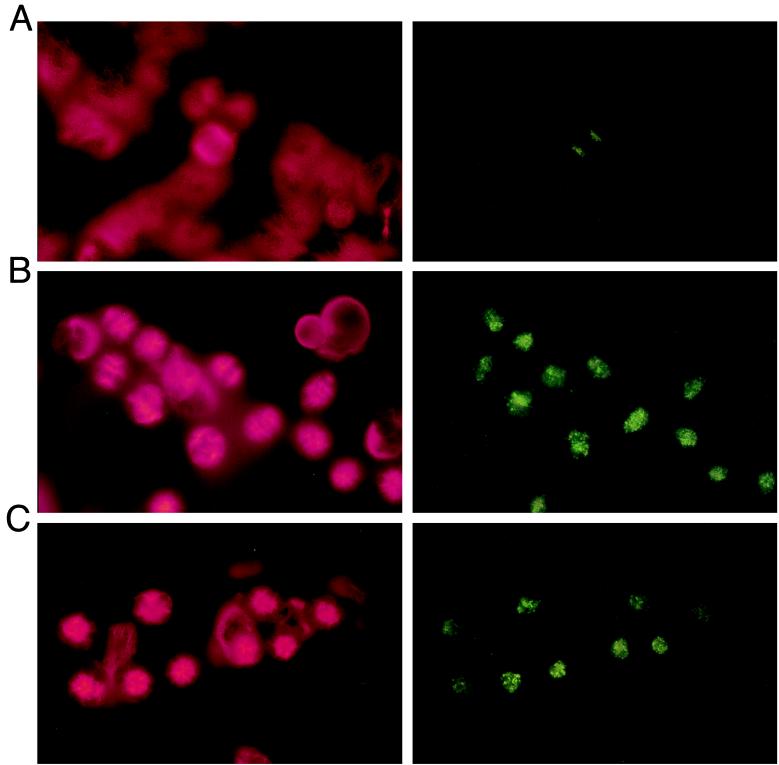
Figure 5.
Immunofluorescence microscopy of mitotic progression. MCF-7 cells were seeded on fibronectin-treated glass slides and treated the following day simultaneously with the experiment in Fig. 3 and in the same experimental arms. After 24 h, cells were washed and fixed in cold methanol and double-stained with anti-tubulin and anti-centromere antibodies as described in Materials and Methods. Conjugated secondary antibody staining was done such that red fluorescence identifies tubulin (Left) and green fluorescence identifies centromeres (Right) in the same microscopic view. A (0 taxol, 0 FTI) shows normal mitosis in progression. B (10 nM taxol, 0 FTI) shows cells blocked in metaphase due to taxol alone. C (6 nM taxol, 1 μM FTI) shows cells blocked in metaphase largely caused by the addition of FTI. The cells are blocked in a metaphase characterized by chromosome alignment but disorderly spindle formation similar to that of B.
Role of Drug Transport.
The interaction observed between FTI and agents that stabilize tubulin polymerization could be based on an effect of FTI on the intracellular accumulation of these drugs. Synergy is not detectable between FTI and other drugs that are transported through a multi-drug-resistant, dependent process such as doxorubicin and vinblastine. We found that FTI had no significant effect on the cellular accumulation of [3H]-taxol (Table 2). We conclude that the mechanism of FTI enhancement of the effects of taxol is probably not caused by effects on cellular transport. The synergy observed between epothilones and taxol is consistent with this conclusion. Multi-drug-resistant cells remain sensitive to epothilones, so these agents are likely to be transported by a different mechanism than taxol (15).
Table 2.
Effect of FTI on cellular taxol uptake
| Experimental arm | Counts ± SD |
|---|---|
| 1) no [3H]-taxol - control | 13 ± 5 |
| 2) 7nM [3H]-taxol - control - no cells | 182 ± 33 |
| 3) 7nM [3H]-taxol - control - no FTI | 16,956 ± 767 |
| 4) 7nM [3H]-taxol - 1 μM FTI | 17,290 ± 924 |
DISCUSSION
FTIs are novel anti-cancer agents that were designed to inhibit tumor growth by interfering with Ras processing. They suppress the growth of a broad range of tumor cell lines whether or not they contain mutations in the ras gene. Preclinical studies of FTI in animal models show that the drug has little toxicity at concentrations that have potent anti-tumor effects. These and other results imply that the FTI may affect multiple targets within the cell and that the antineoplastic activity may not be mediated via a Ras-dependent mechanism. They also suggest that FTI is a potentially useful anticancer agent and that, given its low toxicity, it might be particularly effective when given in combination with traditional cytotoxic drugs. We now show that FTI has additive effects on tumor cell lines in combination with several commonly used chemotherapeutic agents. In addition, FTI and taxol or epothilones act synergistically to inhibit tumor cell growth and arrest cells in metaphase.
These findings suggest a role for a farnesylated protein in regulating the mechanism whereby these drugs kill cells. The effect does not seem to occur at the level of drug transport. FTI could affect the interaction of taxol or epothilones with microtubules, regulate the sensitivity of the mitotic checkpoint, or directly inhibit mitotic progression. The last is unlikely because neither MCF-7 nor MDA-468 cells accumulate in mitosis when treated with high doses of FTI. FTI could affect certain aspects of mitosis through effects on MAP kinase, which is required for mitotic checkpoint function (17), or lamins A and B, two farnesylated proteins in the nuclear envelope (8), or RhoB, which is involved in regulating the actin cytoskeleton (10). However, the lack of obvious synergy of FTI with nocodazole (data not shown) suggests that these general mechanisms are less likely and that the effects of FTI may be related to mechanisms specific to the depolymerization of microtubules required for progression through mitosis.
We have hypothesized that synergistic growth inhibition might be secondary to synergistic induction of mitotic block. For technical reasons, the assay for enhancement of mitosis is done differently than the assay for growth synergy, each under conditions that optimize the effect. Enhancement of mitotic block was measured in cells treated with both drugs for 24 h, whereas growth experiments were assayed after 7–10 days of incubation. The synergistic block in M phase is easily demonstrable in MCF-7 cells, but the effect on cell number is subtle. The reverse is true in MDA-468 cells, in which potent synergistic cytotoxicity is observed but enhanced mitotic accumulation is marginal. It is possible that the synergistic mitotic block and growth inhibition are unrelated synergistic findings. The more likely explanation relates to the observation we have made that, in MDA-468 cells, mitotic block is associated with rapid apoptosis, whereas MCF-7 cells can arrest in M in a relatively stable fashion (data not shown). Thus, it is very difficult to measure an increased accumulation of MDA-468 cells in M, but the killing effects of the combination are easy to observe in growth assays. These findings also suggest the likelihood that the cellular response to FTI or to FTI-containing combinations also will be dependent on the constellation of other mutated genes in the cell.
These data have potentially important clinical implications. FTI is effective in vitro and in animal models in a variety of tumor types that are sensitive to cytotoxic chemotherapeutic agents. Preclinical toxicity profiles imply that FTIs do not exacerbate the toxicity of these agents. These characteristics suggest that the addition of FTI to chemotherapeutic agents could improve their therapeutic index. The identification of an agent that synergizes with taxol without obvious overlapping toxicities defines a new potentially potent combination of anti-cancer drugs. Because taxol is one of the most broadly active agents in use, our findings suggest new strategies for clinical testing that may affect the treatment of many types of human tumors. In addition, comparable synergy is seen with other microtubule-stabilizing drugs such as epothilones. The epothilones are a new class of water-soluble compounds that are more potent and more specific than taxol in vitro (15, 18) and may prove to be clinically superior to taxol. The data shown here were from a breast cancer model. Breast cancer cells rarely contain ras mutations, yet in these experiments they are inhibited by FTI and are exquisitely sensitive to the taxol-FTI combination, further supporting the notion that FT inhibition has applicability well beyond the realm of the mutant ras genotype.
Acknowledgments
This work was supported by National Institutes of Health Breast Cancer Specialized Programs of Research Excellence (SPORE) Grant P50CA68425-02 (N.R.). M.M. is supported by the American Society of Clinical Oncology Career Development Award. L.S.L. is supported by the National Institutes of Health Breast Cancer SPORE Career Development Award.
ABBREVIATIONS
- FTI
farnesyl transferase inhibitor
- FACS
flow-assisted cell sorter
References
- 1.James G L, Goldstein J L, Brown M S, Rawson T E, Somers T C, McDowell R S, Crowley C W, Lucas B K, Levinson A D, Marsters J C., Jr Science. 1993;260:1937–1942. doi: 10.1126/science.8316834. [DOI] [PubMed] [Google Scholar]
- 2.Garcia A M, Rowell C, Ackermann K, Kowalczyk J J, Lewis M D. J Biol Chem. 1993;268:18415–18418. [PubMed] [Google Scholar]
- 3.Kohl N E, Mosser S D, deSolms S J, Giuliani E A, Pompliano D L, Graham S L, Smith R L, Scolnick E M, Oliff A, Gibbs J B. Science. 1993;260:1934–1937. doi: 10.1126/science.8316833. [DOI] [PubMed] [Google Scholar]
- 4.Nigam M, Seong C M, Qian Y, Hamilton A D, Sebti S M. J Biol Chem. 1993;268:20695–20698. [PubMed] [Google Scholar]
- 5.Kohl, N. E., Conner, M. W., Gibbs, J. B., Graham, S. L., Hartman, G. D. & Oliff, A. (1995) J. Cell. Biochem. 22, Suppl., 145–150. [DOI] [PubMed]
- 6.Kohl N E, Omer C A, Conner M W, Anthony N J, Davide J P, deSolms S J, Giuliani E A, Gomez R P, Graham S L, Hamilton K, et al. Nat Med. 1995;1:792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- 7.Kohl N E, Wilson F R, Mosser S D, Giuliani E, deSolms S J, Conner M W, Anthony N J, Holtz W J, Gomez R P, Lee T J, et al. Proc Natl Acad Sci USA. 1994;91:9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepp-Lorenzino L, Ma Z, Rands E, Kohl N E, Gibbs J B, Oliff A, Rosen N. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 9.Whyte D B, Kirschmeier P, Hockenberry T N, Nunez-Oliva I, James L, Catino JJ, Bishop W R, Pai J K. J Biol Chem. 1997;272:14459–14464. doi: 10.1074/jbc.272.22.14459. [DOI] [PubMed] [Google Scholar]
- 10.Lebowitz P F, Davide J P, Prendergast G C. Mol Cell Biol. 1995;15:6613–6622. doi: 10.1128/mcb.15.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nusse M, Beisker W, Hoffmann C, Tarnok A. Cytometry. 1990;11:813–821. doi: 10.1002/cyto.990110707. [DOI] [PubMed] [Google Scholar]
- 12.Chou T C. In: The Median Effect Principle and the Combination Index for Quantitation of Synergism and Antagonism. Chou T C, Rideout D C, editors. Vol. 2. San Diego: Academic; 1991. pp. 61–102. [Google Scholar]
- 13.Jordan M A, Toso R J, Thrower D, Wilson L. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowinsky E K, Donehower R C, Jones R J, Tucker R W. Cancer Res. 1988;48:4093–4100. [PubMed] [Google Scholar]
- 15.Bollag D M, McQueney P A, Zhu J, Hensens O, Koupal L, Liesch J, Goetz M X, Lazarides E, Woods C M. Cancer Res. 1995;55:2325–2333. [PubMed] [Google Scholar]
- 16.Danesi R, Figg W D, Reed E, Myers C E. Mol Pharmacol. 1995;47:1106–1111. [PubMed] [Google Scholar]
- 17.Minshull J, Sun H, Tonks N K, Murray A W. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 18.Muhlradt P F, Sasse F. Cancer Res. 1997;57:3344–3346. [PubMed] [Google Scholar]