Abstract
The electrophysiological properties of acute and chronic methylphenidate (MPD) on neurons of the prefrontal cortex (PFC) and caudate nucleus (CN) have not been studied in awake, freely behaving animals. The present study was designed to investigate the dose-response effects of MPD on sensory evoked potentials recorded from the PFC and CN in freely behaving rats previously implanted with permanent electrodes, as well as their behavioral (locomotor) activities. On experimental day 1, locomotor behavior of rats was recorded for 2 h post saline injection, and sensory evoked field potentials were recorded before and after saline and 0.6, 2.5, and 10 mg/kg, i.p., MPD administration. Animals were injected for the next five days with daily 2.5 mg/kg MPD to elicit behavioral sensitization. Locomotor recording was resumed on experimental days 2 and 6 after the MPD maintenance dose followed by three days of washout. On experimental day 10, rats were connected again to the electrophysiological recording system and rechallenged with saline and the identical MPD doses as on experimental day 1. On experimental day 11, rat’s locomotor recording was resumed before and after 2.5 mg/kg MPD administration. Behavioral results showed that repeated administration of MPD induced behavioral sensitization. Challenge doses (0.6, 2.5, and 10.0 mg/kg) of MPD on experimental day 1 elicited dose-response attenuation in the response amplitude of the average sensory evoked field potential components recorded from the PFC and CN. Chronic MPD administration resulted in attenuation of the PFC’s baseline recorded on experimental day 10, while the same treatment did not modulate the baseline recorded from the CN. Treatment of MPD on experimental day 10 resulted in further decrease of the average sensory evoked response compared to that obtained on experimental day 1. This observation of further decrease in the electrophysiological responses after chronic administration of MPD suggests that the sensory evoked responses on experimental day 10 represents neurophysiological sensitization. Moreover, two different response patterns were obtained from PFC and CN following chronic methylphenidate administration. In PFC, the baseline and effect of methylphenidate expressed electrophysiological sensitization on experimental day 10, while recording from CN did not exhibit any electrophysiological sensitization.
Keywords: Psychostimulants, Electrophysiology, Evoked potential, Prefrontal cortex, Caudate nucleus, Sensitization
1. INTRODUCTION
Psychostimulants were initially proposed for the treatment of attention deficit hyperactivity disorder (ADHD) in 1937 and is currently used as the treatment of choice (Eichlseder 1985; Swanson et al., 1999; Challman and Lipsky, 2000). The psychostimulant methylphenidate (MPD; ritalin) has become the most prescribed medication for ADHD (Solanto 1998; Challman and Lipsky, 2000; Accardo and Blondis, 2001; Swanson and Volkow, 2002). It is estimated that 5–15% (2–6 million) of the U.S. population between 5 and 18 years old is being treated with MPD (Anderson et al., 1987; Rowland et al., 2001; Barbaresi et al., 2002). Since ADHD persists into adulthood, these individuals continue to take MPD well into their adulthood (Himelstein and Halperin, 2000). Yet little is known of the long-term consequences of MPD (Musser et al., 1998; MacDonald and Kollins, 2005; Kollins et al., 2001; Carlezon and Konradi, 2004; MacDonald and Kollins, 2005).
Behavioral experiments in animals using repeated exposure to psychostimulants demonstrated that these drugs elicit behavioral sensitization. Behavioral sensitization refers to the progressive augmentation of the initial, behavioral responses to a psychostimulant (Leith and Kuczenski, 1982; Kalivas and Stewart, 1991; Wolf 1998). A sensitized response to a psychostimulant is elicited by the intermittent administration of a low dose of the psychostimulant, while higher doses produce tolerance (Leith and Kuczenski, 1982; Robinson and Berridge, 1993). Behavioral sensitization is believed to be an early manifestation of the neuronal plasticity associated with repeated administration of a drug of abuse and may provide evidence for the underlying alterations that resulted in craving as well as relapse that occurs after a period of abstinence and play a central role in the development of addictive behavior (Robinson and Berridge, 1993). Thus, behavioral sensitization can serve as an animal model to study drug craving (Robinson and Berridge, 1993; Kalivas et al., 1998) and the induction of persistent changes in the neuronal circuitry of motivation and reward following chronic exposure to psychostimulants (NIHCDCS, 2000). Repeated administration of amphetamine, cocaine, and methamphetamine has been shown to elicit behavioral sensitization (Robinson and Becker, 1986; Segal and Kuczenski, 1987; Pierce and Kalivas, 1997; Crawford et al., 1998). However, findings of behavioral sensitization resulted from repeated exposure to MPD have been inconsistent (McNamara et al., 1993; Gaytan et al., 1997a; Izenwasser et al., 1999; Kuczenski and Segal, 2002; Yang et al., 2003).
Psychostimulants exert their effect mainly on the CNS. Therefore, it is important to study the effects of the drug on the brain itself. Most of the investigators studying brain functions use procedures to evoke brain related events. To obtain brain related events, they stimulate the animal to evoke responses before and after treatments (Dafny and Feldman, 1970; Dafny 1975b; Dafny et al., 1975; Dafny 1975c, 1998; Dafny and Gildenberg, 1984). One of the “best” approaches to obtain events in the CNS is to use physiological sensory stimulation such as acoustic. These stimulations do not require any surgical procedure or cause pain to the animals. Any sensory information entering the CNS spreads to many brain areas (Dafny et al., 1975; Dafny 1998). Each area processes this information for different functions. Moreover, MPD exerts its effects by altering sensory inputs to the subject (Kaufmann et al., 1984; Eichlseder 1985; Gittelman et al., 1985; Biederman et al., 1994; Laviola et al., 1999; Swanson et al., 1999; Greenhill 2001). Therefore, it is essential to study the effect of psychostimulants on sensory input.
The “ideal” preparation to study the effects of drug on the CNS is in an unanesthetized, freely behaving animals previously implanted with permanent electrodes and to use physiological stimulation. The use of intact, freely behaving animals made it possible to study the underlying neurophysiological mechanisms of acute and chronic MPD treatment at the cellular level (i.e., electrophysiology) and systems level (behavior) without interference of anesthesia. Therefore, in the present study, unanesthetized, freely behaving animals previously implanted with permanent electrodes (under anesthesia) and sensory stimulation will be used to investigate the effects of acute and chronic MPD on behavior and electrophysiology from the same animals.
Patients diagnosed with ADHD exhibit dopaminergic and noradrenergic abnormalities/dysfunction in the prefrontal cortex (PFC) and caudate nucleus (CN) (Arnsten et al., 1996; Sergeant et al., 2002; Seidman et al., 2004; Bush et al., 2005). Yet little is known about the mechanisms contributing to MPD’s therapeutic efficacy or the possible enduring neuroadaptational consequences of long-term drug exposure at these sites (National Institutes of Health Consensus Development Conference Statement, 2000; Greenhill, 2001). Specifically, there is lack of data demonstrating, in freely behaving rats, the neurophysiological outcome from acute and chronic treatment of MPD on sensory evoked potentials. Because the main site of MPD action is the CNS, it is essential to study the effect of MPD on the brain regions suggested to be the CNS sites for psychostimulant action and whether sensory information and processing in these regions are influenced by the acute and chronic exposure to MPD. Therefore, the present study set out to investigate the effect of MPD on sensory input at the PFC and CN sites known to be the targets for psychostimulants in awake, freely behaving rats previously implanted with permanent electrodes.
2. RESULTS
2.1. Locomotor behavior
Controls – Locomotor activity of eight rats were recorded continuously (24 h) for 42 days without any treatment. Locomotor activity count was collected for each 10-min samples (bin at 10 min) and summed into light (12 h), dark (12 h), daily (24 h), and weekly locomotor activity in order to determine whether the locomotor activity would change over time without treatment. Figure 1 summarizes the horizontal activity of this experiment and demonstrates that the locomotor activity during the 42 days without treatment remained essentially the same with minor fluctuations during the 12 h light period (Fig. 1A), 12 h night period (Fig. 1B), or 24 h during the light and dark cycle, and weekly activity for six weeks (Fig. 1D), respectively. Figure 2 summarizes the horizontal and vertical activities of ten rats that were implanted with electrodes in the PFC and CN and had received saline and repeated MPD administration (see Table 1). These animals were also used in the electrophysiological experiment. Values are presented as the mean + S.E.M. with * P < 0.05. The bar graphs summarize the 2-h cumulative horizontal and vertical activities under the temporal curve of experimental days 1, 2, 6, and 11. An injection of 2.5 mg/kg MPD on experimental day 2 exhibited similar activity level as that of baseline on experimental day 1. However, after five days of repeated 2.5 mg/kg MPD administration, both the horizontal and vertical activities on experimental day 6 were significantly augmented compared to day 2 [F 3,39 = 4.31, *P = 0.021 (horizontal); F 3,39 = 3.72, *P = 0.046 (vertical)]. This augmentation persisted on experimental day 11 compared to day 2 (*P < 0.05). This augmentation demonstrated that the chronic MPD administration of 2.5 mg/kg MPD elicited behavioral sensitization.
Figure 1.
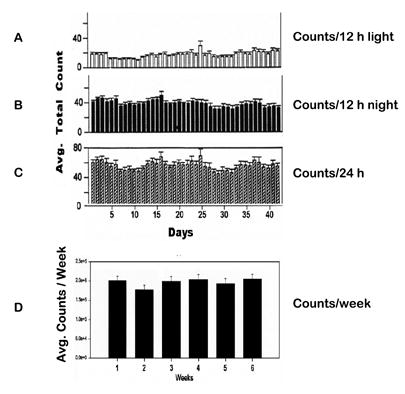
A–C summarizes the 24 h horizontal activity (upper histogram) and the 12 h night time (dark) activity (middle histogram) and the 12 h daytime activity (lower histogram). Figure 1D summarizes the total activity/week (N = 8). This figure demonstrates that the locomotor activity during the 42 days remained essentially the same with minor fluctuations.
Figure 2.
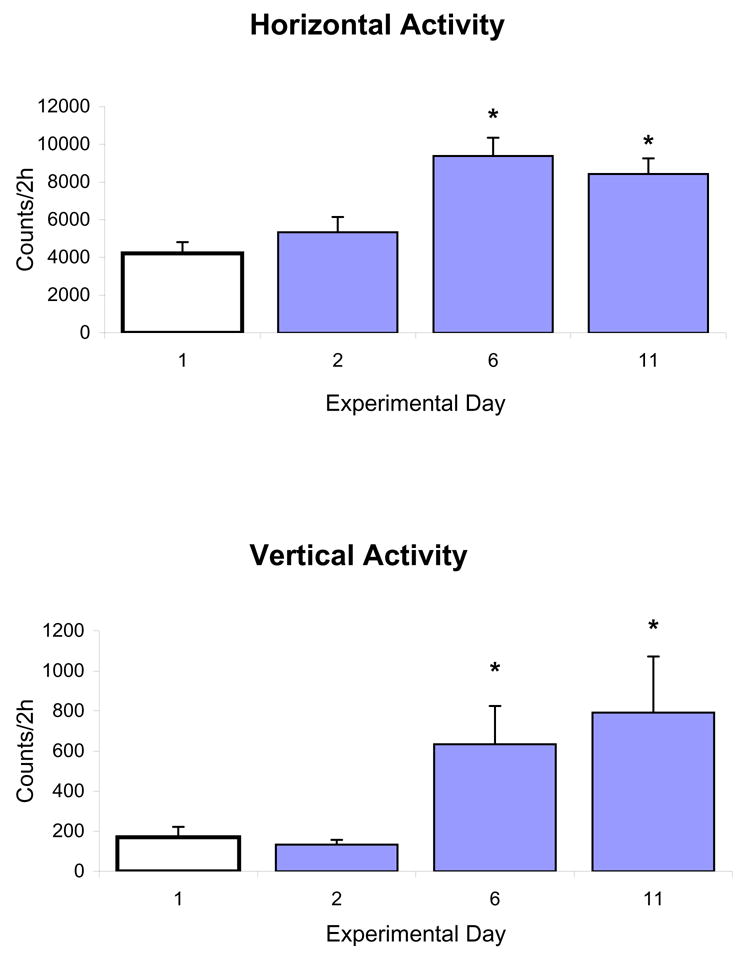
summarizes the horizontal and vertical activities (n = 10) that had received saline and repeated MPD administration. Values are presented as the mean + S.E.M. with * P < 0.05. The bar graphs summarize the 2-h cumulative horizontal and vertical activities on experimental days 1, 2, 6, and 11.
Table 1.
Experimental protocol
| Exp’l Day | 1 | 2 | 3–5 | 6 | 7–9 | 10 | 11 |
|---|---|---|---|---|---|---|---|
| Treatment | Saline – B
Saline – E 0.6* – E 2.5* – E 10.0* – E |
2.5*– B | 2.5* – M | 2.5* – B | Washout | Saline – E
0.6* – E 2.5* – E 10.0* – E |
2.5* – B |
= mg/kg MPD B = Behavioral recording E = Electrophysiological recording M = Maintenance dose
On experimental day 1, behavioral recording (B) was performed for 2 h after saline injection. The rat was then placed in the electrophysiological testing chamber where electrophysiological recordings were performed after saline (baseline) and 0.6, 2.5, and 10.0 mg/kg MPD administration. On experimental day 2, behavioral testing (2 h) was carried out after 2.5 mg/kg MPD injection. Maintenance (M) dose of 2.5 mg/kg MPD was given on days 3–5 without any recording. Behavioral testing (2 h) occurred after 2.5 mg/kg MPD injection on day 6. Days 7–9 were washout days (drug abstinence). Electrophysiological recordings resumed on day 10 after saline (baseline) and 0.6, 2.5, and 10.0 mg/kg MPD. On experimental day 11, behavioral testing resumed after 2.5 mg/kg MPD. Time between injections on experimental days 1 and 10 was 120 min.
2.2. Sensory evoked field responses
Controls – Three electrophysiological control recordings were obtained. Figure 3A shows a representative of the average acoustic evoked responses (AAER) recording from the CN beginning 1 h after the animals were connected to the electrophysiological recording system, and recordings were obtained every 30 min. The data showed that the AAER remained stable over time if no treatment was given. Figure 3B shows four consecutive AAER recording every 10 min (control) and an additional four AAER recorded following two consecutive saline injections (middle and right columns). Time between injections was 120 min. This recording demonstrated that handling of the animal (i.e., inserting the needle into the animal and injection volume) did not modulate the AAER. Figure 3C shows simultaneous recordings of AAER from CN and PFC. Each column shows four consecutive AAER recordings every 10 min post-saline injection throughout 4 consecutive weeks from the same animals. This figure indicates that the AAER over long period of time (4 weeks) remained stable and expressed similar response amplitudes. Therefore, every significant deviation from baseline indicated the effect of the drug.
Figure 3.
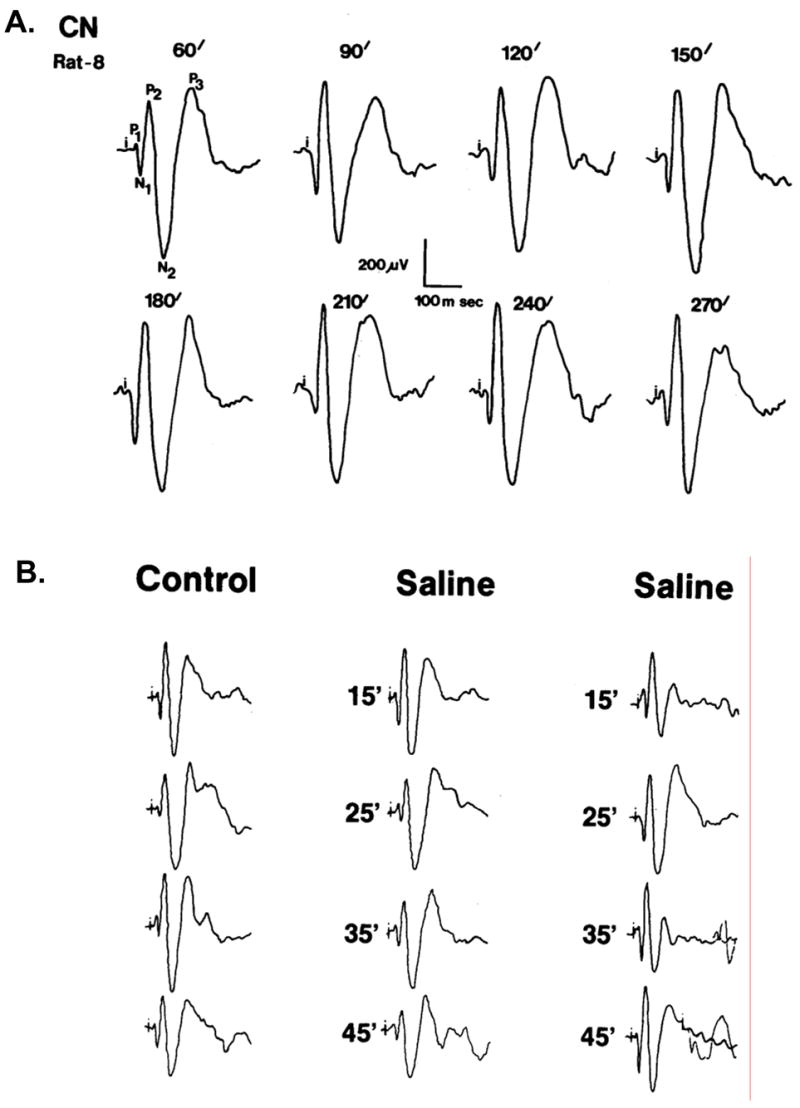
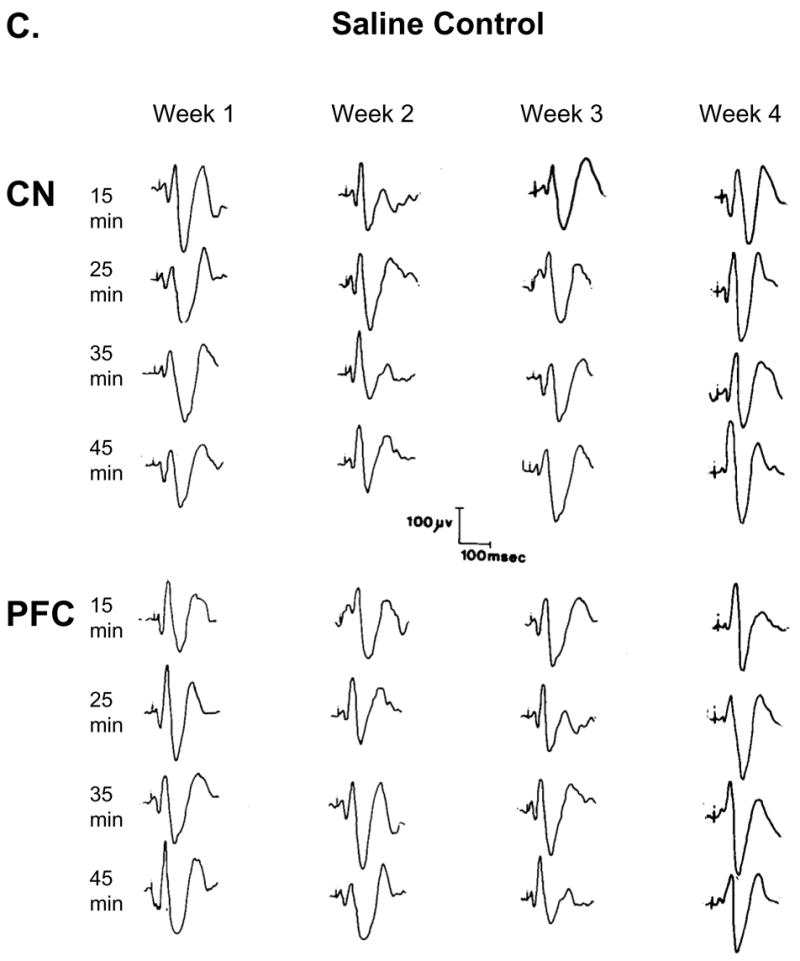
shows that the average acoustic evoked responses (AAER; n = 50) during the experiment (time control) remained the same if no treatment was given, and saline injection did not alter the AAER. Figure 3A shows a representative recording from the CN beginning 1 h after the animal was connected to the electrophysiological recording system, and recordings were obtained every 30 min. Figure 3B shows four consecutive recording every 15 min (control) before and after two saline injections. The number indicates the recording time (min) post-saline injection and shows that animal handling during injection did not alter the AAER. Figure 3C shows simultaneous recordings from CN and PFC. Each column shows four consecutive recordings every 10 min post-saline injection throughout 4 consecutive weeks from the same animals and that the AAER over time did not change.
Figure 4 shows representatives AAER recorded simultaneously in the PFC and CN following the administration of saline (baseline) and a single injection of 0.6, 2.5, and 10.0 mg/kg, i.p., MPD on experimental day 1. All three MPD doses attenuated significantly (P < 0.05) the amplitude response of the AAER components (P2, N2, and P3) as compared to their baseline in both brain sites. Furthermore, this attenuation exhibited dose-response characteristics, i.e., further attenuation in the AAER amplitude was observed with increased MPD dosage.
Figure 4.
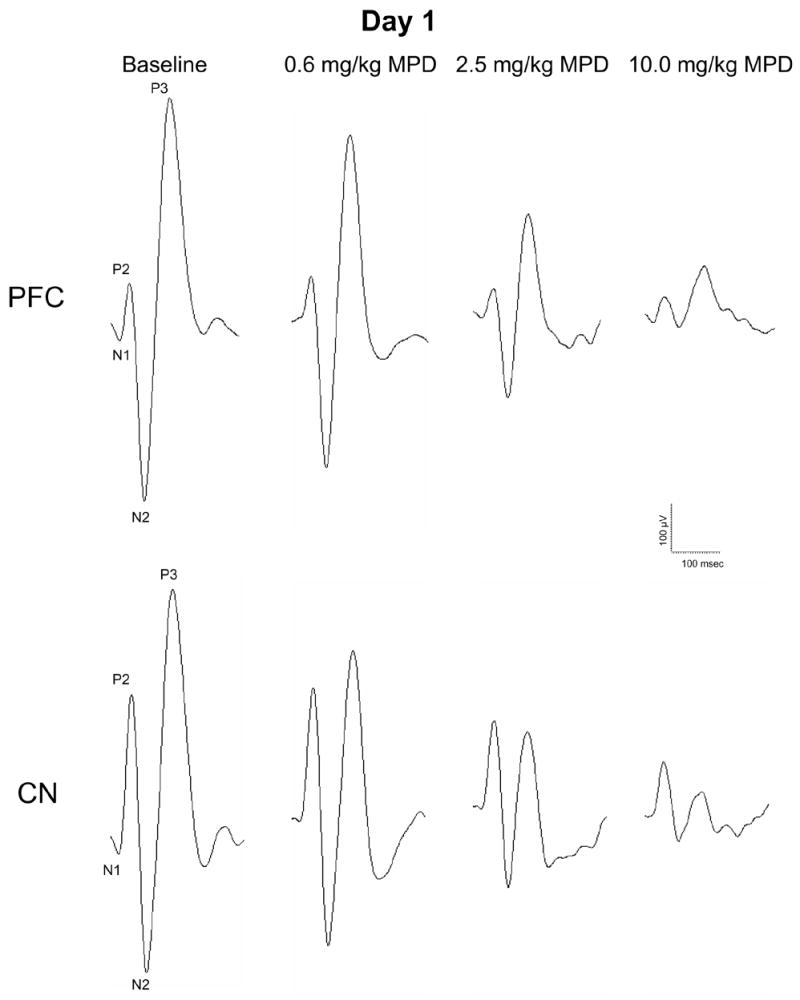
shows representatives of the averaged (n = 50) acoustic evoked responses (AAER) recorded simultaneously in the PFC and CN following the administration of saline (baseline) and a single injection of 0.6, 2.5, and 10.0 mg/kg, i.p., MPD on experimental day 1. Recordings were obtained between 10 and 40 min post-injection of saline and MPD.
Figure 5 summarizes the percent attenuation from baseline of the AAER amplitude for P2, N2, and P3 components recorded from the PFC and CN following 0.6, 2.5, and 10.0 mg/kg MPD, i.p., on experimental day 1. The control amplitude after saline administration (baseline) was arbitrarily set as 100%. Each component’s percent attenuation from baseline following MPD administration was calculated as the average of four post-injection time points recorded (10, 20, 30, and 40 min post-injection) and is presented as the mean ± S.E.M. Figure 5A shows that an injection of 0.6, 2.5, and 10.0 mg/kg MPD, in general, significantly decreased the PFC’s AAER amplitude of P2 [F3,15= 23.86, ΔP < 0.01], N2 [F3,15= 77.16, ΔP < 0.01], and P3 [F3,15= 91.33, ΔP < 0.01] components compared to those of baseline. Additionally, each of these AAER components was attenuated differently in dose-response characteristics with N2 and P3 components exhibiting greater percent attenuation from baseline than P2 component [0.6 mg/kg (F2,11= 39.96, *P < 0.01); 2.5 mg/kg (F2,11= 12.92, *P < 0.01); and 10.0 mg/kg (F2,11= 7.75, *P < 0.05)]. Similar to PFC, an acute administration of 0.6, 2.5, and 10.0 mg/kg MPD also significantly reduced the CN’s AAER amplitude of P2 [F3,15= 39.06, ΔP < 0.01], N2 [F3,15= 127.96, ΔP < 0.01], and P3 [F3,15= 41.32, ΔP < 0.01] components compared to those of baseline (Fig. 5B). When similar comparison for the effect of escalating doses of MPD was performed, it was observed that the three doses of MPD also attenuated the CN’s AAER amplitude of these components in dose-response characteristics. However, there was no significant difference in the level of attenuation among the three peak components at each MPD dose as observed in the PFC, i.e., all three components for each MPD dose were similarly affected.
Figure 5.
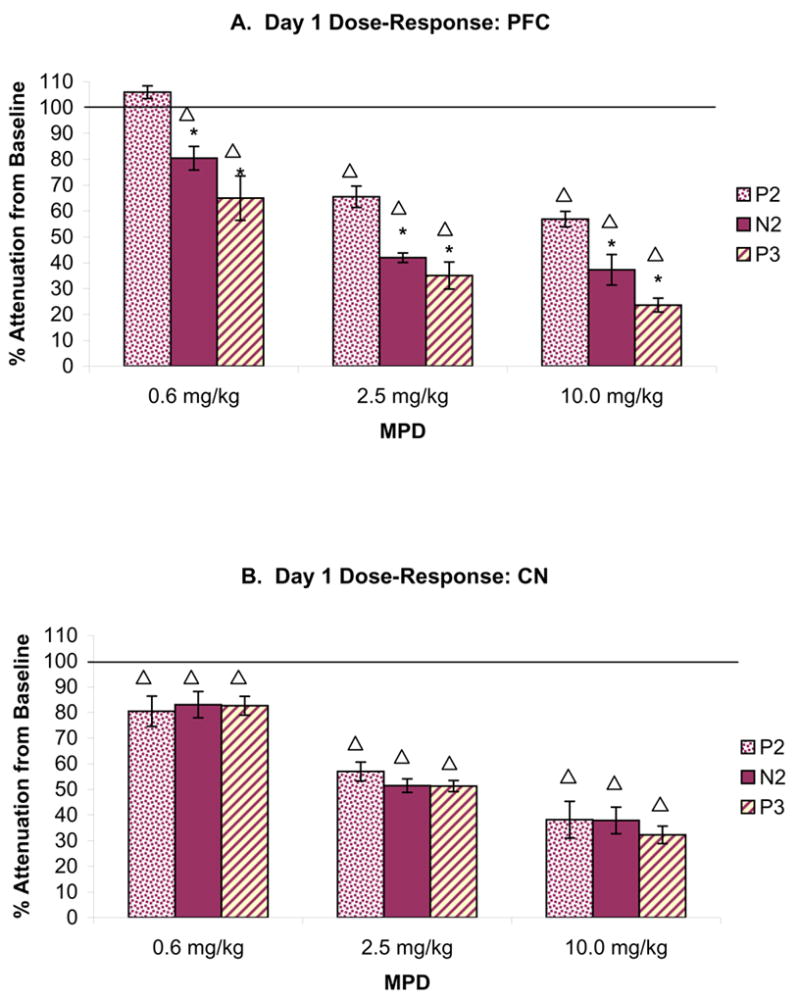
summarizes the percent attenuation from baseline of the AAER amplitude for P2, N2, and P3 components recorded from the PFC (Fig. 5A; n = 7) and CN (Fig. 5B; n = 19) following 0.6, 2.5, and 10.0 mg/kg MPD, i.p., on experimental day 1. The control amplitude after saline administration (baseline) was arbitrarily set as 100%. Each component’s percent attenuation from baseline following MPD administration was calculated as the average of four post-injection time points (10, 20, 30, and 40 min) and is presented as the mean ± S.E.M. ΔP < 0.05 compared to baseline at 100%; *P < 0.05 compared to P2 component.
Figure 6A shows representatives AAER recorded simultaneously in the PFC and CN after the administration of saline (baseline) on experimental days 1 and 10. In general, six days of repeated MPD administration followed by three days of washout significantly reduced the baseline of the AAER amplitude of the N2 and P3 components on experimental day 10 compared to that of day 1 in the PFC, while the baseline recording from the CN at experimental day 10 remained similar to that of experimental day 1. Figure 6B summarizes the data for all recordings and compares the percent change of the AAER amplitude of P2, N2, and P3 recorded simultaneously in the PFC and CN on day 10 to that of day 1. The response amplitude after saline administration on experimental day 1 was arbitrarily set as 100%. There was significant attenuation of AAER baseline on day 10 compared to day 1 for N2 and P3 components of the PFC [N2: F1,13= 4.76, *P = 0.05; P3: F1,13= 13.86, *P = 0.003] but not for the AAER components of the CN.
Figure 6.
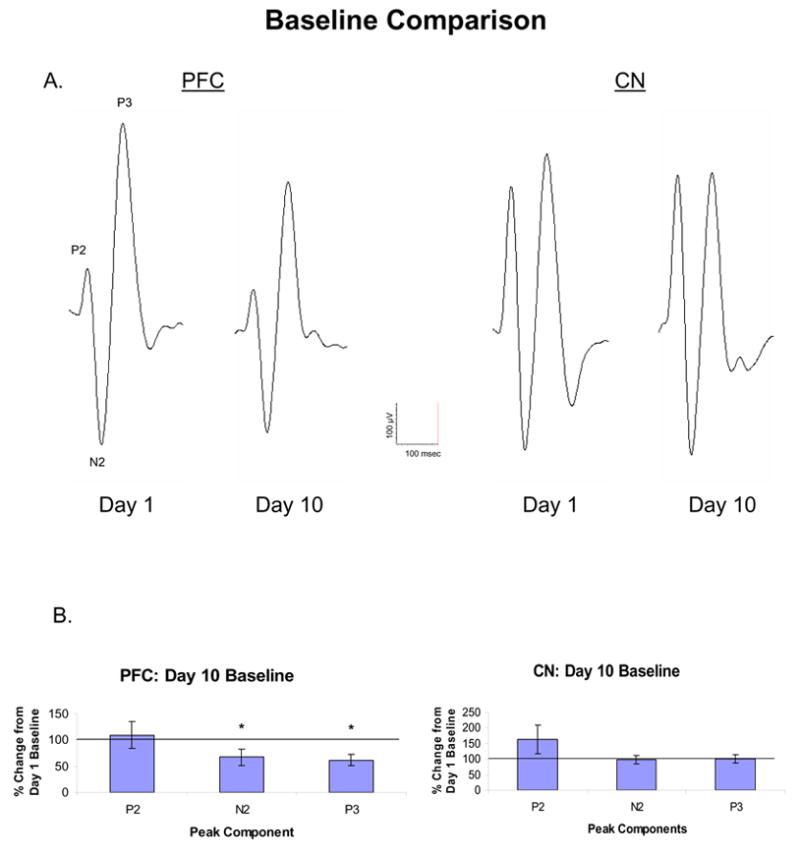
A shows representatives of the AAER recorded simultaneously in the PFC and CN after the administration of saline (baseline) on experimental days 1 and 10. Figure 6B summarizes the data for all recordings and compares the percent change of the AAER amplitude of P2, N2, and P3 recorded simultaneously in the PFC (n = 7) and CN (n = 19) on day 10 to that of day 1. The amplitude after saline administration on experimental day 1 was arbitrarily set as 100%. All recordings were obtained between 10 and 40 min following saline injection. *P < 0.05 compared to day 1 baseline at 100%.
Figure 7 shows representatives of AAER recorded simultaneously in the PFC and CN of a rat following the administration of saline (baseline) and 0.6, 2.5, and 10.0 mg/kg MPD on experimental days 1 and 10. The figure shows the control recording and the effect of drug 20 min post-injection and demonstrates that, on experimental day 1, the recording of AAER from MPD-naïve animal following each incremental MPD injection elicited further attenuation of all the AAER components recorded from the PFC and CN as compared to control recording and to the previous MPD dose. The recording on experimental day 10 was performed after chronic MPD administration (see Table 1) when the animals became behaviorally sensitized to MPD (Fig. 2). The baseline recording on experimental day 10 exhibited differences in AAER recordings between PFC and CN. The baseline AAER obtained from the PFC on experimental day 10 was attenuated for all the response components as compared to the control on experimental day 1; whereas the baseline control recorded from the CN on experimental days 1 and 10 was similar. In a comparison of all recordings of the control AAER components from the PFC on experimental day 10 with those of experimental day 1, significant (P < 0.05) attenuation was observed. Moreover, when incremental MPD doses were given on experimental day 10 (i.e., in MPD behaviorally sensitized animals), each MPD dose (0.6, 2.5, and 10.0 mg/kg) elicited further attenuation of all AAER components (Fig. 7 – PFC: day 10). Such further attenuation could represent electrophysiological sensitization to MPD in the PFC. The AAER recording from the CN exhibited different characteristics on experimental day 10. The CN’s control AAER on experimental day 10 was the same as that obtained on experimental day 1. The level of AAER attenuation following incremental MPD treatment on experimental day 10 was also similar to that on experimental day 1 in MPD-naïve animals. This observation indicates that electrophysiological sensitization to MPD was not expressed in the CN.
Figure 7.
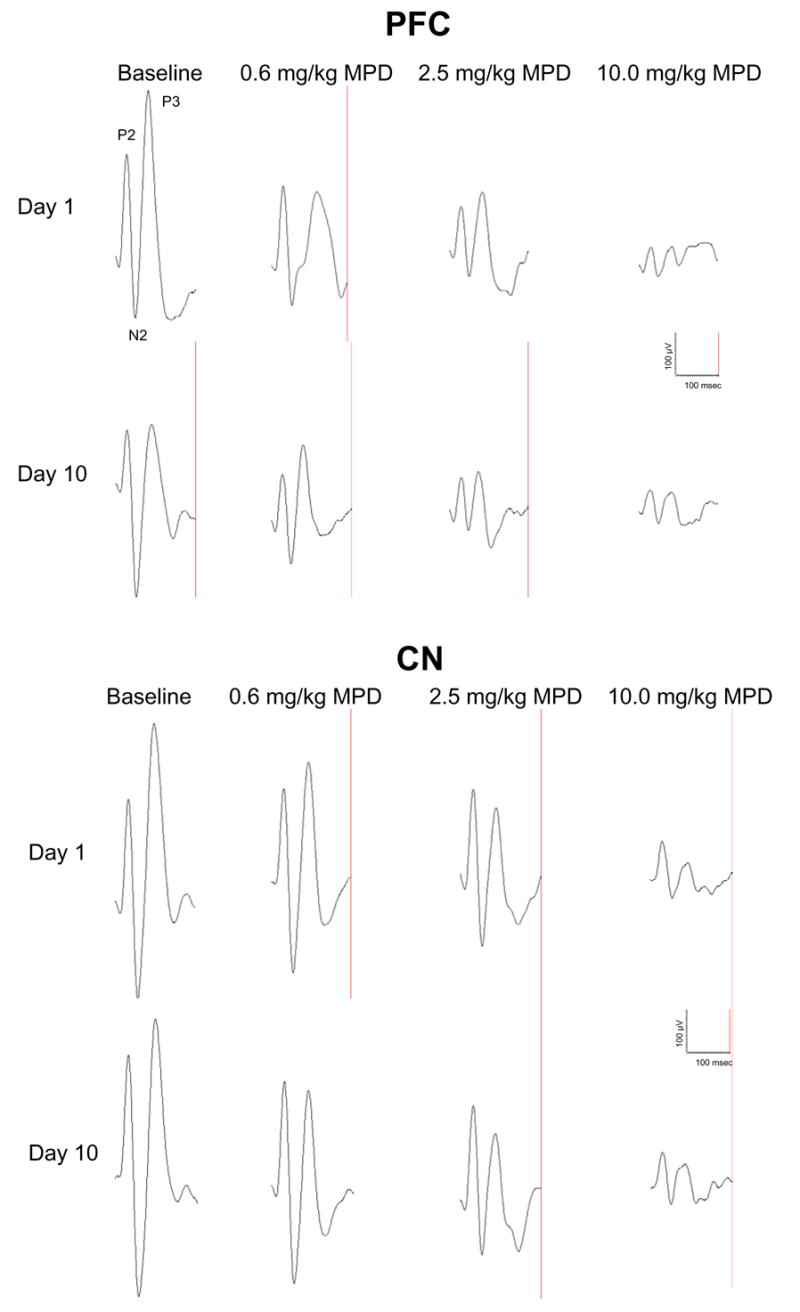
shows representatives of AAER (n = 50) recorded simultaneously in the PFC and CN of a rat following the administration of saline (baseline) and 0.6, 2.5, and 10.0 mg/kg MPD on experimental days 1 and 10.
3. DISCUSSION
In the present study, we investigated neurophysiologically the dose-response characteristics of MPD in MPD-naïve animals (experimental day 1) and when these same animals became behaviorally sensitized to MPD (experimental day 10). We accomplished this by recording the MPD effects on AAER obtained simultaneously in the PFC and CN of non-anesthetized, freely behaving rats previously implanted with permanent electrodes, as well as recording the locomotor activity before and after repeated administration of this psychostimulant to verify that the drug treatment elicits behavioral sensitization. Indeed, all of the experimental animals exhibited behavioral sensitization to the MPD treatment (Fig. 2).
In the behavioral experiment, we found that repeated administration of saline did not have any effect on locomotion, while repeated administration of MPD induced behavioral sensitization as indicated by the progressive augmentation of the horizontal and vertical activities (Fig. 2) on experimental days 6 and 11 compared to experimental day 2. The horizontal and vertical activities on experimental day 2 following 2.5 mg/kg MPD administration were similar to that of saline on experimental day 1. However, after five consecutive days of a daily injection of the maintenance dose of 2.5 mg/kg MPD, the horizontal and vertical activities of the rats increased significantly on experimental day 6 compared to that of day 2. This increase in locomotor response persisted on experimental day 11. These results are in accordance with previous studies that had demonstrated the development of behavioral sensitization following repeated administration of MPD (Gaytan et al., 1997a; Crawford et al., 1998; Kuczenski and Segal, 2001; Yang et al., 2003). However, other investigators had reported that chronic exposure to MPD in rats failed to induce behavioral sensitization (McNamara et al., 1993; Izenwasser et al., 1999; Kuczenski and Segal, 2002). A possible explanation for these inconsistent findings is methodological variations. Specifically, factors such as dose, route, time, regimen of drug administration, and the locomotor indices recorded can critically affect the observation to show whether behavioral sensitization was developed (Robinson and Becker, 1986; Gaytan et al., 1997a; White and Kalivas, 1998; Kuczenski and Segal, 2001, 2002; Dafny and Yang, 2006).
The present study investigated the effect of MPD on sensory input recorded simultaneously from the PFC and CN. Sensory evoked field potential is an electrophysiological measure often used for analyzing information processing in the brain (Picton et al., 2000). A major advantage of using this electrophysiological technique is that it provides both an average sampling of evoked activity in a neuronal population (Dafny et al., 1975; Dafny 1975a, 1978) and the means to study sensory input and processing in millisecond temporal resolution time locked to the stimulus occurrence (Winsberg et al., 1997).
The amplitude of the sensory responses consists of P1, N1, P2, N2, and P3 components. Although the cognitive processes and their underlying mechanisms associated with these components have not been definitely established, it is believed that P1 and N1 components represent the presynaptic incoming impulses, while P2, N2, and P3 components reflect the postsynaptic activity within the recording site (Dafny et al., 1975; Dafny 1978). Moreover, it has been suggested that the P2 represents the beginning of a central process responsible for stimulus identification and the initiation of decision, while N2 and P3 represent the end of this process (Lindholm and Koriath, 1985). The P3 component is suggested to also indicate further processing and evaluation of the relevant stimuli, such as stimulus categorization (Teo and Ferguson, 1986). A reduction of P3 amplitude implies cognitive dysfunction (Michie et al., 1990; Solowij et al., 1991).
The electrophysiological experiment revealed that MPD treatment in MPD-naïve rats (experimental day 1) attenuated the AAER’s recorded from the PFC and CN in dose-response characteristics, i.e., each incremental MPD dose caused further decrease in the AAER. Moreover, similar doses of MPD in MPD behaviorally sensitized animals (experimental day 10) further attenuated the AAER while the locomotor activity increased. These electrophysiological findings suggest that the further decreases in the AAER expressed neurophysiological sensitization in the PFC following the chronic treatment of 2.5 mg/kg MPD, while this MPD dose did not elicit neurophysiological sensitization in the CN. Moreover, chronic treatment of MPD attenuated the AAER baseline recording from the PFC on experimental day 10, while the AAER recording from the CN remained the same on both experimental days (1 and 10).
The electrophysiological findings from the present study suggest that there are differences in the neuronal response to MPD between the PFC and CN. Functionally, the CN plays a key role in motor activity and in the processing of sequential information (Aldridge and Berridge 1998; Nakamura et al., 2001), while the PFC has been implicated in the regulation of cognition and emotion in rats, monkeys, and humans (Mazei et al., 2002), as well as in nearly every major mental disorder in humans (Mazei et al., 2002). In particular, the PFC is thought to support vigilance, selective and divided attention, attention shifting, planning, executive control, working memory, and behavioral inhibition (Rubia et al., 1999; Duncan and Owen, 2002; Aron et al., 2003). The mesocortical dopaminergic projection, which originates in the ventral tegmental area (Emson and Koob, 1978), ascends to the PFC and has an important role in stress, drug abuse, and schizophrenia (Goeders and Smith, 1983; Weinberger 1987; Abercrombie et al., 1989), while the CN receives mainly DA projections from the substantia nigra.
In addition to anatomical and functional differences between the PFC and CN, there are differences in the effects of MPD in the dopamine transporter (DAT) densities and rates of dopamine (DA) release and uptake between these two brain regions, as well as blockade of the norepinephrine transporter (NET) (Carboni et al., 1990). Synaptic DA levels are regulated by DAT, which is a critical protein for DA regulation since it is responsible for the reuptake of DA from the synapse. The DAT is expressed in both the PFC and CN; however, its distribution is not homogenous. The DAT content in the PFC is found to be lower than that of the CN (Mazei et al., 2002). The rate of DA release and uptake in the PFC is about 8 times less than that in the CN (Garris and Wightman, 1994). Moreover, it has been reported that blockade of the norepinephrine transporter (NET) increases extracellular DA levels in the PFC, implying that NET is also involved in clearing DA in the PFC of the rat (Carboni et al., 1990; Pozzi et al., 1994; Gresh et al., 1995). The ability of NET to clear DA is significant since there is an increased innervation of NE terminals compared to DA terminals in the PFC (Slopsema et al., 1982). These differences in DAT and NET densities and rates of DA reuptake between PFC and CN may account for the differences in their response to MPD as observed by the effects of MPD on AAER.
Studies have demonstrated that psychostimulants induce changes in the release characteristics of DA (Robinson et al., 1988; Kalivas and Duffy, 1990; Vezina 1993) and/or alterations in DA-stimulated signal transduction mechanisms (Steketee et al., 1991; Steketee 1994; Miserendino and Nestler, 1995), and these changes play a crucial role in the drug effect on locomotor and neurophysiological sensitization. We posit that the increase in extracellular DA resulted from MPD administration could activate postsynaptic DA receptors in motor nuclei and thereby enhance the motor-activating effects. As the rat was repeatedly exposed to MPD, more extracellular DA became available at the synaptic cleft and activated a greater number of postsynaptic DA receptors. This activation of the motor nuclei could result in behavioral sensitization. In contrast, activation of the presynaptic DA autoreceptors has been found to exert a strong inhibitory effect on the neuronal activity (Ruskin et al., 2001) and thereby result in the inhibition of the firing of DA neurons in the CNS (Bunney et al., 1973; Einhorn et al.,1988; Shi et al., 2000, 2004). This inhibitory role attenuated the sensory information arriving at these sites, which may explain the result in attenuation of all three AAER components recorded from the PFC and CN. Using amphetamine and in vivo microdialysis, it was reported that other neurotransmitters and/or mechanism could contribute to the chronic effect of the psychostimulants (Segal and Kuczenski, 1992).
In conclusion, this is the first study that investigated, in the same freely moving subjects, the animal behavior at the systems level and the sensory evoked field potentials at the neuronal level of the PFC and CN. This study provides the dose-response characteristics of acute and chronic MPD administration recorded from freely behaving animals implanted with permanent electrodes and suggests that chronic MPD elicited plasticity in the PFC, as well as neurophysiological sensitization to MPD, while MPD exerted different effects in the CN. It is clear from the data that further investigations into the sensory evoked field responses at the neuronal level are warranted in the elucidation of the mechanisms of drug action of MPD and its long-term effects on the CNS.
4. MATERIALS AND METHODS
4.1. Subjects
Twenty six male Wistar-Kyoto rats (WKY) were used in the experiment. Eight animals were used for behavioral time control experiment (Fig. 1), eight for electrophysiological experiment to obtain the average acoustic evoked responses (AAER) over time (Fig. 3A), following animal handling (Fig. 3B) and recording over several weeks (Fig. 3C), and ten for the acute and chronic dose response effects of MPD on AAER. The animals weighed 180–190 g at arrival from the vendor (Harlan Laboratories, Indianapolis, IN, USA). They were housed 2 per Plexiglas cage and maintained at an ambient temperature of 21 ± 2 °C and a relative humidity of 37–42% in the vivarium on a 12-h light/dark cycle (lights on from 05:30 to 17:30 h). The animals were allowed 3–7 days of acclimation to this environment before commencement of any experimental manipulation. They received food and water ad libitum throughout experimentation. All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, as well as our institution’s Animal Welfare Committee Guidelines.
4.2. Drug
Methylphenidate hydrochloride (MPD) was supplied by Mallinckrodt, Inc. (St. Louis, MO, USA). The drug was dissolved in 0.9% saline, and the dosages were calculated as free-base. All injections were administered intraperitoneally (i.p.) between 07:00 h and 12:00 h and equalized to a volume of 0.8 ml with 0.9% saline so that the total volume of each injection would be the same for all animals. Time between injections was 120 min.
4.3. Surgical procedure
Rats were anesthetized with 50.0 mg/kg pentobarbital and fitted to a stereotaxic apparatus. An incision of 2.0 cm was made to expose the skull. One millimeter holes were drilled through the skull above the target sites to insert electrodes (stainless steel 80 μm in diameter) bilaterally (in both hemispheres) at the PFC (Bregma 2.7 mm, lateral 0.6 mm, depth 3.8 mm) and CN (Bregma 0.2 mm, lateral 3.2 mm, depth 4.4 mm) using coordinates derived from the Paxinos and Watson (1986) atlas. An additional electrode was implanted in the frontal sinus as a reference electrode. All electrodes were fixed permanently to the rat’s skull with dental acrylic cement and attached to terminals in an Amphenol plug.
4.4. Experimental Design
Each rat participated in both behavioral and electrophysiological recordings (Table 1). On experimental day 1 upon 5–7 recovery days from the surgical implantation of electrodes, the rat was put inside the computerized, automated locomotor activity monitoring cage where its behavioral baseline activity was recorded for 2 h after saline administration (Gaytan et al., 1997a; Yang et al., 2003). It was then transferred to the electrophysiological test chamber where its sensory evoked field potentials were recorded in response to acoustic stimulation following the administration of saline and escalating doses of 0.6, 2.5, and 10.0 mg/kg MPD. On experimental day 2, the rat was placed back inside the locomotor activity open field monitoring cage and its behavioral activity recording was resumed for 2 h after 2.5 mg/kg MPD administration (see Table 1). On experimental days 3 to 6, animals were treated with 2.5 mg/kg MPD, which was the maintenance dose to elicit behavioral sensitization (Gaytan et al., 1997 a,b; Yang et al., 2003). Drug administration always occurred in the testing cage and not in the home cage. Behavioral activity resumed on experimental day 6 following the last maintenance of 2.5 mg/kg MPD. Washout period occurred on experimental days 7 to 9 in which the rats did not receive any treatment. Experimental day 10 was identical as experimental day 1. Behavioral testing of the rat resumed again on experimental day 11 after the administration of 2.5 mg/kg MPD (see Table 1) to observe whether the animals expressed behavioral sensitization.
4.5. Behavioral apparatus
The computerized, automated, open field activity monitoring cage (Accuscan, Columbus, OH, USA) consisted of a clear, acrylic box (40.5 × 40.5 ×31.5 cm) fitted with two levels of infrared motion sensors located 6.0 and 12.5 cm above the floor of the box. The apparatus recorded interruptions of each infrared beam at a frequency of 100 Hz. Interruptions of any infrared beam were recorded as an activity score. Simultaneous interruptions of two or more consecutive beams separated by at least 1 sec were recorded as a movement. Cumulative counts were compiled and downloaded every 10 min into the OASIS data collection software (Accuscan) that organized and differentiated these counts into various locomotor indices. Horizontal and vertical activities were the locomotor indices evaluated in the present study. Horizontal activity measured the total number of beam interruptions that occurred in the horizontal sensor (lowest tier), while vertical activity measured the amount of rearing (upper tier sensor) during a given sample period.
4.6. Behavioral testing
Control
After 3–4 days of adaptation to the experimental room, each rat was placed in the open-field testing cage for 42 days. This cage becomes the animal’s home cage. Once a day at 07:00 h, each cage was cleaned and water and food were given after which locomotor activity resumed, i.e., continuous recording for 42 days. Data was evaluated for 42 days of daylight activities, 42 nights of dark activities, and 42 24-h day and night together and weekly activities.
MPD experiment
On every testing day (see Table 1), each rat was placed in an open-field cage and habituated for 15 to 20 minutes, after which saline/MPD injection was administered and subsequent locomotor activity was recorded for 120 minutes. On experimental day 1 (see Table 1), all animals were injected with saline prior to recording activity. On experimental days 2, 6, and 11, locomotor activity was obtained following injection of 2.5 mg/kg MPD.
4.7. Analysis of behavioral data
The behavioral activity following saline administration on experimental day 1 served as the control baseline activity. Previous studies showed that the duration of MPD effects on the rat’s locomotor activity was approximately 50–80 min, depending on the MPD dose (Gaytan et al., 1997a; Yang et al., 2003); therefore, cumulative locomotor activity was recorded for 120 min after each saline/MPD treatment. Observations within a treatment group and between treatment groups were analyzed using Analysis of Variance (ANOVA: experimental days and treatment). Any statistical significance was determined with the post-hoc Fischer’s LSD method. The statistical significance was set at P < 0.05 for all comparisons.
4.8. Sensory evoked field potentials recording
Three days before commencement of the experiment, rats were transferred to the electrophysiological testing cage connected to the electrophysiological system via a commutator for habituation to the electrophysiological experiment. Fifty acoustic click stimuli were given and repeated every 10 min for 2 h on each of the three adaptation days. On experimental day 1 after the behavioral recording, the rats were transferred to a cubic Plexiglas box (23 cm3) located inside a sound-insulated, electrophysiological test chamber and connected to the electrophysiological system for acclimation to the electrophysiologically testing conditions.
Animal was allowed to habituate for 30 to 40 min before commencement of the experiment. The electrodes in the rat’s head were connected to a Grass P511 amplifier through the emitter follower by means of low noise leads to a commutator mounted on a counterbalanced arm that allowed the rat to move about freely in the Plexiglas box. The amplifier output was connected to the Micro 1041 (Cambridge Electronic Design, Cambridge, UK) for data collection and to multi-beam oscilloscopes to monitor neuronal activities. The Micro 1041 was connected to a PC computer equipped with the Spike 2 software (Cambridge Electronic Design) for digitizing, averaging, and storing the data in the hard drive for off-line evaluation. Acoustic stimulation was in the form of ‘clicks’ produced by a Grass AC-5 ultralinear, audio-stimulator connected to a remote speaker (10.0 cm in diameter), which was placed 1 cm in front of the Plexiglas box and triggered by a 4–5 volt square pulse of 0.1 ms in duration. Measuring the intensity of the click stimulus in the Plexiglas before the experiment was from peak to peak amplitude of 80 DB SPL (re 0.0002 dyne/cm2). A digitimer device (Medical System #3290) triggered the acoustic stimulator and all other electronic equipments. Fifty consecutive click stimuli, each at every 2.4 second, was presented (1 set) and used to produce one average. Four such sets were presented at 10-min intervals after each treatment (saline, 0.6, 2.5, and 10.0 mg/kg MPD, respectively). These four averages, each of 50 stimuli, were averaged to produce final amplitude for comparison between the control and the different drug doses (see Fig. 3B and 3C). Each stimulating session lasted 2 min (50 stimuli every 2.4 sec = 120 sec = 2 min), i.e., time interval between each set was 10 min.
4.9. Histological verification of electrode placement
At the conclusion of the experiments, the rats were overdosed with sodium pentobarbital. A lesion was produced at the tip of each of the electrodes by passing a 50 μA direct current for 30 sec. The rats’ brain was transcardially perfused with a 10% formalin solution containing 3% potassium ferrocyanide. Brain slices were cut serially at a thickness of 80–100μ using a vibrotome (OTS-3000-03; FHC, Brunswick, ME, USA) and histologically stained with Cresyl violet. The position of the electrode tips were identified by the location of the lesion and the Prussian blue spot. After histological verification, it was found that only 7 electrodes were located within the PFC and 19 electrodes within the CN. Recordings from these verified PFC and CN location were analyzed and presented.
4.10. Electrophysiological data analysis
Fifty sensory evoked responses (1 set) were averaged off-line using the Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Four sets of recordings, each following saline, 0.6, 2.5, and 10.0 mg/kg MPD, were obtained. Each average of acoustic evoked responses (AAER) was evaluated in terms of amplitude (μv) of the characteristic components. Amplitudes were measured from peak to peak. The AAER of a rat consisted of five main amplitude/components: P1, N1, P2, N2, and P3 (Dafny 1975a, 1978; Dafny et al., 1981; Yang et al., 2006). The ‘P’ indicates positive amplitude, while the ‘N’ indicates negative amplitude. The integers indicate the first, second, or third positive or negative component. That is, P1 refers to the first positive component of the evoked field response amplitude. The P1 and N1 components were not consistent within and between rats and therefore were not evaluated. The P2, N2, and P3 components were the most consistent components within and between rats and therefore were analyzed.
Changes induced by each of the three doses of MPD were analyzed by comparing the control amplitude of each component averaged (e.g., P2) to that obtained following drug injection recording. Results were analyzed by repeated measures ANOVA with the post-hoc Fischer’s LSD method for treatment, day, and brain site comparisons. A significant level of 0.05 was applied throughout all analyses. Each animal served as its own control.
Acknowledgments
The authors wish to thank Mallinckrodt, Inc. for its gift of methylphenidate. This research was supported in part by the Pat Rutherford Chair in Psychiatry (ACS) and the National Research Service Award from the National Institutes of Health (Grant # F31-DA14441: PBY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Accardo P, Blondis TA. What’s all the fuss about Ritalin? J Pediatrics. 2001;138:6–9. doi: 10.1067/mpd.2001.111505. [DOI] [PubMed] [Google Scholar]
- Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: a “natural action” approach to movement sequence. J Neurosci. 1998;18:2777–2787. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JC, Williams S, McGee R, Silva PA. DSM-III disorders in preadolescent children. Prevalence in a large sample from the general population. Arch Gen Psychiatry. 1987;44:69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Barbaresi W, Katusic S, Colligan R, Oankratz V, Weber K, Mrazek D, et al. How common is attention-deficit/hyperactivity disorder? Incidence in a population-based birth cohort in Rochester, Minn. Arch Pediatric Adolesc Medicine. 2002;156:217–224. doi: 10.1001/archpedi.156.3.217. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV, Spencer T, Wilens T, Mick E, Lapey KA. Gender differences in a sample of adults with attention deficit hyperactivity disorder. Psychiatry Res. 1994;53:13–29. doi: 10.1016/0165-1781(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiari G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47:47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings. 2000;75:711–721. doi: 10.4065/75.7.711. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougal SA, Meier TL, Collins RL, Watson JB. Repeated methylphenidate treatment induces behavioral sensitization and decreases protein kinase A and dopamine-stimulated adenylyl cyclase activity in the dorsal striatum. Psychopharmacology. 1998;136:34–43. doi: 10.1007/s002130050536. [DOI] [PubMed] [Google Scholar]
- Dafny N. Catecholamine-modulated field potentials in the hypothalamus. Neuroendocrinology. 1975a;18:42–54. doi: 10.1159/000122382. [DOI] [PubMed] [Google Scholar]
- Dafny N. Effect of substantia nigra stimulation on spontaneous unit activity in the caudate nucleus. Exp Neurol. 1975b;47:503–508. doi: 10.1016/0014-4886(75)90082-5. [DOI] [PubMed] [Google Scholar]
- Dafny N. Electrophysiological properties of caudate neurons following substantia nigra, motor cortex, and amygdaloid nuclear complex stimulation of the rat. Appl Neurophysiol. 1975c;38:259–272. doi: 10.1159/000102668. [DOI] [PubMed] [Google Scholar]
- Dafny N. Neurophysiological approach as a tool to study the effects of drugs on the central nervous system: dose effect of pentobarbital. Exp Neurol. 1978;59:263–274. doi: 10.1016/0014-4886(78)90155-3. [DOI] [PubMed] [Google Scholar]
- Dafny N. Is interferon-alpha a neuromodulator? Brain Res Rev. 1998;26:1–15. doi: 10.1016/s0165-0173(97)00029-5. [DOI] [PubMed] [Google Scholar]
- Dafny N, Dauth G, Gilman S. Differential effects of agents which alter CNS monoamine levels upon acoustic responses in the basal ganglia of freely moving rats. International J Neurology. 1975;10:53–67. [PubMed] [Google Scholar]
- Dafny N, Feldman S. Single cell activity in the hypothalamus in intact and adrenalectomized rats. Physiol Behav. 1970;5:873–878. doi: 10.1016/0031-9384(70)90174-5. [DOI] [PubMed] [Google Scholar]
- Dafny N, Gildenberg P. Morphine effects on spontaneous, nociceptive, antinociceptive and sensory evoked responses of parafasciculus thalami units in morphine naive and morphine dependent rats. Brain Res. 1984;323:11–20. doi: 10.1016/0006-8993(84)90260-9. [DOI] [PubMed] [Google Scholar]
- Dafny N, Marchand J, McClung R, Salamy J, Sands S, Wachtendorf H, Burks TF. Effects of morphine on sensory-evoked responses recorded from central gray, reticular formation, thalamus, hypothalamus, limbic system, basal ganglia, dorsal raphe, locus ceruleus, and pineal body. J Neurosci Res. 1981;5:399–412. doi: 10.1002/jnr.490050505. [DOI] [PubMed] [Google Scholar]
- Dafny N, Yang PB. The role of age, genotype, sex, and route of acute and chronic administration of methylphenidate: A review of its locomotor effects. Brain Res Bul. 2006;68:393–405. doi: 10.1016/j.brainresbull.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eichlseder W. Ten years of experience with 1,000 hyperactive children in a private practice. Pediatr. 1985;76:176–184. [PubMed] [Google Scholar]
- Einhorn LC, Johansen PA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: studies in the ventral tegmental area. J Neurosci. 1988;8:100–112. doi: 10.1523/JNEUROSCI.08-01-00100.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emson PC, Koob GF. The origin and distribution of dopamine-containing afferents to the rat frontal cortex. Brain Res. 1978;142:249–267. doi: 10.1016/0006-8993(78)90634-0. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaytan O, Al-Rahim S, Swann A, Dafny N. Dose-related effects of MK-801 on acute and chronic methylphenidate administration. Life Sci. 1997a;61:PL101–PL107. doi: 10.1016/s0006-8993(98)01035-x. [DOI] [PubMed] [Google Scholar]
- Gaytan O, Ghelani D, Martin S, Swann A, Dafny N. Methylphenidate: diurnal effects on locomotor and stereotypic behavior in the rat. Brain Res. 1997b;777:1–12. doi: 10.1016/s0006-8993(97)00880-9. [DOI] [PubMed] [Google Scholar]
- Gittelman R, Marmuzza S, Shcnker R, Bonagura N. Hyperactive boys almost grown up. I. Psychiatric status. Arch Gen Psychiat. 1985;42:937–947. doi: 10.1001/archpsyc.1985.01790330017002. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Clinical effects of stimulant medication in attention deficit/hyperactivity disorder (ADHD) In: Solanto MV, Arnsten AFT, Castellanos FX, editors. Stimulant drugs and ADHD: basic and clinical neuroscience. New York: Oxford UP; 2001. pp. 31–71. [Google Scholar]
- Gresch PJ, Sved AF, Zigmond MJ, Finlay JM. Local influence of endogenous norepinephrine on extracellular dopamine in rat medial prefrontal cortex. J Neurochem. 1995;65:111–116. doi: 10.1046/j.1471-4159.1995.65010111.x. [DOI] [PubMed] [Google Scholar]
- Himelstein J, Halperin JM. Identification of AD/HD subtypes using laboratory-based measures: a cluster analysis. CNS Spectrums. 2000;5:58–64. [Google Scholar]
- Izenwasser S, Coy AE, Ladenheim B, Loeloff RJ, Cadet JL, French D. Chronic methylphenidate alters locomotor activity and dopamine transporters differently from cocaine. Eur J Pharmacol. 1999;373:187–193. doi: 10.1016/s0014-2999(99)00274-5. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Effect of acute and daily cocaine treatment on extracellular dopamine in the nucleus accumbens. Synapse. 1990;5:48–58. doi: 10.1002/syn.890050104. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Pierce RC, Cornish J, Sorg BA. A role for sensitization in craving and relapse in cocaine addiction. J Psychopharmacol. 1998;12:49–53. doi: 10.1177/026988119801200107. [DOI] [PubMed] [Google Scholar]
- Kaufmann MW, Cassem NH, Murray GB, Jenike M. Use of psychostimulants in medically ill patients with neurological disease and major depression. Can J Psychiatry. 1984;29:46–49. doi: 10.1177/070674378402900112. [DOI] [PubMed] [Google Scholar]
- Kollins SH, MacDonald EK, Rush CR. Assessing the abuse potential of methylphenidate in nonhuman and human subjects: a review. Pharmacol Biochem Behav 2001. 2001;68:611–627. doi: 10.1016/s0091-3057(01)00464-6. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Locomotor effects of acute and repeated threshold doses of amphetamine and methylphenidate: relative roles of dopamine and norepinephrine. J Pharmacol Exp Ther. 2001;296:876–883. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Exposure of adolescent rats to oral methylphenidate: preferential effects on extracellular norepinephrine and absence of sensitization and cross-sensitization to methamphetamine. J Neurosci. 2002;22:7264–7271. doi: 10.1523/JNEUROSCI.22-16-07264.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacol (Berl) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Lindholm E, Koriath JJ. Analysis of multiple event related potential components in a tone discrimination task. Int J Psychophysiol. 1985;3:121–129. doi: 10.1016/0167-8760(85)90032-7. [DOI] [PubMed] [Google Scholar]
- MacDonald EK, Kollins SH. Assessing the reinforcing effects of methylphenidate in children diagnosed with attention deficit hyperactivity disorder using a choice procedure. Drug Alcohol Dependence 2000. 2000;60:S134. [Google Scholar]
- MacDonald FE, Kollins SH. A pilot study of methylphenidate preference assessment in children diagnosed with attention deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2005. 2005;15:729–741. doi: 10.1089/cap.2005.15.729. [DOI] [PubMed] [Google Scholar]
- Mazei MS, Pluto CP, Kirkbride B, Pehek EA. Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 2002;936:58–67. doi: 10.1016/s0006-8993(02)02542-8. [DOI] [PubMed] [Google Scholar]
- McNamara CG, Davidson ES, Schenk S. A comparison of the motor-activating effects of acute and chronic exposure to amphetamine and methylphenidate. Pharmacol Biochem Behav. 1993;45:729–732. doi: 10.1016/0091-3057(93)90532-x. [DOI] [PubMed] [Google Scholar]
- Michie PT, Fox AM, Ward SV, Catts N, McConaghy N. Event-related potential indices of selective attention and cortical lateralization in schizophrenia. Psychophysiology. 1990;27:209–227. doi: 10.1111/j.1469-8986.1990.tb00372.x. [DOI] [PubMed] [Google Scholar]
- Miserendino MJD, Nestler EJ. Behavioral sensitization to cocaine: modulation by the cyclic AMP system in the nucleus accumbens. Brain Res. 1995;674:299–306. doi: 10.1016/0006-8993(95)00030-t. [DOI] [PubMed] [Google Scholar]
- Musser CJ, Ahmann FW, Mundt P, Broste SK, Mueller-Rizner N. Stimulant use and the potential for abuse in Wisconsin as reported by school administrators and longitudinally followed children. J Dev Behav Pediatr. 1998;19:187–192. doi: 10.1097/00004703-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Behav Brain Sci. 1990;13:201–288. [Google Scholar]
- Nakamura T, Ghilardi MF, Mentis M, et al. Hum Brain Mapp. 2001;12:42–60. doi: 10.1002/1097-0193(200101)12:1<42::AID-HBM40>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health Consensus Development Conference Statement (NIHCDCS) J Am Acad Child Adolesc. Psychiatry. 2000;39:182–193. doi: 10.1097/00004583-200002000-00018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard R, Johnson R. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Pierce CR, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Invernizzi R, Cervo L, Vallebuona F, Samanin R. Evidence that extracellular concentrations of dopamine are regulated by noradrenergic neurons in the frontal cortex of rats. J Neurochem. 1994;63:195–200. doi: 10.1046/j.1471-4159.1994.63010195.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rowland A, Umbach D, Catoe K, Stallone L, Long S, Rabiner D, et al. Studying the epidemiology of attention-deficit hyperactivity disorder: screening method and pilot results. Canadian J Pyschiatry. 2001;46:931–940. doi: 10.1177/070674370104601005. [DOI] [PubMed] [Google Scholar]
- Rubia K, Overmeyer S, Taylor E, Brammer M, Williams SC, Simmons A, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am J Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, Bergstrom DA, Shenker A, Freeman LE, Baek D, Walters JR. Drugs used in the treatment of attention-deficit/hyperactivity disorder affect postsynaptic firing rate and oscillation without preferential dopamine autoreceptor action. Biol Psychiatry. 2001;49:340–350. doi: 10.1016/s0006-3223(00)00987-2. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. Behavioral and neurochemical characteristics of stimulant-induced augmentation. Psychopharmacological Bulletin. 1987;23:417–424. [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Bush G. Brain function and structure in adults with attention-deficit/hyperactivity disorder. Psychiatr Clin North Am. 2004;27:323–347. doi: 10.1016/j.psc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Sergeant JA, Geurts H, Oosterlaan J. How specific is a deficit of executive functioning for attention-deficit/hyperactivity disorder? Behav Brain Res. 2002;130:3–28. doi: 10.1016/s0166-4328(01)00430-2. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Smith PL, Bunney BS. Endogenous DA-mediated feedback inhibition of DA neurons: involvement of both D(1)- and D(2)-like receptors. Synapse. 2000;35:111–119. doi: 10.1002/(SICI)1098-2396(200002)35:2<111::AID-SYN3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Shi WX, Pun CL, Zhou Y. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- Slopsema JS, Van Der Gugten J, DeBruin JPC. Regional concentrations of noradrenaline and dopamine in the frontal cortex of the rat: dopaminergic innervation of the prefrontal subareas and lateralization of prefrontal dopamine. Brain Res. 1982;250:197–200. doi: 10.1016/0006-8993(82)90970-2. [DOI] [PubMed] [Google Scholar]
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94:127–152. doi: 10.1016/s0166-4328(97)00175-7. [DOI] [PubMed] [Google Scholar]
- Solowij N, Michie PT, Fox AM. Effects of long-term cannabis use on selective attention: an event-related potential study. Pharmacol Biochem Behav. 1991;40:683–688. doi: 10.1016/0091-3057(91)90382-c. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Intra-A10 injection of H7 blocks the development of sensitization to cocaine. Neuroreport. 1994;6:69–72. doi: 10.1097/00001756-199412300-00019. [DOI] [PubMed] [Google Scholar]
- Steketee JD, Striplin CD, Murray TF, Kalivas PW. Possible role for G-proteins in behavioral sensitization to cocaine. Brain Res. 1991;545:287–291. doi: 10.1016/0006-8993(91)91299-g. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Gupta S, Guinta D, Flynn D, Agler D, Lerner M, Williams L, Shoulson I, Wigal S. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305. doi: 10.1016/S0009-9236(99)70038-X. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–78. doi: 10.1016/s0166-4328(01)00433-8. [DOI] [PubMed] [Google Scholar]
- Teo RK, Ferguson DA. The acute effects of ethanol on auditory event-related potentials. Psychopharmacology (Berl) 1986;90:179–184. doi: 10.1007/BF00181237. [DOI] [PubMed] [Google Scholar]
- Vezina P. Amphetamine injected into the ventral tegmental area sensitizes the nucleus accumbens dopaminergic response to systemic amphetamine: an in vivo microdialysis study in the rat. Brain Res. 1993;605:332–337. doi: 10.1016/0006-8993(93)91761-g. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;5:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Winsberg BG, Javitt DC, Shanahan/Silipo G. Electrophysiological indices of information processing in methylphenidate responders. Biol Psychiatry. 1997;42:434–445. doi: 10.1016/s0006-3223(96)00429-5. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Yang PB, Amini B, Swann AC, Dafny N. Strain differences in the behavioral responses of male rats to chronically administered methylphenidate. Brain Res. 2003;971:139–152. doi: 10.1016/s0006-8993(02)04240-3. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Dose-response characteristics of methylphenidate on locomotor behavior and on sensory evoked potentials recorded from the VTA, NAc, and PFC in freely behaving rats. Behavioral and Brain Functions. 2006;2:3. doi: 10.1186/1744-9081-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


